Abstract
Purpose
To investigate the roles of a selective MMP-2 and -9 inhibitor (SB-3CT) in corneal inflammatory lymphangiogenesis.
Methods
The expression of MMP-2 and -9 in the cornea after suture inplacement, treated with SB-3CT or negative control, was detected by real-time polymerase chain reaction (PCR). Inflammatory corneal neovascularization (NV) was induced by corneal suture placement. Mice were treated with SB-3CT eye drops (twice daily for 1 week, 5 μL per drop; 50, 100, or 200 μM). The outgrowth of blood and lymphatic vessels, and macrophage recruitment were analyzed by immunofluorescence assay. The expressions of vascular endothelial growth factor-C (VEGF-C) and its receptor VEGFR-3 were tested by real-time PCR.
Results
MMP-2 and -9 expression were suppressed significantly by treatment with SB-3CT. The data demonstrated, for the first time, that SB-3CT strongly reduced corneal lymphangiogenesis and macrophage infiltration during inflammation. Furthermore, expressions of VEGF-C and its receptor VEGFR-3 were significantly inhibited by SB-3CT during corneal lymphangiogenesis.
Conclusions
These novel findings indicated that blockade of MMP-2 and -9 could inhibit lymphangiogenesis. Further investigation of this factor may provide novel therapies for transplant rejection and other lymphatic disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphatic vessels are critical to organ and tissue maintenance. The lymphatic networks play an important role in the regulation of tissue fluid homeostasis and the return of macromolecules and immune cells to the bloodstream [1, 2]. In its normal state, the cornea is devoid of blood and lymphatic vessels. However, corneal lymphangiogenesis (LG; the development of new lymphatic vessels) can be induced during a number of pathological disorders, such as corneal chronic inflammation, alkali burns and graft rejection [3, 4]. It has also been demonstrated that LG is a primary mediator of corneal transplant rejection [5].
LG is a complex process involving lymphatic endothelial cell (LEC) proliferation, migration, adhesion and interaction with the extracellular matrix (ECM). Matrix metalloproteinases (MMPs), a family of Zn-dependent enzymes, play key roles in degrading ECM associated with angiogenesis and tumor invasion [6, 7]. MMPs are intimately involved in the degradation of ECM, which allows endothelial cells to invade and form vessels [8, 9]. MMPs, especially MMP-2 (gelatinase A) and MMP-9 (gelatinase B), are responsible for regulating most of the turnover of matrix proteins by degrading ECM [10, 11]. In addition, studies using carcinoma cells showed that MMP-2 and -9 increased the release of VEGF through proteolysis of the ECM and promoted tumor progression by enhancing angiogenic actions [12]. Among MMPs, MMP-2 and -9 were produced by LEC, and synthetic MMP inhibitors inhibited LEC tube formation [13, 14]. However, how a blockade of MMP-2 and -9 regulates LG remains an intriguing enigma.
SB-3CT, a highly selective inhibitor of MMP-2 and -9, is known to target gelatinases with high selectivity due to a unique mechanism of inhibition that is catalyzed by the target gelatinases themselves [15]. The prototype compound selectively inhibits MMP-2 with high potency and MMP-9 with somewhat lower activity [16]. It was reported that SB-3CT exhibited profound neuroprotection in mouse models of cerebral ischemia by attenuating ECM proteolysis [17, 18]. In addition, SB-3CT significantly reduced flow-induced outward vascular remodeling, which revealed its critical role in the process [19]. Most angiogenic factors also induce LG, suggesting that the mechanism of LG is partially similar to angiogenesis [20,21,22]. Given these facts, whether SB-3CT reduces LG needs to be investigated.
Hence, we set out to determine whether SB-3CT in vivo could reduce corneal LG. The expressions of MMP-2 and -9, which were highly selectively inhibited by SB-3CT, were determined by real-time PCR. In addition, we further investigated whether SB-3CT reduced the numbers of CD11b+ macrophage infiltration in inflammatory corneas. Finally, we investigated whether VEGF-C/VEGFR-3 signaling pathway was involved in the molecular pathway of corneal LG, after application of SB-3CT.
Materials and methods
Animals and anesthesia
All animal protocols were approved by the local animal care committee of our institute, and were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Male C57BL/6 mice (6–8 weeks old) were purchased from the Laboratory Animal Center (Harbin, China). Mice were anesthetized with intraperitoneal injection of a combination of ketamine and xylazine (120 mg/kg and 20 mg/kg bodyweight, respectively).
Suture-induced inflammatory corneal NV
The mouse model of suture-induced inflammatory corneal NV was used as previously described [23]. The central cornea was marked with a 2-mm diameter trephine and three 11–0 nylon sutures (Lingqiao, Ningbo, China) and then placed in the intrastromal position. The outer point of suture placement was chosen near the limbus, and the inner point was chosen near the corneal center equidistant from the limbus. Sutures were removed after 7 days. Treatment groups received SB-3CT eye drops (twice daily for 1 week, 5 μL per drop; 50, 100, or 200 μM). Control mice received equal amounts of saline solution. Experiments were repeated twice with a total of 12 mice in each group.
Corneal immunofluorescence microscopy assays and quantification
The immunofluorescence experiments were performed as previously described [24]. The excised corneas from the corneal NV assay were rinsed in PBS and fixed in acetone for 30 min. After washing and blocking with 2% BSA in PBS, for 2 h, corneas were stained overnight at 4 °C, with a rabbit anti-mouse LYVE-1 antibody (1:500; Abcam) and a rat anti-mouse CD31 antibody (1:100; BD PharMingen, San Diego, CA, USA). On day 2, the tissue was washed three times and stored at 4 °C in the dark. LYVE-1 antibody was detected with an Alexa Fluor 647-conjugated goat anti-rabbit IgG antibody (1:200; Invitrogen) and a CD31 antibody was detected with Alexa Fluor 488-conjugated goat anti-rat IgG antibody (1:200; Invitrogen). To detect the recruitment of macrophages into the inflamed corneas, a FITC-conjugated rat anti-mouse CD11b antibody (1:100; BD PharMingen) was used.
The stained whole mounts were analyzed with a fluorescence microscope (EVOS f1; AMG, Seattle, WA, USA). Each whole mount picture was quantified using NIH Image analysis software. A detailed explanation of this method was described previously [25]. Each whole mount was assembled from nine pictures taken at 100× magnification. Vascular structures stained as CD31+LYVE-1- were identified as blood vessels, whereas those stained as CD31+LYVE-1+ were defined as lymphatic vessels. The mean vascularized area of the control groups was defined as being 100%; vascularized areas were then related to this value (vessel ratio). For macrophage analysis, 10 areas (eight from the periphery, two from the center) of each cornea were randomly picked and examined, and total numbers of CD11b+ cells were counted throughout the whole thickness of the picked area. The mean number of CD11b+ cells of the control groups was set as 100%; the numbers of macrophages per whole mount were then related to this value (cell ratio).
RNA isolation and real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Total RNA (400 ng) was reverse transcribed using the PrimeScript™ RT reagent kit with gDNA Eraser (Takara, Dalian, China). Quantitative real-time PCR was performed using SYBR® Premix Ex Taq™ II (Takara) in a LightCycler 480 Real-time PCR System (Roche, Basel, Switzerland). Real-time PCR was performed under the following conditions: initial denaturation step of 95 °C for 30 s, 40 cycles of 95 °C for 5 s, and of 60 °C for 30 s, followed by an additional denaturation step of 95 °C for 5 s and 60 °C for 60 s, as a subsequent melt curve analysis to check amplification specificity. All assays were conducted three times, and were performed in triplicate. Results were derived from the comparative threshold cycle method and normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The following primers were used for real-time PCR, as shown in Table 1.
Statistical analysis
Statistical analysis was performed by the Student’s t-test using SPSS version 13.0 software (Armonk, NY, USA). Results were expressed as mean ± SEM and a value of p<0.05 was considered statistically significant. Graphs were drawn using GraphPad Prism, version 5.0 software (San Diego, CA, USA).
Results
Blockade of MMP-2 and -9 by SB-3CT inhibits LG after suture placement
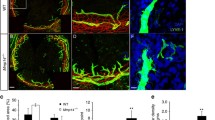
The healthy cornea lacks both blood vessels and lymphatic vessels, but these can be induced when the cornea suffers from severe inflammation. To investigate the effect of SB-3CT on inhibition of MMP-2 and -9, we used a standard suture-induced corneal NV assay. Mice were randomized to receive SB-3CT eye drops (5 μL per drop; 50, 100, or 200 μM) or saline solution after suture placement. On day 7, the densities of CD31-positive blood vessels and LYVE-1-positive lymphatic vessels were detected by immunohistochemistry as described previously [26]. Quantitative immunohistochemical and morphometric analyses clearly revealed that corneal LG were induced by using standard suture placement, and treatment with SB-3CT resulted in a significant reduction of LG in vivo. Corneal LG was significantly inhibited by 25% when used at a dose of 100 μM (p=0.003), and the highest dose (200 μM) showed an even stronger inhibition (34% less; p=0.004), compared with control group. Lower concentrations of SB-3CT (50 μM) eye drops had no significant effect on lymphatic vessel growth (p=0.151; Fig. 1).
Blockade of MMP-2 and -9 by SB-3CT leads to reduced corneal inflammatory LG in vivo. (A−D): Representative images showing corneal whole mounts in eyes treated with saline solution or SB-3CT (50, 100, or 200 μM). Red: LYVE-1. Original magnification, 40×. (E): Summarized data showing that corneal LG was significantly inhibited when used at a dose of 100 μM (by 25%; **p<0.01), and the highest used dose (200 μM) showed an inhibition of 34% (**p<0.01), compared with control group
Effect of MMP-2 and -9 blockade on corneal macrophage infiltration
It is known that macrophages contribute to corneal LG [27]. To test whether the anti-lymphangiogenic effect of SB-3CT was also partially caused by an indirect effect on macrophages (which have been shown to be essential mediators of inflammatory corneal LG [28]), we examined CD11b+ macrophage infiltration into inflamed corneas by using corneal suture model. SB-3CT eye drops were used at the effective dose of 100 μM in the suture-induced inflammatory corneal NV, which could lead to a significant suppression of LG.Treatment with SB-3CT (100 μM) resulted in a significantly reduced recruitment of CD11b+, compared with saline control group (reduction by 39%; p=0.015), as presented in Fig. 2.
Corneal macrophage infiltration was significantly inhibited by treatment of SB-3CT. (A, B, C): Representative images of immunofluorescence microscopy assays show the number of CD11b+ macrophages in the periphery of corneas treated with SB-3CT, saline solution or only suture placement. Original magnification, 200×. (D): Summarized data showing that the number of CD11b+ macrophages was dramatically reduced in eyes treated with SB-3CT, compared with saline control (39% less; *p<0.05)
MMP-2 and -9 expressions in the cornea
We analyzed the impact of SB-3CT on blockage of MMP-2 and -9 expressions in vivo. The Real-time PCR assay clearly demonstrated that MMP-2 and -9 expressions were significantly inhibited in corneas treated with 100 μM SB-3CT (MMP-2: p=0.003; MMP-9: p=0.035), in comparison with the saline control group (Fig. 3). The data indicated that the application of SB-3CT resulted in significant inhibition of MMP-2 and -9 expressions in the inflammatory corneal NV.
MMP-2 and -9 expressions in the cornea. Quantitative real-time PCR analysis of MMP-2 and -9 mRNA were performed in corneas after SB-3CT treatment, saline treatment, or only suture placement. Blockade by SB-3CT (100 μM) led to a significant downregulation of MMP-2 expression (**p<0.01) and MMP-9 expression (*p<0.05), in comparison with saline control group
Treatment with SB-3CT inhibits the expressions of VEGF-C and VEGFR-3
LG were driven by the production of lymphangiogenic growth factors, and the VEGF-C/VEGFR-3 signaling pathway was central to these processes [29]. To further investigate the roles of SB-3CT in LG, we used quantitative real-time PCR to examine VEGF-C ligands and VEGFR-3 receptors. Our results showed that VEGF-C (p=0.021) and VEGFR-3 (p=0.008) expression were significantly reduced in mice treated with SB-3CT, in comparison with the saline control group (Fig. 4).
Discussion
In this study, we provide the first evidence that SB-3CT inhibits corneal LG in vivo in a corneal suture-induced mouse model. We also identified the effects of MMP-2 and -9 on the development of new lymphatic vessels. The results indicated that MMP-2 and -9 are critically involved in corneal LG during inflammatory response, and their blockage is effective in suppressing LG processes in vivo.
MMPs are a group of zinc-binding proteolytic enzymes that participate in ECM remodeling, NV, and LG. Corneal ECM remodeling by MMPs has also been implicated in the maintenance of corneal avascularity. Among 25 MMPs already described, at least 15 have been identified in the cornea, including MMP-2 and -9 [30,31,32,33]. Previous studies have reported that MMP-2 and MMP-9 are produced by LECs [13, 14]. MMP-2 activity contributes to LG in pathologic conditions, during early lymphatic development, and in models of LEC sprouting from lymphatic duct rings or cell spheroids [34]. MMP-2 degrades ECM through its capacity to cleave type IV collagen, and has been considered to play a central role in the process of invasion and metastasis of gynecologic tumors [35, 36]. Furthermore, MMP-9 affects not only invasion and metastasis, but also signal pathways involved both in normal physiology, growth signalling, and LG [37]. Therefore, we postulated that SB-3CT, as a highly selective inhibitor of MMP-2 and -9, might regulate corneal LG.
In our study, corneal LG was significantly reduced in a corneal suture model when SB-3CT was added. We concluded that blockage of MMP-2 and -9 played an important role in the development of new lymphatic vessels. SB-3CT (50 μM) had no significant inhibitory effect on corneal LG, whereas inhibition of LG by SB-3CT started at a dose of 100 μM. There was an even stronger inhibition on LG when SB-3CT was used at the higher dose of 200 μM. We can conclude that the inhibitory effect of SB-3CT on corneal LG needs certain concentrations, so 100 μM SB-3CT was used in subsequent experiments to investigate its potential mechanism. In our study, MMP-2 and -9 expressions were significantly inhibited when SB-3CT (100 μM) was added. Our results demonstrated that SB-3CT might result in significant inhibition of MMP-2 and -9 expressions in the inflammatory corneal NV. This was in agreement with previous results showing that MMP-2 and -9 expressions were significantly reduced with the use of SB-3CT [38, 39]. Taken together, these results demonstrated that MMP-2 and -9 were involved in inflammatory corneal LG, and SB-3CT was an effective inhibitor of MMP-2 and -9.
It is widely accepted that macrophages also strongly contribute to LG [23, 27]. Previous studies have confirmed that large numbers of activated CD11b+ macrophages induced LG during corneal inflammation by trans-differentiating into lymphatic endothelium and by releasing lymphangiogenic growth factors [27]. In our study, treatment with SB-3CT significantly diminishes the numbers of CD11b+ macrophage infiltration in inflammatory corneas, compared with the control group. An earlier study reported that active MMPs might recruit CD11b+ inflammatory cells to the corneal stroma, which is consistent with our findings [40]. We speculated that the anti-lymphangiogenic effect of SB-3CT might also be partially caused by an indirect effect on macrophages.
To further investigate the mechanism through which MMP-2 and -9 regulate corneal LG, we examined whether SB-3CT inhibited corneal LG through the VEGF-C/VEGFR-3 signaling pathway. We detected a marked downregulation of VEGF-C and VEGFR-3 expressions in sutured corneas treated with SB-3CT. In previous studies, VEGF-C is thought to be a dominant factor stimulating LG through binding to its VEGFR-3 receptor. The outgrowth of lymphatic vessels is primarily triggered by VEGF-C [41, 42], and the specific inhibition of VEGFR-3 alone is sufficient enough to block corneal LG [43]. These investigations suggest that the VEGF-C/VEGFR-3 signaling pathway is critical for corneal LG.
Several studies have shown that there was a close correlation between VEGF-C protein expression and MMP-2 activity, and MMP-2 was significantly elevated in the medium of VEGF-C-treated cells [44, 45]. In addition, MMP-2 activity contributes to LG in pathologic conditions during early lymphatic development, and MMP-2 blockage or down-regulation leads to reduced LG [34]. Previous work have also shown that the inhibition of VEGF-C gene in the T24 cells revealed a decrease in the protein level of MMP-9, and MMP-9 in conjunction with VEGF-C promoted LG and lymph node metastasis of breast cancer [46, 47], which proves their close relations. Our data presented here showed that VEGF-C and VEGFR-3 expressions were significantly reduced, when SB-3CT inhibited corneal LG. It was possible, therefore, that MMP-2 and -9 induced corneal LG by upregulating the expressions of VEGF-C and VEGFR-3.
In summary, through the use of a corneal sutured model, our data showed that SB-3CT, a highly selective inhibitor of MMP-2 and -9, inhibited corneal LG in vivo. Its important role was closely correlated with CD11b+ macrophage infiltration, and VEGF-C/VEGFR-3 signaling pathway. Although the mechanisms of MMP-2 and -9 involvement in corneal LG are not yet understood completely, our finding will attract more interest and efforts to further investigate their roles in LG.
References
Ji RC (2006) Lymphatic endothelial cells, lymphangiogenesis, and extracellular matrix. Lymphat Res Biol 4:83–100
Hirakawa S, Detmar M (2004) New insights into the biology and pathology of the cutaneous lymphatic system. J Dermatol Sci 35:1–8
He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S et al (2005) Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res 65:4739–4746
Hosseini H, Nejabat M (2007) A potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med Hypotheses 68:799–801
Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G et al (2010) Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol 184:535–539
Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
McCawley LJ, Matrisian LM (2000) Matrix metalloproteinases: multifunctional contributors to tumor progression. Mol Med Today 6:149–156
Ma DH, Chen JK, Zhang F, Lin KY, Yao JY, Yu JS (2006) Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog Retin Eye Res 25:563–590
Saika S (2007) Yin and yang in cytokine regulation of corneal wound healing: roles of TNF-alpha. Cornea 26:S70–S74
Matrisian LM (1992) The matrix-degrading metalloproteinases. Bioessays 14:455–463
Mott JD, Werb Z (2004) Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol 16:558–564
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K et al (2000) Matrix metalloproteinase 9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2:737–744
Bruyere F, Melen-Lamalle L, Blacher S, Roland G, Thiry M, Moons L et al (2008) Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods 5:431–437
Nakamura ES, Koizumi K, Kobayashi M, Saiki I (2004) Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci 95:25–31
Ikejiri M, Bernardo MM, Bonfil RD, Toth M, Chang M, Fridman R et al (2005) Potent mechanism-based inhibitors for matrix metalloproteinases. J Biol Chem 280:33992–34002
Krüger A, Arlt MJ, Gerg M, Kopitz C, Bernardo MM, Chang M et al (2005) Antimetastatic activity of a novel mechanism-based gelatinase inhibitor. Cancer Res 65:3523–3526
Gu Z, Cui J, Brown S, Fridman R, Mobashery S, Strongin AY et al (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25:6401–6408
Cui J, Chen S, Zhang C, Meng F, Wu W, Hu R et al (2012) Inhibition of MMP-9 by a selective gelatinase inhibitor protects neurovasculature from embolic focal cerebral ischemia. Mol Neurodegener 7:21
Ota R, Kurihara C, Tsou TL, Young WL, Yeghiazarians Y, Chang M et al (2009) Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J Cereb Blood Flow Metab 29:1547–1558
Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y et al (2005) Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A 102:15593–15598
Björndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D et al (2005) Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res 65:9261–9268
Chung ES, Chauhan SK, Jin Y, Nakao S, Hafezi-Moghadam A, van Rooijen N et al (2009) Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands. Am J Pathol 175:1984–1992
Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C et al (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization by macrophage recruitment. J Clin Invest 113:1040–1050
Tang XL, Sun JF, Wang XY, Du LL, Liu P (2010) Blocking neuropilin-2 enhances corneal allograft survival by selectively inhibiting lymphangiogenesis on vascularized beds. Mol Vis 16:2354–2361
Bock F, Onderka J, Hos D, Horn F, Martus P, Cursiefen C (2008) Improved semiautomatic method for morphometry of angiogenesis and lymphangiogenesis in corneal flatmounts. Exp Eye Res 87:462–470
Zhang H, Hu X, Tse J, Tilahun F, Qiu M, Chen L (2011) Spontaneous lymphatic vessel formation and regression in the murine cornea. Invest Ophthalmol Vis Sci 52:334–338
Watari K, Nakao S, Fotovati A, Basaki Y, Hosoi F, Bereczky B et al (2008) Role of macrophages in inflammatory lymphangiogenesis: Enhanced production of vascular endothelial growth factor C and D through NF-kappaB activation. Biochem Biophys Res Commun 377:826–831
Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita T et al (2005) Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest 115:2363–2372
Folkman J (1995) Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1:27–31
Dong Z, Ghabrial M, Katar M, Fridman R, Berk RS (2000) Membrane-type matrix metalloproteinases in mice intracorneally infected with Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci 41:4189–4194
Kato T, Kure T, Chang JH, Gabison EE, Itoh T, Itohara S et al (2001) Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett 508:187–190
Dushku N, John MK, Schultz GS, Reid TW (2001) Pterygia pathogenesis: corneal invasion by matrix metalloproteinase expressing altered limbal epithelial basal cells. Arch Ophthalmol 119:695–706
Maguen E, Zorapapel NC, Zieske JD, Ninomiya Y, Sado Y, Kenney MC (2002) Extracellular matrix and matrix metalloproteinase changes in human corneas after complicated laser-assisted in situ keratomi-leusis (LASIK). Cornea 21:95–100
Detry B, Erpicum C, Paupert J, Blacher S, Maillard C, Bruyère F et al (2012) Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood 119:5048–5056
Ueda M, Terai Y, Kumagai K, Ueki K, Kanemura M, Ueki M (2001) Correlation between thymidine phosphorylase expression and invasion phenotype in cervical carcinoma cells. Int J Cancer 91:778–782
Ueda M, Terai Y, Kumagai K, Ueki K, Yamaguchi H, Akise D et al (2001) Vascularendothelial growth factor-C gene expression is closely related to invasion phenotype in gynecological tumor cells. Gynecol Oncol 82:162–166
Kessenbrock K, Plaks V, Werb Z (2010) Matrix Metalloproteinases: Regulators of the Tumor Microenvironment. Cell 141:52–67
Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM et al (2013) Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One 8:e76904
Yu F, Kamada H, Niizuma K, Endo H, Chan PH (2008) Induction of MMP-9 expression and endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma 25:184–195
Ebrahem Q, Chaurasia SS, Vasanji A, Qi JH, Klenotic PA, Cutler A et al (2010) Cross-talk between vascular endothelial growth factor and matrix metalloproteinases in the induction of neovascularization in vivo. Am J Pathol 176:496–503
Ji RC (2012) Macrophages are important mediators of either tumor- or inflammation-induced lymphangiogenesis. Cell Mol Life Sci 69:897–914
Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH et al (2007) The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer 96:541–545
Bock F, Onderka J, Dietrich T, Bachmann B, Pytowski B, Cursiefen C (2008) Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol 246:115–119
Ueda M, Hung YC, Terai Y, Kanda K, Kanemura M, Futakuchi H et al (2005) Vascular endothelial growth factor-C expression and invasive phenotypein ovarian carcinomas. Clin Cancer Res 11:3225–3232
Zhao T, Zhao W, Meng W, Liu C, Chen Y, Sun Y (2014) Vascular endothelial growth factor-C: its unrevealed role in fibrogenesis. Am J Physiol Heart Circ Physiol 306:H789–796
Zheng SQ, Huang RQ, Zhang YJ (2010) Role of matrix metalloproteinase (MMP)-2 and -9 and vascular endothelial growth factor C in lymph node metastasis of breast cancer. Zhonghua Bing Li Xue Za Zhi 39:240–244
Zhang HH, Qi F, Zu XB, Cao YH, Miao JG, Xu L et al (2012) A proteomic study of potential VEGF-C-associated proteins in bladder cancer T24 cells. Med Sci Monit 18:BR441–449
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Rights and permissions
About this article
Cite this article
Du, HT., Du, LL., Tang, XL. et al. Blockade of MMP-2 and MMP-9 inhibits corneal lymphangiogenesis. Graefes Arch Clin Exp Ophthalmol 255, 1573–1579 (2017). https://doi.org/10.1007/s00417-017-3651-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3651-8