Abstract
Purpose
To evaluate a modified treat-and-extend (TAE) regimen of intravitreal aflibercept injection (IAI) for treatment-naïve patients with neovascular age-related macular degeneration (AMD).
Methods
Thirty-six eyes (36 patients) treated with the modified TAE regimen were evaluated at 12 months retrospectively. The modified TAE regimen consisted of three steps: 1) an induction phase, during which patients were treated with ≥ 3-monthly IAIs until exudative activity disappeared, 2) an observation phase, during which patients were monitored until exudative activity appeared, and 3) a TAE phase, for which the initial treatment interval was determined based on the disease recurrence interval, followed by treatment intervals changing by 2 weeks.
Results
Mean logMAR BCVA improved significantly from 0.48 ± 0.51 at baseline to 0.40 ± 0.53 at 12 months (P < 0.01), and was maintained (losing <0.3 logMAR units) in 35 eyes (97.2 %). Mean central retinal thickness and central choroidal thickness decreased significantly after 12 months. In the TAE phase, the distribution of treatment intervals was ≥8 weeks in 64.7 % (11 eyes) at 12 months. The mean number of injections was 4.53.
Conclusion
A modified TAE regimen of IAI for neovascular AMD produced good functional outcomes over 12 months with the small number of injections.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the elderly populations of industrialized countries [1, 2]. Vascular endothelial growth factor (VEGF) plays a key role in the pathogenesis of AMD [3]. Today, intravitreal anti-VEGF injection is the primary treatment for neovascular AMD. In Japan, pegaptanib (Macugen; OSI/Eyetech, Melville, NY, USA), ranibizumab (Lucentis; Genentech, Inc., South San Francisco, CA, USA) and aflibercept (Eylea; Regeneron, Tarrytown, NY, USA, and Bayer, Berlin, Germany) are the approved anti-VEGF agents for neovascular AMD. Although pegaptanib, which inhibits VEGF165 selectively, has a favorable safety profile in the treatment of patients with neovascular AMD [4], few patients achieve improvements in visual acuity with intravitreal pegaptanib injections [5]. Thus, most patients with neovascular AMD receive intravitreal ranibizumab or aflibercept injections to obtain improvements in VA [6–8].
Aflibercept appears to be one of the most effective agent, because some studies have reported that aflibercept is also effective for patients with neovascular AMD refractory to ranibizumab [9–12]. Nevertheless, most patients need continuous treatment to maintain the initial visual gain, even if treated with aflibercept. According to the VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD (VIEW 1 and 2) Studies, aflibercept (2 mg every 2 months after three initial monthly doses) is noninferior and clinically equivalent to ranibizumab 0.5 mg monthly [13]. Thus, a bimonthly after three initial monthly dosing regimen has been recommended when treatment-naïve patients with neovascular AMD are treated with aflibercept in Japan. However, this regimen is overtreatment for patients who never show signs of recurrence after the initial loading dose of three monthly intravitreal injections, and might be undertreatment for patients who show signs of recurrence within 2 months, resulting in deterioration of visual function over the long term.
Many studies have demonstrated that the treat and extend (TAE) regimen, which is an individualized proactive dosing regimen, allowed for similar visual improvements with fewer injections and visits compared with monthly treatments when used for patients treated with bevacizumab (Avastin; Genentech Inc., South San Francisco, CA, USA) and ranibizumab [14, 15]. Therefore, medical facilities using the TAE regimen for patients with neovascular AMD have also increased in Japan. However, the standard TAE regimen might also be overtreatment for patients who do not show signs of recurrence after the initial loading dose, as well as the regimen in the VIEW study, because of lacking the observation phase, during which patients are observed without treatment until exudative activity reappears, as well as the pro re nata (PRN) dosing regimen.
It is very important to minimize the number of injections, because frequent injections result in an increased risk of severe ocular or systemic adverse events, and place a heavy burden on patients, institutions, and the medical economy.
Mantel and colleagues reported the outcomes of the observe-and-plan regimen with ranibizumab for neovascular AMD [16]. This regimen contained an observation phase after the induction phase, and patients were treated in an individually adapted treatment plan of a series of injections with a fixed interval if exudative activity appeared. That study demonstrated that the observe-and-plan regimen allowed for similar visual improvements with fewer clinic visits compared with other regimens, although the number of injections was similar among the observe-and-plan, PRN, and TAE regimens.
We explored a beneficial treatment regimen for all patients with neovascular AMD. The standard TAE regimen is feasible for determining an individual treatment interval, because interindividual recurrence intervals are variable. It might be useful to observe patients until exudative activity appears after the induction phase in order to avoid overtreatment. From these considerations, when our patients with neovascular AMD received intravitreal ranibizumab or aflibercept injections, we used a modified TAE regimen, which consists of an induction phase, an observation phase, and a TAE phase. This is a report of the 1-year results of a modified TAE regimen of aflibercept using.
Materials and methods
Study patients
We retrospectively reviewed 56 eyes with treatment-naïve neovascular AMD which underwent an initial loading dose of at least three monthly intravitreal injections of aflibercept 2 mg at Kansai Medical University between August 2013 and December 2014, and completed 1 year of follow-up. Two eyes were excluded from this study due to the use of other treatment regimens, which were fixed dosing and standard TAE regimens. Thirty-six eyes (36 patients) were treated with the modified TAE regimen, and 37 eyes (37 patients) were treated with the pro re nata (PRN) dosing regimen. Each regimen included 19 eyes (19 patients), which underwent only an initial loading dose, because there was not any sign of recurrence until 12 months. These eyes were evaluated at 12 months.
The diagnosis of typical neovascular AMD was confirmed by slit-lamp biomicroscopy, fluorescein angiography (FA) with a Topcon fundus camera (TRC-50DX; Topcon Medical Systems Inc., Oakland, NJ, USA), indocyanine green angiography (ICGA) with a confocal laser scanning ophthalmoscope (HRA2; Heidelberg Engineering GmbH, Heidelberg, Germany), and optical coherence tomography (OCT) with a spectral-domain OCT (Spectralis; Heidelberg Engineering GmbH or RTVue; Optovue, Freemont, CA, USA). Patients with polypoidal choroidal vasculopathy, retinal angiomatous proliferation, pathologic myopia, angioid streaks, idiopathic choroidal neovascularization (CNV), and other secondary CNV were excluded. Exudative activity was confirmed by hemorrhage, choroidal neovascular leakage on FA, and intraretinal or subretinal fluid on OCT.
All patients underwent measurement of best-corrected VA (BCVA) with a Landolt chart, slit-lamp biomicroscopy, and OCT at every visit. The greatest linear dimension (GLD) of CNV at baseline was measured on the basis of FA and ICGA. The central retinal thickness (CRT), defined as the distance between the inner limiting membrane (ILM) and the retinal pigment epithelium (RPE) at the center of the fovea, and the central choroidal thickness (CCT), defined as the distance between Bruch’s membrane and chorioscleral interface at the center of the fovea, were measured on OCT and enhanced depth imaging (EDI)-OCT.
Treatment design
The modified TAE regimen consisted of the following three steps:
-
(1)
An induction phase, during which patients received three or more monthly IAIs (aflibercept 2.0 mg in 0.05 ml volume) until a dry macula was achieved, defined as complete resolution of intraretinal and subretinal fluid without new retinal hemorrhage. Any change on pigment epithelial detachment (PED) was not included on dry macula.
-
(2)
An observation phase, during which patients were monitored monthly until the first signs of exudative activity appeared. The signs of recurrence were defined as any fluid on OCT and new hemorrhage. Decreased BCVA and expansion of PED were not defined as signs of recurrence.
-
(3)
A TAE phase, during which patients received monthly IAIs until a dry macula was achieved, and then the next injection was administered after the initial treatment interval was determined based on the disease recurrence interval, which was the time from when a dry macula was achieved in the induction phase to when the first signs of recurrence appeared. The initial treatment interval was designed to be 1 week or 2 weeks less than the disease recurrence interval. If no signs of recurrence appeared at the point when the initial treatment interval passed since last the IAI before a dry macula was achieved, a new injection was administered, and the period until the next injection was extended by 2 weeks at a time, up to a maximum interval of 12 weeks during the 12 months after the initial IAI in the TAE phase. If signs of recurrence appeared, the treatment interval was shortened by 2 weeks at a time, until a dry macula was achieved. Even if a dry macula was achieved, the treatment interval was not extended, and treatment was continued during the 12 months in order to avoid multiple recurrences. However, if there was no sign of recurrence three consecutive times at 4-week treatment intervals, the treatment interval was extended by 2 weeks. After the fixed treatment intervals during the 12 months, the treatment intervals were extended by 2 weeks at a time, up to a maximum interval of 16 weeks. If there was no sign of recurrence, three consecutive times at 16-week treatment intervals, the proactive treatment was interrupted (Fig. 1).
In the PRN dosing regimen, the need for retreatment is determined at monthly or bimonthly visits. The retreatment criteria included recurrence of intra-/subretinal fluid, or hemorrhages.
Outcome measures
The outcome measures included the following: mean BCVA, CRT, and CCT change, the initial treatment interval, the treatment interval change, and the number of injections over 12 months.
Statistical analysis
All data are presented as mean ± standard deviation. The measured BCVA values were converted to the logarithm of the minimum angle of resolution (logMAR) units for statistical analysis. The proportion of patients with changes in the BCVA of 0.3 logMAR vision or more was also compared. Student’s t-test was used to compare mean age, VA, GLD, CRT, and CCT. Values of P < 0.05 were considered statistically significant.
Results
Of 56 eyes that completed 1 year of follow-up after the induction phase, 36 eyes were evaluated as the modified TAE group retrospectively; 19 eyes (33.9 %) underwent only an initial loading dose, because there was not any sign of recurrence until 12 months, which means that these eyes remained in the observation phase. Seventeen eyes were treated in the TAE phase because signs of recurrence appeared within 1 year. The former group (19 eyes) was defined as the observation phase group, and the latter group (17 eyes) was defined as the TAE phase group. Thirty-seven eyes were evaluated as the PRN group retrospectively.
Baseline characteristics
In the modified TAE group, 22 (22 eyes) were men, and 14 (14 eyes) were women. The mean patient age was 72.3 ± 8.4 years (range, 53–86 years). Eight eyes had predominantly classic, five eyes had minimally classic, and 23 eye had occult with no classic. Patient clinical data at baseline are shown in Table 1. There were no substantial differences between the observation phase group and the TAE phase group at baseline.
In the PRN group, 21 (21 eyes) were men, and 16 (16 eyes) were women. The mean patient age was 72.4 ± 8.38 years (range, 53–82 years). Nine eyes had predominantly classic, and 28 eye had occult with no classic. Patient clinical data at baseline are shown in Table 1. There were no substantial differences between the modified TAE group and the PRN group.
Best-corrected visual acuity
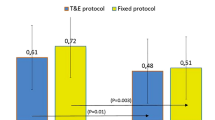
In the modified TAE group, the mean logMAR BCVA improved significantly (P = 0.037) from 0.48 ± 0.51 at baseline to 0.40 ± 0.53 at 12 months. In the PRN group, the mean logMAR BCVA improved significantly (P = 0.039) from 0.47 ± 0.46 at baseline to 0.41 ± 0.46 at 12 months (Fig. 2a).
In the TAE phase group, the mean logMAR BCVA significantly improved (P = 0.038) from 0.35 ± 0.44 at baseline to 0.23 ± 0.46 at 12 months; however, in the observation phase group, there was no significant difference at 12 months (0.56 ± 0.54) compared with baseline (0.60 ± 0.54). The mean logMAR BCVA in the observation phase group was lower than that in the TAE phase group, except at baseline (Fig. 2b).
In the modified TAE group, the BCVA improved 0.3 or more logMAR units in five eyes (13.9 %), decreased 0.3 or more logMAR units in one eye (2.8 %), and remained unchanged in 30 eyes (83.3 %). In the PRN group, the BCVA improved 0.3 or more logMAR units in three eyes (8.1 %), decreased 0.3 or more logMAR units in one eye (2.7 %), and remained unchanged in 33 eyes (89.2 %). There were no substantial differences between the groups (Fig. 3a).
Visual acuity change. The proportion of patients with changes in the BCVA of 0.3 logMAR vision or more at 12 months compared with baseline. a The modified TAE group and the PRN group. b The TAE phase group and the observation phase group in the modified TAE. Improvement = increased 0.3 or more logMAR units, no change = changed less 0.3 logMAR units, deterioration = decreased 0.3 or more logMAR units
There were no substantial differences between the TAE phase group and the observation phase group (Fig. 3b).
Central retinal thickness and central choroidal thickness
In the modified TAE group, the mean CRT decreased significantly from 278 ± 116 μm at baseline to 154 ± 59 μm at 3 months (P < 0.001), and this decrease was maintained to month 12 (167 ± 70 μm, P < 0.001). In the PRN group, the mean CRT decreased significantly from 294 ± 133 μm at baseline to 165 ± 63 μm at 3 months (P < 0.001), and this decrease was maintained to month 12 (175 ± 73 μm, P < 0.001). There were no significant differences between the groups (Fig. 4a).
There were no significant differences between the TAE phase group and the observation phase group (Fig. 4b).
In the modified TAE group, the mean CCT significantly decreased from 254 ± 111 μm at baseline to 225 ± 102 μm at 3 months (P < 0.001), and this decrease was maintained to month 12 (230 ± 108 μm, P < 0.001). In the PRN group, the mean CCT significantly decreased from 240 ± 100 μm at baseline to 209 ± 86 μm at 3 month (P < 0.001), and this decrease was maintained to month 12 (219 ± 95 μm, P < 0.001) (Fig. 5a).
There were no significant differences between the TAE phase group and the observation phase group (Fig. 5b).
Injection frequency
The mean number of IAIs was 4.53 ± 2.02 over 12 months in the modified TAE group; 3.00 in the observation phase group (three in 19 eyes), and 6.24 ± 1.75 in the TAE phase group (four in four eyes, five in two eyes, six in four eyes, seven in two eyes, eight in three eyes, nine in two eyes). In the PRN group, the mean number of IAIs was 4.57 ± 2.50 over 12 months.
In the TAE phase group, the distribution of initial treatment intervals was 4 weeks in 5.9 % (one eye), 6 weeks in 5.9 % (one eye), 8 weeks in 17.6 % (three eyes), 10 weeks in 11.8 % (two eyes), and 12 weeks in 58.8 % (ten eyes). The distribution of treatment intervals at 12 months was 6 weeks in 35.3 % (six eyes), 10 weeks in 17.6 % (three eyes), and 12 weeks in 47.1 % (eight eyes) (Fig. 6). Treatment intervals were extended in one eye (5.9 %; 2 weeks in one eye), shortened in six eyes (35.3 %; 2 weeks in five eyes and 4 weeks in one eye), and remained stable in ten eyes (58.8 %) over 12 months.
Adverse events
During the first year of this study, there were no severe ocular or systemic adverse events.
Discussion
In this study, we demonstrated the 1-year visual and morphological outcomes of IAIs for treatment-naïve patients with neovascular AMD with a modified TAE regimen and PRN dosing regimen.
The mean BCVA improved significantly at 12 months compared with baseline, and was maintained (losing <0.3 logMAR units) in 97.2 % (35 eyes) of 36 eyes over 12 months according to this modified TAE regimen. The VIEW 2 study, in which IAIs were administered every 2 months after three initial monthly doses, demonstrated that the mean BCVA improved by 8.9 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at 52 weeks from baseline (51.6 letters), and was maintained in 95.4 % (losing <15 ETDRS letters) over 52 weeks [13]. In this modified TAE regimen, the mean BCVA was 65.0 letters (+4.0 letters from baseline) at 12 months when the measured logMAR VA values were converted to ETDRS letters. Although the BCVA improvement in this study was less during 1 year because of better VA at baseline, this modified TAE is useful, because the mean BCVA at 1 year was better compared with that in the VIEW 2 study.
In addition, the mean number of IAIs was fewer in this modified TAE regimen (4.5) than in the VIEW 2 study (7.5) over 1 year [13]. One of the reasons for the fewer IAIs in this study could be because the patients using the modified TAE regimen were observed without injections until exudative activity appeared after the induction phase. In the SUSTAIN study, a 12-month, phase III, multicenter, single-arm, open-label trial conducted in ten European countries and Australia to evaluate the safety and efficacy of ranibizumab in treating subfoveal CNV secondary to AMD, 20.5 % of patients who received three initial monthly treatments did not receive any additional dose for months 3 to 11 [17]. Kuroda and colleagues reported that the rate of no recurrence during 1 year was 34.3 % of the eyes which showed complete resolution of retinal exudative change after the three loading intravitreal ranibizumab injections [18]. In this modified TAE regimen, the rate of no recurrence during the 12 months after the induction phase was 33.9 % (19 eyes of 56 eyes). Thus, the bimonthly-after-three-initial-monthly dosing regimen in the VIEW 2 study might be overtreatment for patients who never show signs of recurrence after the induction phase.
To our knowledge, there is no report describing the use of aflibercept for treatment-naïve patients with neovascular AMD using a standard TAE regimen because of the good results obtained in the VIEW study, though there are many reports describing the use of bevacizumab and ranibizumab [14, 15, 19]. In the LUCAS study, patients received monthly injections until exudative changes disappeared, followed by increasing or decreasing intervals between injections depending on disease activity. The mean BCVA improved to 67.2 letters (+7.9 letters) with bevacizumab and 69.6 letters (+8.2 letters) with ranibizumab at 1 year compared with baseline, and was maintained (losing <15 ETDRS letters) in 96.2 % of the eyes treated with bevacizumab and in 95.7 % of the eyes treated with ranibizumab over 1 year. The mean number of injections was 8.9 in the bevacizumab group and 8.0 in the ranibizumab group during the first year [15]. Although it is not suitable to compare the standard TAE and our modified TAE because of the different drugs and treatment protocols in the induction phase, our modified TAE regimen could be expected to produce good functional outcomes with fewer injections compared with the standard TAE regimen during the first year. Reducing the number of injections results in an advantage in terms of medical safety and costs [16].
In addition, the distribution of treatment intervals at 12 months was over 8 weeks in 64.7 % of eyes in this modified TAE regimen. In the LUCAS study, the distribution of treatment intervals at 1 year was over 8 weeks in 41.3 % of eyes for bevacizumab and in 52.4 % of eyes for ranibizumab [15]. These results indicate that aflibercept allows for longer treatment intervals and fewer injections than bevacizumab or ranibizumab when AMD patients were treated according to the standard TAE regimen. Thus, treatment with aflibercept using our modified TAE regimen might be encouraged.
In this modified TAE regimen, the treatment intervals at 12 months changed by more than 4 weeks as compared to the initial treatment interval in one eye (5.9 %). This result suggested that this method of determining the initial treatment intervals was reasonable.
One of the advantages with regard to a standard TAE regimen is that a standard TAE regimen is an individualized proactive dosing regimen, which could be expected to maintain visual function over a long period by preventing a reactivation of disease. In contrast, our modified TAE regimen may raise concerns that the existence of an observation phase with no treatment until the first signs of recurrence may result in aggravation of disease or deterioration of visual function. However, the number of eyes with VA that decreased significantly at the point when first signs of exudative activity appeared compared with at the point when a dry macula was achieved in the induction phase was zero. In addition, the number of eyes that required multiple injections until a dry macula was achieved after the first recurrence was only one (3.9 %; twice in one eye). These results indicate that the observation phase is not likely to generate more resistance to IAIs.
Another advantage with regard to a standard TAE regimen is the reduction in the number of visits. In contrast, our modified TAE regimen requires monthly visits until signs of recurrence appeared, at least 12 weeks after a dry macula was achieved, in order to determine the adequate initial treatment intervals, which resulted in an increase in the number of visits. However, this burden would be necessary to provide ideal, individualized medicine.
The problem with a proactive dosing regimen is that there is no evidence with regard to when the injections should be interrupted, although a guideline on interruption of injection has been issued in Europe [20]. In our protocol, the injections would be interrupted when a dry macula was maintained during 1 year at 16-week treatment intervals, though it is impossible to show a result because of the small number of cases.
In this study, there were no substantial differences between the modified TAE regimen and the PRN dosing regimen over 12 months. However, real-life data on the long-term outcomes for ranibizumab and bevacizumab with the PRN regimen revealed poor functional results [21, 22]. Long-term follow-up will be important to evaluate a difference between the modified TAE regimen and the PRN dosing regimen.
We acknowledge that this study has several limitations. It was an uncontrolled retrospective study with a small number of cases in a short follow-up period. Further prospective studies with larger study populations and longer follow-up would be needed to confirm the usefulness of our regimen. Furthermore, a randomized clinical trial comparing ranibizumab and aflibercept should be planned.
In conclusion, we designed a modified TAE regimen as a treatment protocol of IAI for patients with treatment-naïve neovascular AMD in order to avoid excessive injections, based on the existing proactive dosing regimen, and demonstrated that this regimen allowed for similar visual improvements with fewer injections compared with the bimonthly-after-three-initial-monthly dosing regimen in the VIEW study or the standard TAE regimen during 1 year. This regimen might be the ideal individualized protocol for neovascular AMD with diversity.
References
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP (2004) Global data on visual impairment in the year 2002. Bull World Health Organ 82:844–851
Congdon N, O’Colmain B, Klaver CC, Klein R, Muñoz B, Friedman DS, Kempen J, Taylor HR, Mitchell P, Eye Diseases Prevalence Research Group (2004) Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122:477–485
Augustin AJ, Kirchhof J (2009) Inflammation and the pathogenesis of age-related macular degeneration. Expert Opin Ther Targets 13:641–651
Singerman LJ, Masonson H, Patel M, Adamis AP, Buggage R, Cunningham E, Goldbaum M, Katz B, Guyer D (2008) Pegaptanib sodium for neovascular age-related macular degeneration: third-year safety results of the VEGF Inhibition Study in Ocular Neovascularisation (VISION) trial. Br J Ophthalmol 92:1606–1611
Chakravarthy U, Adamis AP, Cunningham ET Jr, Goldbaum M, Guyer DR, Katz B, Patel M, VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group (2006) Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 113:1508–1521
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, MARINA Study Group (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, ANCHOR Study Group (2009) Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 116:57–65
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD, Ho AC, Ogura Y, Simader C, Jaffe GJ, Slakter JS, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Sowade O, Zeitz O, Norenberg C, Sandbrink R, Heier JS (2014) Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 121:193–201
Yonekawa Y, Andreoli C, Miller JB, Loewenstein JI, Sobrin L, Eliott D, Vavvas DG, Miller JW, Kim IK (2013) Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am J Ophthalmol 156:29–35
Cho H, Shah CP, Weber M, Heier JS (2013) Aflibercept for exudative AMD with persistent fluid on ranibizumab and/or bevacizumab. Br J Ophthalmol 97:1032–1035
Grewal DS, Gill MK, Sarezky D, Lyon AT, Mirza RG (2014) Visual and anatomical outcomes following intravitreal aflibercept in eyes with recalcitrant neovascular age-related macular degeneration: 12-month results. Eye (Lond) 28:895–899
Kawashima Y, Oishi A, Tsujikawa A, Yamashiro K, Miyake M, Ueda-Arakawa N, Yoshikawa M, Takahashi A, Yoshimura N (2015) Effects of aflibercept for ranibizumab-resistant neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 253:1471–1477
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, VIEW 1 and VIEW 2 Study Groups (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548
Abedi F, Wickremasinghe S, Islam AF, Inglis KM, Guymer RH (2014) Anti-VEGF treatment in neovascular age-related macular degeneration: a treat-and-extend protocol over 2 years. Retina 34:1531–1538
Berg K, Pedersen TR, Sandvik L, Bragadóttir R (2015) Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 122:146–152
Mantel I, Niderprim SA, Gianniou C, Deli A, Ambresin A (2014) Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br J Ophthalmol 98:1192–1196
Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO, Weichselberger A, Staurenghi G, SUSTAIN Study Group (2011) Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 118:663–671
Kuroda Y, Yamashiro K, Miyake M, Yoshikawa M, Nakanishi H, Oishi A, Tamura H, Ooto S, Tsujikawa A, Yoshimura N (2015) Factors associated with recurrence of age-related macular degeneration after anti-vascular endothelial growth factor treatment: A retrospective cohort study. Ophthalmology 122:2303–2310
Rayess N, Houston SK III, Gupta OP, Ho AC, Regillo CD (2015) Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol 159:3–8
McKibbin M, Devonport H, Gale R, Gavin M, Lotery A, Mahmood S, Patel PJ, Ross A, Sivaprasad S, Talks J, Walters G (2015) Aflibercept in wet AMD beyond the first year of treatment: recommendations by an expert roundtable panel. Eye (Lond) 29:S1–S11
Wolf A, Kampik A (2014) Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol 252:647–655
Wecker T, Ehlken C, Bühler A, Lange C, Agostini H, Böhringer D, Stahl A (2016) Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. Br J Ophthalmol. doi:10.1136/bjophthalmol-2016-308668
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ohnaka, M., Nagai, Y., Sho, K. et al. A modified treat-and-extend regimen of aflibercept for treatment-naïve patients with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 255, 657–664 (2017). https://doi.org/10.1007/s00417-016-3507-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-016-3507-7