Abstract
Purpose
This prospective study aimed to investigate macular structural characteristics in children with Down syndrome compared to those in healthy children.
Methods
Two groups of children (aged 6–16 years) were enrolled: children with Down syndrome (Down syndrome group, N = 17) and age-matched healthy children who were full-term at birth (control group, N = 18). Eligible patients had visual acuity of 20/100 or better and gestational age at birth of ≥ 36 weeks. Fourier domain optical coherence tomography was used for imaging of the macular retinal structure, and retinal volume scans centered on the macula were obtained. Central subfield thickness (CST) and the thickness of the inner and outer retinal layer regions were analyzed using the instrument’s segmentation software. The analysis of data is provided for the right eye only, since there was no significant difference between right and left eyes for either the Down syndrome or control groups.
Results
Children in the Down syndrome group generally had identifiable retinal structure. The CST for the full retina and inner and outer retinal layers were all significantly greater in the Down syndrome group than the control group (independent t test, all p < 0.05). Despite the significantly thicker macula, only about 29 % (5 of 17) of the right eyes of patients with Down syndrome had macular thickness outside the normal range. Visual acuity in the Down syndrome group was not directly correlated with increased CST (t = 1.288, r = 0.326, p = 0.202).
Conclusions
On average, CST in the Down syndrome group was greater than that in the control group, suggesting abnormal macular development in children with Down syndrome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Children with Down syndrome are known to be at increased risk for ocular abnormalities, including significant refractive error, strabismus, nystagmus, and even cataracts [1–5]. Poor optical quality at an early age has implications for retinal and cortical image quality. Visual functions in these children are also often abnormal, including visual, low-contrast, and vernier acuity, and accommodative ability [6–9, 2, 10–12]. The etiology of this poor visual performance associated with Down syndrome is not fully understood.
Foveal development begins around 24–28 weeks after conception [13–15], and fovea continue to mature until children are at least 4 years of age [16, 17]. Eye growth during childhood is a visually guided process, and degradation or alteration of visual input may disrupt growth [18]. Numerous studies have evaluated the effects of early abnormal visual experience in relation to macular thickness in children with strabismic and anisometropic amblyopia [19–24]. Despite the obvious ocular problems, in light of the unique challenges of retinal imaging in children, there are few reports on retinal structure in children with Down syndrome. We occasionally see case reports on Down syndrome children with bilateral macular coloboma [25], optic disc elevation, or other retinal abnormalities [26–28]. Although there are published reports discussing these issues, much of the current data are considerably variable. For example, Stirn et al. noted that among a total of 65 Down syndrome children who visited their hospital, 32 % suffered from abnormalities of the retinal vessels, foveal hypoplasia, or retinal pigment epithelium hyperplasia [29]. Fimiani et al., however, reported retinal anomalies in only 6 % of 157 Italian children with Down syndrome [30]. As such, it is uncertain whether abnormalities in retinal structure are common in these children, or whether it is simply that our knowledge of the foveal structure in children with Down syndrome is extremely limited.
Optical coherence tomography (OCT) is an increasingly employed non-invasive and high-resolution imaging technique that offers the potential to quantitatively and objectively evaluate the tissue architecture of the fovea. In the present study, using Fourier domain optical coherence tomography (fdOCT), we investigated retinal macular structural features in children with Down syndrome (Down syndrome group) compared to those of age-matched healthy children (control group).
Methods
The institutional review board of Indiana University approved this research protocol as well as the Health Insurance Portability and Accountability Act (HIPAA)-compliant forms. Informed consent was obtained from the subjects' parents, and assent was obtained from subjects over 7 years of age. The ocular conditions of all participants were diagnosed and continually cared for by pediatric ophthalmologists at Indiana University School of Medicine.
Participants
Two groups of children aged 6 to 16 years were enrolled: Down syndrome group (N = 17) and control group (N = 18).
Inclusion criteria in the Down syndrome group
Participants were born with Down syndrome, with the diagnosis of Down syndrome usually reported by a primary care physician before the patient was recommended to our ophthalmologists. Down syndrome can be diagnosed during pregnancy or after birth. For children born with Down syndrome, there is a common blood test to determine the number of the baby’s chromosomes. Prenatal screenings/diagnostic tests can take place during pregnancy in order to determine the likelihood of the child being born with Down syndrome. Gestational age at birth of all participants was over 36 weeks.
Inclusion criteria in the control group
We enrolled healthy age-matched children who were full-term at birth as the control group. Because Down syndrome patients often have hyperopia [1–5], we allowed several age-matched children with hyperopia to be enrolled in the study, as well as children with emmetropia. The control group had a range of spherical equivalents (−1.75 D to 6.5 D) similar to that in the children with Down syndrome.
Exclusion criteria
Children with glaucoma, cataracts, corneal transplant, or co-existing ocular or congenital infections were excluded.
Study procedures
At the time of retinal imaging, a cycloplegic refraction was performed using streak retinoscopy. Refraction was recorded as the spherical equivalent (SEQ, the sum of the sphere power and half of the cylinder power). Visual acuity (VA) was measured with Snellen letters.
Imaging procedures
Macular thickness was measured using high-resolution fdOCT (iVue; Optovue Inc., Fremont, CA, USA). Two or three macular volume scans across the fovea were obtained for each eye, and the volume scan with the highest signal-to-noise ratio was selected. Average sectional total thickness was automatically determined by the instrument’s software using a modified Early Treatment Diabetic Retinopathy Study (ETDRS) circle grid (center, middle, and outer rings of 1, 2, and 3 mm, respectively). The instrument’s segmentation software was used to calculate the thickness of a central subfield 1 mm in diameter, and the thickness of the inner and outer retinal layer regions were measured. The sectional thickness of the total, inner, and outer retinal layer regions of the middle ring (superior, nasal, inferior, and temporal sections) were also measured and compared to those of the control group. The inner layers included the retinal nerve fiber layer, ganglion cell layer, inner plexiform layer, and inner nuclear layer (INL), while the outer layers comprised the outer plexiform layer (OPL), outer nuclear layer, photoreceptor inner segment layer, photoreceptor outer segment layer, and retinal pigment epithelial layer. The dividing line for the inner versus outer layer regions was between the INL and OPL.
Sample size
The sample size was planned to provide 0.80 power 1-β with α = 0.05 to permit detection of ≥10 μm interocular difference in macular thickness. A value of 10 μm was chosen, as coefficients of repeatability for macular thickness measured by fdOCT in eyes with healthy retinas have been reported at 1.0 to 3.5 %, or up to about 9.5 μm [31, 32]. Interocular differences in macular thickness of 0.0 ± 13.4 μm as measured by fdOCT have been reported for children 6–17 years of age [21]. Using this standard deviation, we determined that a sample size of 16 in each group was sufficient.
Data analysis
Central subfield thickness (CST) was compared between the two groups using an independent t test. In addition, we compared our data with the normative control data of healthy children published by Wu et al. [33].
Results
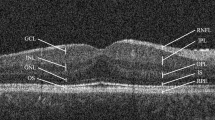
The demographic characteristics of children are reported in Table 1. All children in the Down syndrome group showed identifiable retinal structure, including a foveal pit. A sample picture from each group is presented in Fig. 1a and b. Because of similarities between the right and the left eyes, we reported results only for the right eye.
a OCT image from the right eye of a healthy 11-year-old full-term child from the control group. The full retina central subfield thickness (CST) is 235 μm, and visual acuity was 20/20 at the time of testing. This child had a spherical equivalent of 0.75 D and a normal retina. The foveal pit and central thickness were normal. b OCT image from the right eye of a 13-year-old patient born at 38 weeks of gestational age with Down syndrome. CST is 281 μm, and visual acuity was 20/65 at the time of testing. The patient had a spherical equivalent of −1.5 D and the full retina was significantly thicker as compared to the control group
Retinal thickness
CST and sectional average thickness for the full retina are summarized in Table 2. CST for the full retina was significantly greater in the Down syndrome group (281 ± 17 μm) than the control group (246 ± 21 μm; independent t test, t = 5.90, p < 0.001). After Bonferroni correction, all regions immediately surrounding the full retina CST were significantly thicker except for the temporal section. The CST for the inner and outer retinal layers were both significantly greater in the Down syndrome group as well (independent t test, t = 4.34, p < 0.001; independent t test, t = 3.33, p = 0.002) (Table 2).
When compared to control patients in the study published in Wu et al. [33], the results are similar. The CST of the full retina for the right and left eyes was again significantly greater in the Down syndrome group (right eye: t = 5.9, p < 0.001; left eye: t = 5.38, p < 0.001). For the right eyes, approximately 29 % of patients (5/17) in the Down syndrome group were outside the normal range (normal range provided by the control group or Wu et al. [33]).
We also compared CST of the right eyes to CST of the left eyes in the Down syndrome group, and no significant interocular difference was found (paired t test, t = 0.253, p = 0.802).
Qualification of foveal pit depth
Because the total thickness in the central subfield and the superior and nasal sections of the Down syndrome group were thicker than those of the control group, we used a simple method to qualify foveal pit depth. We subtracted the total thickness of the CST from the four surrounding sections (superior, nasal, inferior, temporal) to estimate foveal pit depth. Except for the inferior section, the sectional thickness differences between surrounding sections and CST in the Down syndrome group were significantly lower than those in the control group, indicating that the depth of the foveal pit was shallower in the Down syndrome group (Table 3).
Correlation of CST with visual acuity and SEQ
Since many patients with Down syndrome suffer from poor visual acuity, we investigated the correlation between visual acuity and CST values. Our results demonstrate no significant correlation between visual acuity and CST (t = 1.288, r = 0.326, p = 0.202). Furthermore, there was no significant correlation between spherical equivalent (SEQ) and CST in patients with Down syndrome (r = 0.087, p = 0.740).
In addition, we compared the correlation of visual acuity and SEQ to that of CST in our control group. Again, there was no significant correlation between CST and visual acuity (t = 0.518, r = 0.163, p = 0.518), and there was also no significant correlation between SEQ and CST (t = 1.407, r = 0.366, p = 0.135).
Discussion
This prospective study evaluated the characteristics of macular retinal structure in children with Down syndrome and compared them with an age-matched and spherical equivalent-matched control group. We are not aware of similar reports in the literature. Although most eyes remained within the published normal range, CST values for children in the Down syndrome group were significantly greater than those in the control group. The thicker macula was also attributable to a thicker central subfield in both the inner and outer retinal layers. Our results suggest that an OCT finding of an apparently thicker macula in the Down syndrome group does not necessarily correlate with degraded visual acuity, which is consistent with previous reports describing a lack of association between macular thickness and visual acuity [15, 34, 35, 36].
We previously found that children with retinopathy of prematurity had a thicker fovea due to arrested foveal development by preterm birth [15]. Macular thickness is generally greater in children with congenital and developmental cataracts, particularly in those with unilateral cataracts [37]. In this study, although the gestational age of children in the Down syndrome group was over 36 weeks, and there was no involvement of severe visual deprivation such as cataracts, their CST measurements were significantly greater, suggesting a potential association between Down syndrome and macular abnormalities. Thicker macular structure may be a primary pathology related to the disorder. Stirn et al. reported the presence of foveal hypoplasia or retinal pigment epithelium hyperplasia in 32.2 % of patients with Down syndrome [29]. Our data showed that thickening could result from both inner and outer layers, which agrees with this report. However, Laguna et al. investigated retinal morphology structure in a mouse model of Down syndrome and reported that only the inner layers contributed significantly to retinal thickening [38]. Furthermore, a significantly shallower foveal pit, which is often demonstrated in foveal hypoplasia [36], was present in the Down syndrome group compared with the control group.
Our study had a few limitations. First, in the Down syndrome group, we excluded children with visual acuity worse than 20/100, significant nystagmus, or previous cataract surgery. The 20/100 visual acuity cut-off was determined based on our previous experiments and the patients’ ability to fixate. We have found that many patients with visual acuity over 20/100 are unable to fixate on the target presented in the iVue recording device. More severe macular abnormalities may be associated with those children who were excluded. Aside from central retinal thickening, our data showed no gross alterations in retinal structural organization. Although we occasionally see case reports of abnormalities of the macula (e.g., bilateral macular coloboma [25]) or optic disc (e.g., unilateral morning glory optic disc anomaly [27]), we observed no such abnormal retinal structures in our sample. Wu et al. conducted a study in 47 normal full-term children, similar in age to those in our study, using the same brand of OCT (OptoVue) as was used in our study, and which provided a normal range (fifth percentile was 205 μm and 95th percentile was 289 μm) [33]. Based on these values, approximately one-fourth of Down syndrome patients had a CST beyond the normal range.
Second, axial length was not measured in our study. Although axial length can affect CST results [39], we do not think it would have affected the results of this study, for two reasons. 1) We chose spherical equivalent-matched control subjects. On average, axial length is shorter in children with Down syndrome, similar to children with hyperopia. Approximately 80 % of patients with Down syndrome are hyperopic, and their spherical equivalent is correlated with axial length [40]. 2) According to Odell and colleagues [39], after adjusting for shorter axial length, our data should demonstrate an even thicker central subfield in the Down syndrome group. In other words, adjusting for axial length confirmed our conclusion that CST was significantly different between the two groups. Considering that most OCTs in the clinic do not provide axial length adjustment, we presented our major results with unadjusted axial length measurements for general reference. Because OCT is widely available to ophthalmologists as part of their clinical practice, our findings may help clinicians differentiate foveal abnormalities associated with Down syndrome versus abnormalities for other reasons. For example, a thicker central subfield may frequently be seen in patients with Down syndrome.
Third, due to software limitations, we were not able to report more details on the individual layers or foveal contours in children with Down syndrome. This limitation will be addressed in future studies that characterize the individual layers in order to provide a deeper understanding of abnormalities in retinal structure in this population.
Conclusions
Patients with Down syndrome had significantly greater CST values compared to the control group. In general, both the inner and outer layers contributed to the thicker central subfield. Although patients with Down syndrome tend to suffer from degraded vision, an OCT finding of an apparently thicker macula in a pediatric Down syndrome patient does not necessarily correlate with poorer visual acuity.
References
Cregg M, Woodhouse JM, Stewart RE, Pakeman VH, Bromham NR, Gunter HL, Trojanowska L, Parker M, Fraser WI (2003) Development of refractive error and strabismus in children with Down syndrome. Invest Ophthalmol Vis Sci 44(3):1023–1030
Little JA, Woodhouse JM, Lauritzen JS, Saunders KJ (2007) The impact of optical factors on resolution acuity in children with Down syndrome. Invest Ophthalmol Vis Sci 48(9):3995–4001. doi:10.1167/iovs.06-1387
Ljubic A, Trajkovski V, Stankovic B (2011) Strabismus, refractive errors and nystagmus in children and young adults with Down syndrome. Ophthalmic Genet 32(4):204–211. doi:10.3109/13816810.2011.592175
McCullough SJ, Little JA, Saunders KJ (2013) Higher order aberrations in children with Down syndrome. Invest Ophthalmol Vis Sci 54(2):1527–1535. doi:10.1167/iovs.12-10597
Woodhouse JM, Pakeman VH, Cregg M, Saunders KJ, Parker M, Fraser WI, Sastry P, Lobo S (1997) Refractive errors in young children with Down syndrome. Optom Vis Sci 74(10):844–851
Cregg M, Woodhouse JM, Pakeman VH, Saunders KJ, Gunter HL, Parker M, Fraser WI, Sastry P (2001) Accommodation and refractive error in children with Down syndrome: cross-sectional and longitudinal studies. Invest Ophthalmol Vis Sci 42(1):55–63
Felius J, Beauchamp CL, Stager DR Sr (2014) Visual acuity deficits in children with nystagmus and Down syndrome. Am J Ophthalmol 157(2):458–463. doi:10.1016/j.ajo.2013.09.023
John FM, Bromham NR, Woodhouse JM, Candy TR (2004) Spatial vision deficits in infants and children with Down syndrome. Invest Ophthalmol Vis Sci 45(5):1566–1572
Little JA, McCullough S, McClelland J, Jackson AJ, Saunders KJ (2013) Low-contrast acuity measurement: does it add value in the visual assessment of down syndrome and cerebral palsy populations? Invest Ophthalmol Vis Sci 54(1):251–257. doi:10.1167/iovs.12-10506
Little JA, Woodhouse JM, Lauritzen JS, Saunders KJ (2009) Vernier acuity in Down syndrome. Invest Ophthalmol Vis Sci 50(2):567–572. doi:10.1167/iovs.08-2250
Paudel N, Leat SJ, Adhikari P, Woodhouse JM, Shrestha JB (2010) Visual defects in Nepalese children with Down syndrome. Clin Exp Optom 93(2):83–90. doi:10.1111/j.1444-0938.2010.00458.x
Woodhouse JM, Meades JS, Leat SJ, Saunders KJ (1993) Reduced accommodation in children with Down syndrome. Invest Ophthalmol Vis Sci 34(7):2382–2387
Provis JM, Hendrickson AE (2008) The foveal avascular region of developing human retina. Arch Ophthalmol 126(4):507–511. doi:10.1001/archopht.126.4.507
Springer AD, Hendrickson AE (2004) Development of the primate area of high acuity. 2. Quantitative morphological changes associated with retinal and pars plana growth. Vis Neurosci 21(5):775–790. doi:10.1017/S0952523804215115
Wang J, Spencer R, Leffler JN, Birch EE (2012) Critical period for foveal fine structure in children with regressed retinopathy of prematurity. Retina 32(2):330–339. doi:10.1097/IAE.0b013e318219e685
Yuodelis C, Hendrickson A (1986) A qualitative and quantitative analysis of the human fovea during development. Vis Res 26(6):847–855
Candy TR, Crowell JA, Banks MS (1998) Optical, receptoral, and retinal constraints on foveal and peripheral vision in the human neonate. Vis Res 38(24):3857–3870
Wallman J, Winawer J (2004) Homeostasis of eye growth and the question of myopia. Neuron 43(4):447–468
Huynh SC, Samarawickrama C, Wang XY, Rochtchina E, Wong TY, Gole GA, Rose KA, Mitchell P (2009) Macular and nerve fiber layer thickness in Amblyopia: the Sydney childhood Eye study. Ophthalmology 116(9):1604–1609. doi:10.1016/j.ophtha.2009.03.013
Altintas O, Yuksel N, Ozkan B, Caglar Y (2005) Thickness of the retinal nerve fiber layer, macular thickness, and macular volume in patients with strabismic amblyopia. J Pediatr Ophthalmol Strabismus 42(4):216–221
Al-Haddad CE, El Mollayess GM, Mahfoud ZR, Jaafar DF, Bashshur ZF (2013) Macular ultrastructural features in amblyopia using high-definition optical coherence tomography. Br J Ophthalmol 97(3):318–322. doi:10.1136/bjophthalmol-2012-302434
Wang BZ, Taranath D (2012) A comparison between the amblyopic eye and normal fellow eye ocular architecture in children with hyperopic anisometropic amblyopia. J AAPOS 16(5):428–430. doi:10.1016/j.jaapos.2012.06.006
Pang Y, Goodfellow GW, Allison C, Block S, Frantz KA (2011) A prospective study of macular thickness in amblyopic children with unilateral high myopia. Invest Ophthalmol Vis Sci 52(5):2444–2449. doi:10.1167/iovs.10-5550
Yoon SW, Park WH, Baek SH, Kong SM (2005) Thicknesses of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmol 19(1):62–67
Aziz HA, Ruggeri M, Berrocal AM (2011) Intraoperative OCT of bilateral macular coloboma in a child with Down syndrome. J Pediatr Ophthalmol Strabismus 48:e37–39. doi:10.3928/01913913-20110712-03
Al-Hemidan AI, Al-Hazzaa SA, Chavis P, Al-Hussein H (1999) Optic disc elevation in Down syndrome. Ophthalmic Genet 20(1):45–51
Altun A, Altun G, Kurna SA, Olcaysu OO, Aki SF (2014) Unilateral morning glory optic disc anomaly in a case with Down syndrome. BMC Ophthalmol 14:48. doi:10.1186/1471-2415-14-48
Parsa CF, Almer Z (2008) Supranumerary optic disc vessels may indicate reduced systemic angiogenesis in Down syndrome. Br J Ophthalmol 92(3):432–433. doi:10.1136/bjo.2007.124248
Stirn Kranjc B (2012) Ocular abnormalities and systemic disease in Down syndrome. Strabismus 20(2):74–77. doi:10.3109/09273972.2012.680234
Fimiani F, Iovine A, Carelli R, Pansini M, Sebastio G, Magli A (2007) Incidence of ocular pathologies in Italian children with Down syndrome. Eur J Ophthalmol 17(5):817–822
Bambo MP, Garcia-Martin E, Otin S, Sancho E, Fuertes I, Herrero R, Satue M, Pablo L (2014) Influence of cataract surgery on repeatability and measurements of spectral domain optical coherence tomography. Br J Ophthalmol 98 (1):52–58. doi:10.1136/bjophthalmol-2013-303752
Comyn O, Heng LZ, Ikeji F, Bibi K, Hykin PG, Bainbridge JW, Patel PJ (2012) Repeatability of Spectralis OCT measurements of macular thickness and volume in diabetic macular edema. Invest Ophthalmol Vis Sci 53 (12):7754–7759. doi:10.1167/iovs.12-10895
Wu WC, Lin RI, Shih CP, Wang NK, Chen YP, Chao AN, Chen KJ, Chen TL, Hwang YS, Lai CC, Huang CY, Tsai S (2012) Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology 119(9):1907–1916. doi:10.1016/j.ophtha.2012.02.040
Marmor MF, Choi SS, Zawadzki RJ, Werner JS (2008) Visual insignificance of the foveal pit: reassessment of foveal hypoplasia as fovea plana. Arch Ophthalmol 126(7):907–913. doi:10.1001/archopht.126.7.907
Recchia FM, Recchia CC (2007) Foveal dysplasia evident by optical coherence tomography in patients with a history of retinopathy of prematurity. Retina 27(9):1221–1226. doi:10.1097/IAE.0b013e318068de2e
Meyer CH, Lapolice DJ, Freedman SF (2002) Foveal hypoplasia in oculocutaneous albinism demonstrated by optical coherence tomography. Am J Ophthalmol 133(3):409–410
Wang J, Smith HA, Donaldson DL, Haider KM, Roberts GJ, Sprunger DT, Neely DE, Plager DA (2014) Macular structural characteristics in children with congenital and developmental cataracts. J AAPOS. doi:10.1016/j.jaapos.2014.05.008
Laguna A, Barallobre MJ, Marchena MA, Mateus C, Ramirez E, Martinez-Cue C, Delabar JM, Castelo-Branco M, de la Villa P, Arbones ML (2013) Triplication of DYRK1A causes retinal structural and functional alterations in Down syndrome. Hum Mol Genet 22(14):2775–2784. doi:10.1093/hmg/ddt125
Odell D, Dubis AM, Lever JF, Stepien KE, Carroll J (2011) Assessing Errors Inherent in OCT-Derived Macular Thickness Maps. J Ophthalmol 2011:692574. doi:10.1155/2011/692574
Doyle SJ, Bullock J, Gray C, Spencer A, Cunningham C (1998) Emmetropisation, axial length, and corneal topography in teenagers with Down’s syndrome. Br J Ophthalmol 82(7):793–796
Acknowledgments
We thank Afiah Hasnie, Alexander Walls, Jessica Felker and Paxton Ott for helping obtain patient data.
Conflict of interest
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Financial support
This study is supported by an Indiana University–Purdue University Indianapolis (IUPUI) EMPOWER [Enhanced Mentoring Program with Opportunities for Ways to Excel in Research] award to Dr. Wang and a Research to Prevent Blindness (RPB) unrestricted grant to the Glick Eye Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
O’Brien, S., Wang, J., Smith, H.A. et al. Macular structural characteristics in children with Down syndrome. Graefes Arch Clin Exp Ophthalmol 253, 2317–2323 (2015). https://doi.org/10.1007/s00417-015-3088-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3088-x