Abstract
Purpose
To evaluate vascular endothelial growth factor (VEGF) plasma levels before and after intravitreal injection of ranibizumab in patients with retinopathy of prematurity (ROP).
Methods
Case series study. Eleven infants with type 1 pre-threshold ROP were treated with intravitreal ranibizumab 0.5 mg. Blood samples were collected before intravitreal injection of ranibizumab and 1 day, 1 week, 2 weeks, and 4 weeks after injection. Concentration of plasma VEGF was measured by enzyme-linked immunosorbent assays (ELISA).
Results
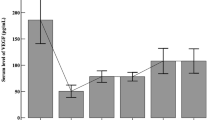
The mean ± standard deviation of plasma VEGF concentration of the available samples before and 1 day, 1 week, 2 weeks, and 4 weeks after a total of 0.5 mg ranibizumab injection were 46.07 ± 9.40 pg/ml (n = 11), 10.59 ± 7.32 pg/ml (n = 5), 45.76 ± 6.75 pg/ml (n = 5), 62.44 ± 15.51 pg/ml (n = 5), and 56.82 ± 12.78 pg/ml (n = 4) respectively. A significant reduction was found in the plasma VEGF levels 1 day after intravitreal injection of ranibizumab (P = 0.002). No significant differences were found between before and 1 week, 2 weeks, and 4 weeks after the injection.
Conclusions
Intravitreal ranibizumab reduced plasma VEGF levels 1 day after injection in infants with ROP. This effect disappeared 1 week after the injection. Intravitreal ranibizumab did not induce prolonged systemic VEGF suppression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Retinopathy of prematurity (ROP) is a neovascular retinal disorder that occurs only in the immature retina and that can progress to retinal detachment, which can result in blindness. Due to improved neonatal care in developing countries, a growing number of preterm infants survive. As a result, the number of severe ROP cases has increased dramatically [1, 2]. The retinal blood supply of premature infants is incomplete and highly vulnerable to decay, particularly when oxygen therapy is administered. This immaturity in vascular development causes retina hypoxia. Angiogenesis is a compensatory mechanism for retina hypoxia, and vascular endothelial growth factor (VEGF) is the most important proangiogenic factor [3]. Conventional laser therapy and anti-VEGF therapy are used to treat eyes with ROP before retinal detachment occurs.
Conventional laser therapy, which ablates peripheral avascular retina, is the standard therapy for the management of ROP [4]. However, it is difficult to implement conventional laser therapy in eyes with poorly dilating pupils, which can occurs in advanced ROP [4]. Moreover, cases with high vascular activity or posterior disorders may progress to retinal detachment despite peripheral retinal laser ablation [5]. In addition, conventional laser therapy has been associated with the modest visual field loss [4] and can induce myopia with long-term observation [6].
The use of anti-VEGF agents, primarily intravitreal bevacizumab, as first-line monotherapy or rescue therapy combined with laser therapy has shown efficacy in highly active, posterior ROP [7]. Non-ablative anti-VEGF therapy allows for the development of peripheral retinal vessels [7, 8], and it has been associated with less myopia than laser therapy [9]. However, several studies have reported that bevacizumab escapes from the vitreous into the circulation and reduces the systemic unbound VEGF concentration for weeks to months in infants and adults [10, 11]. Thus, there are concerns about the prolonged suppression of systemic VEGF, particularly in infants with very low body weight and rapidly developing tissues. Ranibizumab (Lucentis; Genentech Inc., South San Francisco, CA, USA), a humanized monoclonal antibody fragment Fab specifically designed for ocular use, acts as an ideal optional anti-VEGF agent for neovascular disorders, including ROP [8, 12, 13]. Considering the systemic suppression of VEGF, could ranibizumab be a safe choice in the management of ROP? To date, the influence of intravitreal ranibizumab therapy on systemic VEGF levels has not been well studied.
Here, we examined plasma VEGF levels before and after intravitreal injection of ranibizumab.
Methods
This study was designed as a case series study. The fundus of infants with ROP was examined after pupillary dilation using binocular indirect ophthalmoscopy under topical or general anesthesia. During the examinations, fundus photographs were abtained with a RetCam digital fundus camera. The stage of the ROP was based on the International Classification of Retinopathy of Prematurity [14]. All of the diagnoses were confirmed independently by at least two physicians who had sufficient knowledge and experience in treating ROP. Eyes with stage 3+ located in zone 1 or posterior zone 2 received an intravitreal injection of 0.25 mg ranibizumab [7, 8]. The exclusion criteria included (1) major congenital anomalities, mechanical ventilation, sepsis, neurological deficits, and systemic instability, and (2) a history of treatment for ROP. A follow-up period, with 3 to 7 days [4] after treatment as the start and 21 weeks [7] after treatment as the end, was provided to all of the patients. We recorded the early treatment outcomes of all eyes. Recurrence was defined as the return of vascular dilation and tortuosity and the stage 3 disorder in zone 1 or 2. Once recurrence was found, additional laser treatment was implemented. Consistent with the BEAT–ROP study, eyes requiring additional laser therapy of a few laser applications to inadvertently skipped areas within 1 week after initial treatment were not considered to be recurrences [7]. Intraocular pressure (IOP) was measured before injection and at the first follow-up using Schiötz tonometers. The clinical trial was registered by Peking University People’s Hospital in Beijing, China, under number 2012(23). The study was conducted in accordance with the Declaration of Helsinki, and we received approval from the Investigational Review Board of the Peking University People’s Hospital. Informed consent was obtained from the parents of each patient after an explanation of the off-label use of the drug and the purpose and potential adverse effects of the procedure.
Blood samples were collected 1 day before injection and 1 day, 1 week, 2 weeks, and 4 weeks after injection. The blood samples were collected in sterile tubes containing EDTA, and were centrifuged at 3,000 rpm at room temperature for 10 min. The clear supernatant was immediately separated and frozen at −70 °C until the assay. The blood samples exclusion criteria included (1) coagulation, (2) a limited volume insufficient for double-checking, and (3) a history of receiving laser treatment before drawing.
The concentration of VEGF in the plasma was measured by enzyme-linked immunosorbent assays (ELISA) using kits for human anti-VEGF (Quantikine VEGF ELISA Kit; R&D Systems, Inc., Minneapolis, MN, USA). Each assay was performed according to the manufacturer’s protocol.
Statistical analyses were performed using the SPSS software (SPSS for Windows, version 17.0; SPSS, Inc., Chicago, IL, USA). Data are presented as the mean and standard deviation (SD). Considering the very small sample size we hypothesized that the variables were Gaussian distributed. For the three matched groups, one-way repeated-measures analysis of variance and Greenhouse–Greisser correction were used, and the Holm–Sidak method detected significant differences between each set of data. For two groups, paired t-test was used. A P value less than.05 was considered statistically significant.
Results
General states and early outcomes of patients
Eleven infants (four girls and seven boys) with type 1 pre-threshold ROP were enrolled in the study. The demographics and treatment outcomes of these patients are summarized in Table 1. The mean ± standard deviation of the gestational age and birth weight of all of the patients were 29.18 ± 2.35 weeks and 1329.09 ± 365.80 g respectively. The mean ± standard deviation of postmenstrual age and body weight at initial treatment were 38.05 ± 2.35 weeks and 2913.64 ± 906.67 g respectively. Of the 22 eyes, 11 presented with iris vascular engorgement before treatment. Complete disappearance of iris vascular engorgement in all of the eyes was observed at the first follow-up. Recurrence occurred in ten eyes (45.45 %), and the mean recurrence interval was 7.30 weeks (range: 2 to 13 weeks). The remaining eyes had favorable treatment outcomes without any signs of recurrence, retinal detachment, or macular dragging at the end of the follow-up. No significant difference in IOP was found between before and 3–7 days after injection (mean ± SD IOP: 15.38 ± 2.59 mmHg vs 15.58 ± 2.57 mmHg, P = 0.361). No ocular or systemic adverse events were found at the end of the follow-up.
Plasma vascular endothelial growth factor levels in patients treated with ranibizumab
The mean ± standard deviation of plasma VEGF concentration of the available samples before and at 1 day, 1 week, 2 weeks, and 4 weeks after a total injection of 0.5 mg ranibizumab were 46.07 ± 9.40 pg/ml (n = 11), 10.59 ± 7.32 pg/ml (n = 5), 45.76 ± 6.75 pg/ml (n = 5), 62.44 ± 15.51 pg/ml (n = 5), and 56.82 ± 12.78 pg/ml (n = 4) respectively (Fig. 1 and Table 2). In patients 1, 2, 3, 4, and 5, a significant difference in the plasma VEGF levels between before and 1 day, 2 weeks after intravitreal injection of ranibizumab was observed (P = 0.002). A reduction in plasma VEGF levels was observed in the ranibizumab group 1 day after intravitreal injection compared with before and 2 weeks after injection (P = 0.000, P = 0.004 respectively). The plasma VEGF levels normalized 2 weeks after injection (P = 0.179). Comparing the plasma VEGF levels of each post-injection group with its baseline, no significant differences were found at 1 week (patients 6, 7, 8, 9, and 10), or 4 weeks (patients 3, 4, 6, and 11) in the post-injection group (P = 0.474 and P = 0.413 respectively).
Plasma vascular endothelial growth factor: effects of intravitreal injection of ranibizumab. The abscissa represents the time course before and after treatment. The ordinate represents the plasma level of vascular endothelial growth factor (VEGF). Circles represent the mean. The bars represent the standard deviation from the mean. The numbers of patients of each group were listed over each bar. This figure shows the plasma VEGF levels of all infants with retinopathy of prematurity (ROP) who received intravitreal ranibizumab
Discussion
Anti-VEGF therapy is a potentially promising option for the management of ROP. VEGF is essential for numerous physiological functions, such as the survival of vascular endothelial cells and the formation of the blood–brain barrier. Therefore, the suppression of systemic VEGF should be considered, particularly in premature patients. It is important to investigate the systemic VEGF levels in response to intravitreal ranibizumab administration.
Our results showed that the suppression of VEGF was short-term, as the plasma VEGF levels declined sharply 1 day after the injection and normalized 1 week after the injection. This finding was consistent with a previous study in which the serum VEGF levels decreased 1 day after intravitreal injection of ranibizumab but normalized on the third day after injection in adult patients [15]. The change in the plasma VEGF level is reasonable, and is supported by the following evidence:
-
(1) Radioactive ranibizumab was detected in all of the retinal layers 24 h after intravitreal injection of ranibizumab, including the retinal pigment epithelium [16]. With aqueous humor outflow or through the choroid vessels, ranibizumab egresses to the circulation [16, 17], leading to a reduction in systemic unbound VEGF levels.
-
(2) In rabbits, serum ranibizumab could be detected until 7 days after the injection, with the maximum serum ranibizumab concentration of 0.055 μg/ml at 24 h after injection [16]. The serum ranibizumab level in this study had a negative correlation with the plasma VEGF level in our study.
-
(3) Bakri et al. investigated the tissue distribution and elimination of ranibizumab after intravitreal injection in rabbits. They reported that vitreous concentrations of ranibizumab declined in a monoexponential fashion with a half-life of 2.88 days; concentrations of >0.1 μg/ml ranibizumab were maintained in the vitreous humor for 29 days [18]. Krohne et al. found that the aqueous half-life of 0.5 mg of intravitreal injected ranibizunab was 7.19 days in nonvitrectomized human eyes [19]. Xu et al. demonstrated that due to the slow release of ranibizumab from the stagnant vitreous, the apparent serum half-life following intravitreal ranibizumab administration was 9 days in adult patients. During this steady-state period, the systemic-to-vitreous exposure ratio for ranibizumab was 1:90,000 [20]. Because the bioactivity of ranibizumab operates in a dose-dependent manner [21], the systemic ranibizumab concentration was too low to have an effect on VEGF. It is reasonable that the plasma VEGF returned to baseline levels only 1 week after injection, as we showed in the present study.
In contrast, Hoerster et al. reported a case illustrating that serum VEGF reached a nadir at 2–3 weeks after intravitreal injection of ranibizumab, and normalized 4 weeks after injection [12]. Our study found that the plasma VEGF suppression started as early as 1 day after intravitreal injection of ranibizumab, and normalized 1 week after injection. The difference may be the result of their studying an extremely premature infant with a gestational age of 22.71 weeks and a birth weight of 305 g. The suppression of VEGF might have been affected by the maturation and development of the infant. In addition, the serum VEGF might have been artificially high due to the release of VEGF on platelet activation [22]. We measured the plasma level of VEGF instead.
Suppression of plasma VEGF levels by intravitreal ranibizumab injection was not found at 2 weeks or 4 weeks after treatment. Thus, we did not find any powerful evidence to support the notion that intravitreal injection of ranibizumab had a relatively prolonged effect on systemic VEGF levels.
In contrast to intravitreal bevacizumab, which induced a suppression of systemic VEGF activity for at least 2 weeks post-injection [10], intravitreal ranibizumab showed a shorter influence on systemic VEGF levels. In rabbits, the vitreous half-life of bevacizumab was 1.5-fold longer than that of ranibizumab [18]. In humans, the ocular half-life of bevacizumab was reported to be 1.37-fold longer than that of ranibizumab [19, 23]. Avery et al. investigated the systemic exposure after an intravitreal injection of bevacizumab or ranibizumab in patients with age-related macular degeneration (AMD), and they reported that systemic exposure to bevacizumab was higher than that to ranibizumab [24]. Plasma and serum VEGF have been less suppressed after the intravitreal injection of ranibizumab than after injection of bevacizumab in studies of adults with AMD [24–27]. These results support the viewpoint that ranibizumab has less influence on the suppression of systemic VEGF after intravitreal injection. With regard to the systemic suppression of VEGF, could ranibizumab be better than bevacizumab in ROP? A carefully performed large-sample comprehensive study is necessary.
In our case series, intravitreal ranibizumab was an effective therapy for ROP. Iris vascular engorgement completely resolved in all 11 eyes, indicating the rapid decrease in intra-ocular VEGF levels after intravitreal injection of ranibizumab. The recurrence of ROP after ranibizumab injection occurred at 7.3 ± 3.5 weeks over 21 weeks of observation. With regard to bevacizumab, the early recurrence interval was reported to be 7.6 ± 9.4 weeks [28]. With regard to the IOP, we did not find significant change at the first follow-up after intravitreal injection of 0.25 mg ranibizumab. Neither retinal detachment nor side-effects were found in these 22 eyes over 21 weeks of observation.
There were some limitations to our study. First, the activation or rupture of platelets, which can lead to the release of VEGF, is difficult to avoid, although any blood sample with coagulation was excluded from our study and EDTA was used. Second, the number of patients and samples in this study were limited because of the small number of infants with severe ROP and the technical difficulty in drawing blood, making the statistical analyses difficult. Although it had limitations, our study was noteworthy in illustrating the marked reduction and rapid normalization in plasma VEGF levels after an intravitreal injection of ranibizumab.
In conclusion, we studied the plasma VEGF levels in infants with ROP after intravitreal injection of ranibizumab. The plasma VEGF levels decreased 1 day after injection, and normalized 1 week after injection. Intravitreal injection of ranibizumab for ROP was efficacious, but its safety profile requires further study.
References
Chen Y, Li XX (2006) Characteristics of severe retinopathy of prematurity patients in China: a repeat of the first epidemic? Br J Ophthalmol 90:268–271
Gilbert C (2008) Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 84:77–82
Hartnett ME, Penn JS (2012) Mechanisms and management of retinopathy of prematurity. New Engl J Med 367:2515–2526
Fierson WM, American Academy of Pediatrics Section on Ophthalmology, American Academy of Ophthalmology, American Association for Pediatric Ophthalmology and Strabismus, American Association of Certified Orthoptists (2013) Screening examination of premature infants for retinopathy of prematurity. Pediatrics 131:189–195
Vinekar A, Trese MT, Capone A Jr, Photographic Screening for Retinopathy of Prematurity Cooperative Group (2008) Evolution of retinal detachment in posterior retinopathy of prematurity: impact on treatment approach. Am J Ophthalmol 145:548–555
Early Treatment for Retinopathy of Prematurity Cooperative Group, Good WV, Hardy RJ et al (2010) Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol 128:663–671
Mintz-Hittner HA, Kennedy KA, Chuang AZ, Group B-RC (2011) Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. New Engl J Med 364:603–615
Castellanos MA, Schwartz S, Garcia-Aguirre G, Quiroz-Mercado H (2013) Short-term outcome after intravitreal ranibizumab injections for the treatment of retinopathy of prematurity. Br J Ophthalmol 97:816–819
Hwang CK, Hubbard GB, Hutchinson AK, Lambert SR (2015) Outcomes after intravitreal bevacizumab versus laser photocoagulation for retinopathy of prematurity: a 5-year retrospective analysis. Ophthalmology. doi:10.1016/j.ophtha.2014.12.017
Sato T, Wada K, Arahori H et al (2012) Serum concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol 153(327–333):e321
Ma Y, Zhang Y, Zhao T, Jiang YR (2012) Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol 153(307–313):e2
Hoerster R, Muether P, Dahlke C et al (2013) Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol 91:e74–e75
Mota A, Carneiro A, Breda J et al (2012) Combination of intravitreal ranibizumab and laser photocoagulation for aggressive posterior retinopathy of prematurity. Case Rep Ophthalmol 3:136–141
International Committee for the Classification of Retinopathy of Prematurity (2005) The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 123:991–999
Gu X, Yu X, Dai H (2014) Intravitreal injection of ranibizumab for treatment of age-related macular degeneration: effects on serum VEGF concentration. Curr Eye Res 39:518–521
Gaudreault J, Fei D, Beyer JC et al (2007) Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina 27:1260–1266
Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V (2005) Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci 46:726–733
Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ (2007) Pharmacokinetics of intravitreal ranibizumab (Lucentis). Ophthalmology 114:2179–2182
Krohne TU, Liu Z, Holz FG, Meyer CH (2012) Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol 154(682–686):e682
Xu L, Lu T, Tuomi L et al (2013) Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci 54:1616–1624
Lowe J, Araujo J, Yang J et al (2007) Ranibizumab inhibits multiple forms of biologically active vascular endothelial growth factor in vitro and in vivo. Exp Eye Res 85:425–430
Banks RE, Forbes MA, Kinsey SE et al (1998) Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significant for VEGF measurements ans cancer biology. Br J Cancer 77(6):956–64
Krohne TU, Eter N, Holz FG, Meyer CH (2008) Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol 146:508–512
Avery RL, Castellarin AA, Steinle NC et al (2014) Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 98(12):1636–1641. doi:10.1136/bjophthalmol-305252
Investigators IS, Chakravarthy U, Harding SP et al (2012) Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 119:1399–1411
Carneiro AM, Costa R, Falcao MS et al (2012) Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol 90:e25–e30
Kodjikian L, Souied EH, Mimoun G et al (2013) Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL noninferiority randomized trial. Ophthalmology 120:2300–2309
Wu WC, Kuo HK, Yeh PT, Yang CM, Lai CC, Chen SN (2013) An updated study of the use of bevacizumab in the treatment of patients with prethreshold retinopathy of prematurity in Taiwan. Am J Ophthalmol 155(150–158):e151
Acknowledgments
Publication of this article was supported by Grant 81271027 from the National Natural Science Foundation of China, and Grant RDC 2012–21 from the Research and Development Funds of Peking University People’s Hospital, Beijing, China. All authors declare that no conflict of interest was involved.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Y., Jiang, Y., Bai, Y. et al. Vascular endothelial growth factor plasma levels before and after treatment of retinopathy of prematurity with ranibizumab. Graefes Arch Clin Exp Ophthalmol 254, 31–36 (2016). https://doi.org/10.1007/s00417-015-2996-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2996-0