Abstract
Purpose
To investigate the plasma levels of amyloid beta (Aβ) and select inflammatory mediators in patients with various stages of AMD compared to that of age-matched controls, and discern a relationship to disease severity.
Methods
Plasma samples were obtained from AMD subjects at various stages of disease—early (drusen only), geographic atrophy (GA), neovascular AMD (CNV)—and from controls of similar age without AMD. Samples were analyzed using a commercially available ELISA kit (sixteen cytokines) or LC/MS/MS (Aβ isotypes). Descriptive statistics were compiled on all analytes. Analysis of covariance (ANCOVA) was conducted to compare each analyte across AMD groups while adjusting for sex and age of the patients, and in comparison to the control group. Receiver operating characteristics plots were generated for the strongest predictor variables.
Results
Levels of alternative spliced CC3 proteins were significantly different between controls and CNV groups (p < 0.05), with median levels almost twice higher in CNV than in controls. There was an increasing trend for plasma levels of Αβ isotypes across AMD progressive stages (p values ranged from 0.052 to 0.0012) (ANCOVA). When adjusted for multiple comparisons analysis, plasma Aβ 1–42 levels, and its ratio with Aβ 1–40 were the most significantly associated with late AMD stages. Consistently with the ANCOVA results for Αβ isotypes, the ROC curve showed a moderate prediction (AUC = - ~ 0.78) of AMD vs control using the Aβ 1−42 isotype.
Conclusion
Plasma Aβ 1–42 may have utility as a systemic biomarker for AMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, our understanding of the pathogenic mechanisms involved in drusen formation and AMD has increased with histopathological, biochemical, and genetic discoveries implicating the complement cascade and immune mechanisms in AMD, and has led to a general consensus that inflammation is a key driver in the development of AMD. Major genetic findings strongly implicating inflammatory pathways in the pathogenesis of AMD with several single nucleotide polymorphisms (SNP) within genes involved in the alternate complement pathway, a key pathway in innate immunity and inflammation, have been identified in relation to AMD. These genes include the complement factor H (CFH) gene, where polymorphisms have been involved in over 50 % of AMD in a population [1–5]. As CFH is a key regulator of the complement cascade, it has been hypothesized that defects in CFH may result in excessive stimulation of the complement system, increasing cellular damage, and subsequently sustained retinal pigment epithelium (RPE) and choroidal cell attack, drusen formation, and the disruption of Bruch’s membrane, resulting in the development of late-stage AMD. Other complement-related genes associated with AMD include the CFH paralogous genes (CFHR1-5), the complement factor B (BF) gene, complement component 2 (C2) gene, and complement C3 gene, further solidifying a role for complement in this disease [2, 6, 7] All these genes are implicated in the complement cascade, and may therefore influence the regulation of innate immunity and inflammation [8, 9].
As in several other chronic diseases of aging such as Alzheimer’s disease (AD) and atherosclerosis, it is postulated that AMD is the result of a systemic process involving ongoing low-grade chronic inflammation, or para-inflammation, leading to tissue damage at a local level. This process is implicated in drusen formation, as activated complement components and fragments associated with the terminal complement pathway have been found within them [10, 11]. Other drusen components include activators of the complement cascade such as amyloid, cholesterol, C-reactive protein (CRP) and amyloid beta (Aβ) [12–15].
When such an inflammatory process occurs in the retina, it results in the release of circulating inflammatory mediators [16] and the detection of these in the serum has been considered a potentially useful measure of the underlying disease process. This has led to several studies investigating serum levels of systemic markers of inflammation in AMD including CRP, interleukin 6 (IL-6), interleukin 2 (IL-2), tumour necrosis factor-α (TNF- α), soluble intercellular adhesion molecule 1 (s-ICAM-1), and C3a-desArg, where higher levels were found by some to be associated with AMD in comparison to controls [17–22].
The pathological protein aggregation in extracellular deposits in both diseases consists of Αβ peptides, apolipoprotein E (ApoE), complement, and other inflammatory proteins [10, 13, 23–29]. Most notably, one of the proteins identified in drusen is Aβ, a 38–43 amino acid residue peptide formed via the proteolytic processing of amyloid precursor proteins and identified as the major component of amyloid plaques within the brain. Aβ is considered to play a major role in the pathogenesis of AD. [1, 13, 15, 28, 29]
While it is still not known what triggers the complement cascade in AMD, Aβ presents as an obvious candidate, given its role in initiating the complement cascade in AD [27].
Human RPE primary cell cultures, when treated with Aβ, have shown activation of the complement alternative pathway. Furthermore, ex-vivo evaluation of the RPE/choroid in the macular regions of human donor eyes has demonstrated that Αβ co-localizes with complement in drusen, supporting the premise that it initiates complement cascades and contributes to proinflammatory pathology in AMD [28, 30].
There have been numerous studies that have assessed the plasma levels of Aβ in patients with AD, with the goal of understanding the role of Aβ in the pathogenesis of that disease [31]. However, there is no literature regarding the plasma levels of Aβ in AMD patients. Therefore, we conducted a pilot study, to assess the plasma levels of Aβ in a small cohort of patients with AMD compared to that assessed in age-matched controls, to determine if any difference in plasma Aβ levels could be identified and a relationship to disease severity versus age-related changes alone could be established. Further, we examined a panel of cytokines, pro-inflammatory mediators, and vascular modulators to assess their utility as systemic biomarkers of AMD.
Methods
Study design
Cross-sectional case–control study.
Study population and eligibility
Study participants were recruited as part of our AMD risk factor study from the medical retinal clinics at the Royal Victorian Eye and Ear Hospital (RVEEH) and from referrals into the Macular Research Unit at the Center for Eye Research Australia. In this pilot study we recruited a cohort of normal participants and a disease group with a range of AMD stages. We aimed to recruit only Caucasian participants to limit the ethnic variability of the cohort. In total, 81 participants aged 50 years or older were recruited, with approximately 20 in each of the following groups assigned as follows: 19 normal controls (<5 hard drusen of any size and no pigment abnormalities), 18 with Early AMD (drusen > 125 um); 21 with Geographic Atrophy (GA) (hypopigmentation > 175 um in diameter, in which choroidal vessels can be visualized); and 23 with Choroidal Neovascular Membrane secondary to AMD (CNV).
This research adhered to the tenets of the Declaration of Helsinki and participants provided written informed consent prior to participation. The study was approved by the Human Research and Ethics Committee of the RVEEH..
Patients with any co-existing retinal disease, other ocular diseases, known systemic inflammatory diseases or Alzheimer’s disease, were excluded from the study. No subject had received anti-VEGF treatment within the preceding 30 days of sample collection.
Data collection and clinical examination
All participants were consented and then underwent a full ophthalmic examination including best corrected visual acuity, a dilated fundus examination, digital fundus photography and data collection pertaining to patient demographics, medical and family history. A venous blood sample was collected for plasma extraction. Fundus images were formally graded (international classification and grading system for AMD)[32] using fundus photographs and CNV was confirmed with fluorescein angiography (FFA) and optical coherence tomography (OCT).
Patients were categorized into one of the four AMD categories. Control participants had no signs of AMD in either eye, participants in the early AMD group had to have early disease in both eyes, whilst the presence of GA or CNV needed to be present in at least one eye. Risk factors such as smoking were evaluated per stage of AMD. In general, patterns of smoking was relatively uniform among the groups, and because the sizes of the groups were small, this analysis was not included. In addition, other demographics were collected such as percentage of subjects per group with diabetes and hypercholesterolemia. Due to the small sample size and the relatively small dynamic range of percentages across all groups, ANCOVA statistical analysis was not used to adjust for these differences, apart from age and sex.
Sample processing and bioanalysis
Plasma was extracted by collecting blood samples using EDTA tubes, which were processed within 30 min by spinning at 2000G for 20mins at room temperature, and the supernatant collected and stored immediately at −80 °C. Once the entire cohort was collected, samples were then forwarded to the Pfizer laboratory on dry ice for analysis.
Plasma samples obtained from these subjects were analyzed for quantification of Αβ isotypes 1–42, 1–40, 1–38, and 1-X. The “1” designation refers to the specific amino acid residue present on the protein position counting from the N terminus of the protein. “X” is a designation that reflects an approximation of the sum of all detected Αβ isotypes. The ratio of 1–42/1–40 was also determined as it is considered a more robust measure as it is not so influenced by storage conditions and is considered to better reflect individual Aβ metabolism. Selected cytokines and pro-inflammatory mediators or vascular modulators (IL-1a, IL-1b, IL-3, IL-6, MCP-1, MIP-1a, TNF-a, TNF-b, CRP, CC2, the alternative spliced CC3 protein (C3a and C3d fragments), CFH, MMP1, MMP2, MMP9, sVCAM, sICAM) were also measured..
Quantification of Aβ isotypes in human plasma using immune-precipitation liquid chromatography mass spectrometry (LC/MS/MS)
Briefly, Aβ 1–38, Aβ 1–40 and Aβ 1–42 were extracted from plasma using Dyna-Bead Streptavidin (M-280, Invitrogen) pre-coated with Biotin-6E10 (Covance) as capture antibody. Aβ isotypes were then separated on a liquid chromatography system (Shimadzu LC-20 AC pump and SIL-20 AC autosampler), and detected on a triple-quadrupole mass spectrometer (Applied Biosystems, API4000) by monitoring their specific precursor and respective product ions in negative electrospray ionization mold. The dynamic range was 20 to 5000 pg/mL for Aβ 1–38 and Aβ 1–42 and 40 to 10,000 pg/mL for Aβ 1–40. Plasma levels of Aβ 1-X were estimated by the sum of Aβ 1–38, Aβ 1–40 and Aβ 1–42 concentrations in samples.
Cytokines and pro-inflammatory mediators
A commercially available ELISA from R&D Systems (Cat# SCRP00) was purchased to quantify CRP in EDTA-plasma samples from the patients. Human Complement C2 was measured with an immunoassay developed in-house on an MSD platform. The other cytokines were analyzed using four Multiplex panels from Millipore:
-
1)
sICAM-1 &sVCAM-1 (Cat# HSEP-63 K),
-
2)
IL1a, IL1b, IL3, IL6, TNFa, TNFb, MIP-1a, & MCP-1 (Cat# HCYTOMAG-60 K),
-
3)
MMP1, MMP2, & MMP9 (Cat# HMMP2-55 K), and
-
4)
Alternative spliced CC3 protein (C3a and C3d fragments) & complement factor H (Cat# HNDG1-36 K).
Assays were performed as the vendor suggested, and the Standards and QCs met the acceptance criteria. All samples were analyzed in duplicate, and the mean results were reported.
Statistical analysis
Descriptive statistics were compiled on all analytes. Median and inter-quartile range (difference between the third and the first quartiles) were calculated for each analyte. Assay data distribution was normalized using log10 transformation. Analysis of covariance (ANCOVA) was conducted to compare each analyte across AMD groups while adjusting for sex and age of the patients. Using the Dunnett’s test, we compared means in multiple groups to the same control group. A linear trend test per Αβ isotype as a function of AMD disease severity was also conducted under the ANCOVA model. Statistical significance was established at p-value < 0.05. Plasma Aβ levels for each group were normalized to plasma control Aβ levels to produce the fold-change relative to control plasma level plots of the Aβ isotypes 1–38, 1–40, 1–42, 1-X, and 1–42/1–40. Error bars reflect the 95 % confidence intervals of the mean. Multiplicity adjustment was not performed across multiple parameters.
Analytes with values below the limit of quantification (BLQ) were assigned a uniform random number between 0 and lower limit of quantification (LLOQ). For the few patients with C-reactive protein (CRP) levels above the upper limit of quantification (ALQ), the assay results were assigned a random uniformly distributed value between upper limit of quantification (ULOQ) and 10,000. Analytes were excluded from ANCOVA analysis when greater than 25 % of the samples analyzed were not detectable. For analytes with more than 25 % BLQ samples, a Chi-square test was used to assess whether the proportion of BLQ samples was equal across the AMD stages.
To assess the predictability of biomarkers to the AMD disease stage, logistic regression analysis was performed with stepwise selection of significant predictors from demographics, plasma cytokine levels, and Αβ plasma levels. The logistic regression analysis also incorporated a “leave-one-out” cross-validation to assess performance of the predictive models. Receiver operating characteristics plots were generated for the strongest predictor variable.
Results
Although we targeted ∼20 patients per group, the resultant number of participants did vary slightly between the groups. From the total of 81 participants, 54 (67 %) were female (Table 1). Mean age in the control group was similar to that of early AMD and significantly lower than in the GA or CNV groups, reflecting the fact that age is a significant risk factor for advanced AMD. There were relevant comorbidities in the cohort, with the percentage of subjects per group with diabetes ranging from 4.35 to 15.79 % and hypercholesterolemia from 42.1 to 55.6 % of each cohort. (Table 1).
Cytokines and pro-inflammatory mediators
Descriptive statistics for the cytokines and pro-inflammatory mediators analyzed in each of the defined subject groups are presented in Table 2. Plasma concentrations for some of the cytokines such as the interleukins (i.e., IL-3) were generally at or below the level of quantification (data not shown). Plasma levels of the complement products were robustly quantified and were similar to those reported by Scholl et al. 2008 [19].
Of the sixteen cytokines analyzed, only spliced CC3 protein showed a statistically significant difference in geometric mean levels between the CNV group (higher levels) and control group (p < 0.01, adjusted p = 0.013).
Αβ peptide isotypes
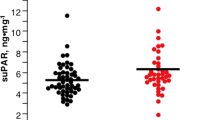
Mean (SD) plasma levels for Aβ 1–40, 1–42, 1–38, and the ratio of Aβ 1–42/1–40 in the control and AMD groups are presented in Table 3. Associations of the mean plasma levels of Αβ 1–38, 1–40, 1–42, 1-X, and ratio of 1–42 to 1–40 (1–42/1–40) with AMD stages are presented as plots of the fold changes relative to control levels with error bars reflecting the 95 % confidence interval (Fig. 1). A trend towards increasing plasma concentrations for Αβ 1–42, 1–40, and 1–42/1–40 with increasing severity of AMD was observed (Fig. 1 and Table 3). The trend test was significant for almost all Αβ isotypes (p values ranged from 0.052 to 0.0012), even after age and sex adjustment (ANCOVA) (Table 4). Aβ 1–42 and Aβ 1–42/1–40 remain significant even after considering the Bonferroni adjustment for multiple comparisons. Aβ 1–42 levels were the most significantly associated with late AMD stages. Further inspection of the scatter plots of the plasma Aβ levels as a function of AMD status are presented in Fig. 2. In general, the levels of plasma Aβ increase as AMD status advances, indicative of the potential utility as a prognostic biomarker. CC3 split product levels were highest in the CNV group (Table 3). CC2 levels were similar across all groups, while CFH levels appeared to numerically decrease from control through CNV groups, consistent with CFH’s inverse relationship with AMD progression.
ANCOVA analysis — fold change by parameters (fold change from control with 95 % CI) for Αβ1-38, 1–40, 1–42, 1–42/1–40 ratio and 1-X. All comparisons with 95 % confidence intervals above 1 are statistically significant. Error bars reflect 95 % CI. AMD = age-related macular degeneration, GA = geographic atrophy, CNV = choroidal neovascularization)
A stepwise logistic regression using demographic variables (age, sex), and Aβ peptides resulted in a model with Aβ 1–42 as the only significant predictor. Receiver operating characteristics (ROC) plot using Aβ 1–42 as the predictive variable in relation to AMD vs. No-AMD, were prepared. Consistent with the ANCOVA results for Αβ isotypes, the ROC curve showed a moderate prediction of control vs. all AMD cases (AUC ∼ 0.78 with 95%CI = (0.65,0.92); Fig. 3). Αβ 1–42 provided a much stronger prediction of AMD than other Αβ isotypes (AUC < 0.73). The ROC analysis on the cross-validated data resulted in an AUC of 0.76 with 95%CI = (0.62,0.90) that still demonstrates a moderate predictability of Αβ 1–42 to the AMD status. Other cross-validation methods such as 10 fold cross-validation and bootstrap re-sampling (n = 2000) generated similar results.
Discussion
In this pilot study, we were able to find a statistically significant increase in C3 split product plasma concentrations in the CNV group in comparison to that of control participants (P < 0.01). The C3 split product results are in line with the findings by Reynolds et al., who investigated plasma levels of various markers of complement activation and regulation in 120 cases of advanced AMD and compared them to 60 control samples [33]. They found that several plasma complement components and activation fragments, including C3, were elevated in cases compared to controls. We were not able to find any differences in the plasma levels of other cytokines. Some groups have found higher levels of systemic markers of inflammation such as CRP, IL6, and s-ICAM1 in AMD subjects in comparison to controls, however this has not been a universal finding [21, 20, 34–38] Plasma levels of the complement products were robustly quantified and were similar to those reported by Scholl et al. [19]. Levels of CRP and CFH were also similar to published results while levels of CC3 split products (3a,3d) and IL6 were found to be higher and ICAM-1 levels lower than previous reports [22, 33, 39]. The varying results reported by different laboratories, which in part will depend on specific laboratory protocols, differences in bioassays, artefacts introduced in the processing of the blood to produce the plasma, and storage of the plasma samples, is an indication of some of the difficulties that will need to be managed if measurements of components of plasma are to be considered as potential biomarker of disease. Furthermore, heterogeneity of the biology of cytokines and marker proteins in humans and the need for large study populations to study, add further challenges for developing robust information to establish such biomarkers..
Stability of Aβ species in plasma has been examined in key studies involving AD plasma samples. [40] Due to differences in storage conditions across studies it is considered that the ratio 1–40/1–42 could provide a more robust reflection of an individual’s Aβ metabolism, in part, due to the fact that both may degrade at similar rates, providing an internal standard of correction. Thus, in addition to quantifying individual Aβ species 1–40 and 1–42, the ratio of their plasma concentrations was analysed. We did however find plasma levels of Αβ isotypes 1–38, 1–40, and 1–42 in AMD subjects to be significantly increased above control plasma levels even though our sample size was small. Moreover, the fold-change analysis and scatter plots show the trend towards higher levels with advancing AMD status. Among the various Αβ isotypes, 1–42, and its ratio with 1–40, had the most robust association to AMD status. Receiver operator characteristic analysis confirmed that the most meaningful predictor for AMD was Αβ 1–42 (ROC ∼0.78).
In our analysis of the association between plasma Αβ and AMD stage, age was found to be significantly associated with Αβ levels. As a result, analysis of covariance was used to adjust for the age effect. This ANCOVA analysis demonstrated that even after adjustment for age, Αβ was still a significant factor associated with AMD stage. The association of age with Aβ levels are similar to those reported in the AD literature [41, 42] As for other potential demographic factors, such as diabetes, due to size of this case control study, the small numbers of diabetes subjects per group, and the relatively uniform percentages of subjects with hypercholesterolemia per group, these factors were not controlled for in the analysis.
After adjustment for the multiple comparisons across the three Αβ isotypes, Αβ 1–42 remained statistically significant. With all three isotypes trending in the same direction, it strengthens the likelihood that our findings are real, rather than a chance finding. Moreover, ANCOVA analysis of the ratio of Aβ 1–42 to 1–40 levels per group further confirmed the significant association of Aβ levels with increasing disease severity. Consistent with the trends of increasing plasma Aβ levels with increasing AMD disease severity, the ratios of 1–42/1–40 Aβ levels for each group was significantly associated with increasing disease severity. In contrast, a study by Lambert et al. of cerebral vascular disease dementia patients [42] showed an increased risk of dementia with decreasing 1–42/1–40 ratio. However, Ertekin et al. 2008, reported that Aβ plasma levels increased in subjects with AD [43]. Thus the biology of Aβ pathogenic mechanisms in AD and other cerebrovascular diseases and its relationship to plasma Aβ 1–42, Aβ-1–40 levels and their ratio is yet emerging. Similarly, the current understanding of AMD pathogenic mechanisms specific to amyloid beta is also being developed. A limitation to the study was the small sample size in this pilot study. This limited our ability to find differences between groups. However, despite the small sample size it is interesting that we were still able to observe consistent and meaningful trends.
Based upon the findings of this exploratory study, isotypes of Αβ should be considered as potential markers of the disease state and further work involving a prospective study with longitudinal follow up of a larger patient cohort would be worthy of consideration.
The possibility of utilizing Alzheimer’s disease based immunotherapies such as anti-amyloid-beta antibodies for use in AMD signifies the importance of further investigation of potentially common pathogenic pathways involved in both of these aging diseases. [44, 45]
All authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty L et al (2005) A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A 102(20):7227–7232
Maller J, George S, Purcell S, Fagerness J, Altshuler D, Daly MJ et al (2006) Common variation in three genes, including a noncoding variant in CFH, strongly influences risk of age-related macular degeneration. Nat Genet 38(9):1055–1059
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C et al (2004) Complement factor H polymorphism in age-related macular degeneration. Science 308(5720):385–389
Robman L, Baird PN, Dimitrov PN, Richardson AJ, Guymer RH (2010) C-reactive protein levels and complement factor H polymorphism interaction in age-related macular degeneration and its progression. Ophthalmology 117(10):1982–1928
Thakkinstain A, Han P, McEvoy M, Smith W, Hoh J, Magnusson K et al (2006) Systemic review and meta-analysis of the association between complement factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet 15(18):2784–2790
Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AH, Gehrs K et al (2006) Variation in factor B(BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet 38(4):458–462
Yates JR, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H et al (2007) Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med 357(6):553–561
Barid PN, Islam FM, Richardson ASH, Cain M, Hunt N, Guymer R (2006) Analysis of the Y402H variant of the complement factor H gene in age-related macular degeneration. Invest Ophthalmol Vis Sci 47(10):4194–4198
Baird PN, Robman LD, Richardson AJ, Dimitrov PN, Tikellis G, McCarty CA et al (2008) Gene-environment interaction in progression of AMD: the CFH gene, smoking and exposure to chronic infection. Hum Mol Genet 17(9):1299–1305
Mullins RF, Russell SR, Anderson DH, Hageman GS (2000) Drusen associated with age and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J 14(7):835–846
Johnson LV, Leitner WP, Staples MK, Anderson DH (2001) Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp Eye Res 73(6):887–896
Anderson DH, Mullins RF, Hageman GS, Johnson LV (2002) A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 134(3):411–431
Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johson LV (2004) Characterization of beta amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res 78(2):243–256
Malek G, Li CM, Guidry C, Medeiros NE, Curcio CA (2003) Apolipoprotein B in cholesterol-containing drusen and basal deposits in human eyes with age-related maculopathy. Am J Pathol 162(2):413–425
Dentchev T, Milam AH, Lee VM, Trojanowski JQ, Dunaief JL (2003) Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol Vis 9:184–190
Xu H, Chen M, Forrester JV (2009) Para-inflammation in the aging retina. Prog Retin Eye Res 28(5):348–368
Boekhoorn SS, Vingerling JR, Witteman JC, Hofman A, de John PT (2007) C-reactive protein level and risk of aging macula disorder: the Rotterdam Study. Arch Ophthalmol 125(10):1396–1401
Klein R, Klein BE, Knudtson MD, Wong TY, Shankar A, Tsai MY (2005) Systemic markers of inflammation, endothelial dysfunction, and age-related maculopathy. Am J Ophthalmol 140(1):35–44
Scholl HPN, Charbel Issa P, Walier M, Janzer S, Pollok-Kopp B et al (2008) Systemic complement activation in age-related macular degeneration. PLOS ONE. doi:10.1371/jornal.pone.0002593
Seddon JM, Gensler G, Milton RC, Klein ML, Rifai N (2004) Association between C-reactive protein and age-related macular degeneration. JAMA 291(6):704–710
Seddon JM, George S, Rosner B, Rifai N (2005) Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol 123(6):774–782
KH W, Tan AG, Rochtchina E, Favaloro EJ, Williams A, Mitchell P et al (2007) Circulating inflammatory markers and hemostatic factors in age-related maculopathy: a population-based case–control study. Invest Ophthalmol Vis Sci 48(5):1983–1988
Ohno-Matsui K (2011) Parallel findings in age-related macular degeneration and Alzheimers disease. Prog Retin Eye Res 30:217–238
Tzekov R, Mullan M (2014) Vision function abnormalities in Alzheimer disease. Surv Ophthalmol 59:414–433
Clemons TE, Rankin MW, McBee WL (2006) Cognitive impairment in the age-related eye disease study: AREDS report no. 16. Arch Ophthalmol 124(4):537–543
Klaver CC, Ott A, Hofman A, Assink JJ, Breteler MM, de Jong PT (1999) Is age-related maculopathy associated with Alzheimer’s disease? The Rotterdam Study. Am J Epidemiol 150(9):963–968
Zetterberg M, Landgren S, Andersson ME, Palmer MS, Gustafson DR, Skoog I et al (2008) Association of complement factor H Y402H gene polymorphism with Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet 147B(6):720–726
Jonson LV, Leitner WP, Rivest AJ, Staples MK, Radeke MJ, Anderson DH (2002) The Alzheimer’s A beta peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc Natl Acad Sci U S A 99(18):11930–11935
Bradt BM, Kolb WP, Cooper NR (1998) Complement-dependent proinflammatory properties of the Alzheimer’s disease beta-peptide. J Exp Med 188(3):431–438
Wang J, Ohno-Matsui K, Yoshida T, Kojima A, Shimada N, Nakahama K et al (2008) Altered function of factor I caused by amyloid beta: implication for pathogenesis of age-related macular degeneration from drusen. J Immunol 181(1):712–720
Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM (2000) Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol 57(1):100–105
Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD et al (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The international ARM epidemiological study group. Surv Ophthalmol 39(5):367–374
Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM (2009) Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci 50(12):5818–5827
Shankar A, Mitchell P, Rochchina E, Tan J, Wang JJ (2007) Association between circulating white blood cell count and long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Am J Epidemiol 165(4):375–382
Despriet DD, Klaver CC, Witterman JC, Bergen AA, Kardys I, de Matt MP et al (2006) Complement factor H polymorphism, complement activators, and risk of age-related macular degeneration. JAMA 296(3):301–309
Schaumberg DA, Christen WG, Buring JE, Glynn RJ, Rifai N, Ridker PM (2007) High-sensitivity C-reactive protein, other markers of inflammation, and the incidence of macular degeneration in women. Arch Ophthalmol 125(3):300–305
Vine AK, Stader J, Branham K, Musch DC, Swaroop A (2005) Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology 112(12):2076–2080
McGwin G, Hall TA, Xie A, Owsley C (2005) The relation between C reactive protein and age related macular degeneration in the Cardiovascular Health Study. Br J Ophthalmol 89(9):1166–1170
Sivaprasad S, Adewoyin T, Bailey TA, Dandekar SS, Jenkins S, Webster AR et al (2007) Estimation of systemic complement C3 activity in age-related macular degeneration. Arch Ophthalmol 125(4):515–519
Bibl M, Welge V, Esselmann H, Wiltfang J (2012) Stability of amyloid-beta peptides in plasma and serum. Electrophoresis 33(3):445–450
Mayeux R, Honig LS, Tang MX, Manly J, Stern Y, Schupf N, Mehta PD (2003) Plasma A[beta]40 and A[beta]42 and Alzheimer’s disease: relation to age, mortality, and risk. Neurology 61(9):1185–1190
Lambert JC, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, Dartigues JF, Tzourio C, Alperovitch A, Buee L, Amouyel P (2009) Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology 73(11):847–853
Ertekin-Taner N, Younkin LH, Yager DM, Parfitt F, Baker MC, Asthana S, Hutton ML, Younkin SG, Graff-Radford NR (2008) Plasma amyloid beta protein is elevated in late-onset Alzheimer disease families. Neurology 70(8):596–606
Ding JD, Lin J, Mace BE, Herrman R, Sullivan P, Bowes RC (2008) Targeting age-related macular degeneration with Alzheimer’s disease based immunotherapies: anti-amyloid-beta antibody attenuates pathologies in an age-related macular degeneration mouse model. Vis Res 48(3):339–345
Ding JD, Johnson LV, Herrmann R, Farsiu S, Smith SG, Groelle M et al (2011) Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc Natl Acad Sci U S A 108(28):E279–E287
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guymer, R., Cipriani, T., Rittenhouse, K.D. et al. Plasma levels of amyloid beta and other proinflammatory mediators in patients with age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 253, 1347–1354 (2015). https://doi.org/10.1007/s00417-015-2970-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2970-x