Abstract
Purpose
To investigate the DNA methylation status of αA-crystallin gene in cataract secondary to pars plana vitrectomy.
Methods
Anterior capsular membranes of 40 eyes of 40 patients with cataract secondary to vitrectomy were collected. Another 20 eyes of 20 patients who received pars plana vitrectomy and phacoemulsification in the primary procedure, were recruited as control. Methylation status of the CpG islands of αA-crystallin gene was analyzed by pyrosequencing. Expression of αA-crystallin was evaluated by real-time polymerase chain reaction and western blot.
Results
In the post vitrectomy group, five patients with posterior subcapsular opacity and four patients with cortical opacity were excluded from further analysis. The remaining 31 patients with nuclear cataract were assigned into two groups according to tamponade types: 19 of octafluoropropane (C3F8) and 12 of silicone oil (SiO). The average nuclear color grading was elevated both in C3F8 and SiO groups after vitrectomy. Compared to the control group, hypermethylation of the CpG islands in the αA-crystallin gene promoter was found in both post vitrectomy groups, accompanied by significantly reduced αA-crystallin expression. No statistically significant differences were found between the C3F8 and SiO groups either for DNA methylation status or αA-crystallin expression.
Conclusions
CpG islands hypermethylation of αA-crystallin gene may be involved in nuclear cataract formation after pars plana vitrectomy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its first introduction in 1971 [1], pars plana vitrectomy (PPV) has been widely applied in treating various posterior segment diseases. Despite technical progress in PPV surgery, cataract formation, other than reoccurrence of primary underlying diseases, remains to be the most common complication of PPV [2], with up to 80 % developing cataract within 2 years [3]. Although the immediate cause is unknown, possible suspicions include age [4], iatrogenic factors, and concurrent systemic diseases like diabetes mellitus [5]. Meanwhile, different types of tamponade were reported to result in various forms of cataract and different interval time between PPV and cataract onset.
Previous studies, including our work, demonstrated that decreased levels of αA-crystallin (CRYAA) are involved in the lens opacification process during aging [6–8]. Alpha-crystallin, composed of two subunits, αA- and αB-crystallin, is the most abundant structural protein in the human lens [9–11]. It possesses chaperon-like function as a member of the small heat-shock protein family [12], which is particularly important in the lens, as there is no protein turnover in the inner part, and, once synthesized, these proteins persist for an individual’s lifespan.
Recently, research on epigenetic regulation of gene expression has drawn more attention. It alters DNA-transcription factor affinity through three mechanisms: DNA methylation, histone modification, and microRNAs. DNA methylation is found to be closely related to growth and development regulation and tumor pathogenesis [13]. Moreover, our previous studies found that down-regulation of CRYAA gene associated with DNA methylation in six CpG islands of CRYAA promoter is an important contributing factor both in the formation of age-related nuclear cataract [8] and high myopia cataract [14].
We, therefore, hypothesized that hypermethylation of CpG islands in the CRYAA promoter is also involved in cataract secondary to PPV. To test our hypothesis, anterior capsular membranes were collected from patients receiving phacoemulsification for secondary cataract after PPV, and DNA methylation status of CpG islands in CRYAA promoter was investigated by pyrosequencing. CRYAA gene expression in lens epithelial cells (LECs) of these eyes was analyzed by real-time polymerase chain reaction (real-time PCR) and western blot assay.
Methods
All the samples obtained during surgery were handled in accordance with the tenets of the Declaration of Helsinki. The ethics committee of the Eye and ENT Hospital, Fudan University, approved our collection and use of anterior capsular membranes from patients undergoing cataract surgeries. Before the sample collection, written informed consent was obtained from all subjects following an explanation of the nature and possible consequences of the study. The consent procedure was approved by the aforementioned ethics committee.
Patient collection
Forty eyes of 40 cataract patients with previous history of PPV because of rhegmatogenous retinal detachment (32 patients, 80 %) or macular holes (eight patients, 20 %) were recruited and received cataract surgery during 1 May 2012 to 30 April 2013. The preoperative exclusion criteria included: (a) cataracts due to etiologies other than PPV-related changes; (b) a history of prior ocular surgery except PPV; (c) glaucoma, uveitis, or choroidal neovascularization; (d) systematic disease such as diabetes. Another 20 eyes of 20 patients, who received PPV and phacoemulsification in the primary procedure, were designated as the control group. The type and severity of cataracts were graded and recorded according to the modified version of the lens opacity classification system III (LOCS III) [15] by one experienced surgeon before surgery, with NC1 to NC5 representing nuclear color (NC) grading. Average grading of control cases was NC (1.4 ± 0.5). As all the control cases were found with mild nuclear sclerosis, only 31 (77.5 %) cases with nuclear sclerosis in the post-PPV group were included for further analysis.
Human lens capsular membrane samples acquisition
Surgery procedures are briefly described below. An approximately 2.6 mm clear cornea incision was made. After the paracentisis, continuous curvilinear capsulorhexis measuring about 5.5 mm in diameter was accomplished using capsulorhexis forceps. Following hydrodissection, phacoemulsification of the nucleus and aspiration of the cortex were performed. Then the intraocular lens (IOL) was inserted into the capsular bag. The cornea incision was sealed with sterilized balanced salt solution. All the anterior capsular membranes were collected and stored at −80 °C immediately until further use.
DNA extraction and bisulfite conversion
DNA isolation was performed adhere to the manufacturer’s instructions of a DNeasy Blood & Tissue kit (Qiagen, Germantown, MD, USA). EZ DNA Methylation-Gold™ Kit (Zymo Research, Irvine, CA, USA) was used for bisulfite conversion of DNA. Each DNA sample (20 μl) mixed with CT Conversion Reagent (130 μl) were heated to 98 °C for 10 min, 64 °C for 2.5 h, and stored at 4 °C up to 20 h before loading onto the Zymo-Spin™ IC Column (Zymo Research). After centrifugation for 30 s at 10,000 × g, desulphonation buffer and elution buffer were added to the column, then centrifuged again for 30 s at 10,000 × g to elute the DNA. After bisulfite conversion, unmethylated cytosines (Cs) changed to thymines (Ts), leaving the methylated cytosines (CGs) unchanged.
PCR design and amplification
The bisulfite-modified DNA (2 μl) was amplified with primers of CRYAA gene as follows: forward, Biotin-TGGGGATATGTAGTTATTTTGATAGGAG; reverse, CTAAACCCCCAACCCCATAACCAT. The methylated primers were selected because the primer sequences are located in upstream sequences of the genes, and the inverse relation between methylation and expression of the gene has been demonstrated in our previous study using these primers [8]. PCR was performed in 25-μl reaction volumes, according to the manufacturer’s instructions (PyroMark PCR Kit, Qiagen), containing bisulfite converted DNA (20 ng), primers (0.2 μM each), 1 × PyroMark PCR Master Mix (containing HotStarTaq DNA Polymerase,1 × PyroMark PCR Buffer, and dNTPs), and MgCl2 (1.5 mM). The conventional amplification protocol of PCR was programmed as follows: initial 15 min first heat activation step at 95 °C followed by 45 amplification cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 30 s. The program was ended with a final elongation of 10 min at 72 °C. Each PCR sample was collected for pyrosequencing.
Pyrosequencing
The pyrosequencing procedure was performed according to previous studies [16] and the manufacturer’s instructions. A PyroMark Q24 Plate (Qiagen) was prepared by adding 0.3 μM sequencing primer (CAACCCCATAACCATC) in 25 μl annealing buffer per well, one for each sample to be tested. A mixture of 40 μl binding buffer and 2 μl streptavidin Sepharose beads (34 μm, 5 ml, Streptavidin Sepharose High Performance, GE Healthcare) was added to a biotinylated PCR product (15 μl), maintaining a total volume of 80 μl. Then the prepared mixture was added into a 24-well PCR plate. After sealing the plate, the mixture was shaken at room temperature to allow biotinylated PCR product capture on streptavidin-coated beads. Immediately after immobilization, the PCR plate and PyroMark Q24 Plate were placed onto a PyroMark Q24 Vacuum Workstation (Qiagen). The streptavidin-coated Sepharose beads containing immobilized templates were captured, washed, denatured, then rewashed with the Vacuum Prep Tool (Qiagen). The prewarmed PyroMark Q24 Plate containing the single-stranded pyrosequencing template DNA was heated at 80 °C for 2 min to denature the template and let the sequencing primer anneal during cooling to the room temperature. The resulting products were sequenced by PyroMark Q24 System (Qiagen). The methylation status of each locus in each pyrogram was analyzed individually as a T/C SNP using PyroMark Q24 Software (Qiagen).
Real-time polymerase chain reaction
Real-time PCR was performed as previously described [17]. Total RNA was extracted from anterior capsular membranes using an RNAprep Pure Cell Kit (DP430; TIANGEN, Beijing, China) according to the manufacturer’s instructions. DNase I was used to eliminate the possibility of genomic DNA contamination. Samples were dissolved in RNase-free water and quantified by the average of duplicate spectrophotometric readings at 260 nm (A260). Total RNA purity was determined by A260/A280 absorption. All the RNA samples were found to have A260/A280 ratios between 1.9 and 2.0. DNase-treated RNA samples were transcribed to cDNA with ReverTra Ace qPCR RT Kit (FSQ-101; TOYOBO, Osaka, Japan). The reaction containing of 1 μg RNA, 0.5 μL Primer Mix, and 0.5 μL RT Enzyme Mix was incubated for 15 min at 37 °C, followed by heating for 5 min at 95 °C.
PCR amplifications were performed in a 20-μL reaction mix containing 10 ng cDNA, 2 μL primers, and 10 μL PowerSYBR Green PCR Master Mix on Mastercycler (Eppendorf AG 22331; Hamburg, Germany). The specificity of the PCR amplification products was checked by performing dissociation melting-curve analysis and by 1 % agarose gel electrophoresis. All quantitation were calculated by the comparative cycle threshold method (ΔΔCt). The results were expressed as ratio between CRYAA and GAPDH gene.
Human primers were designed using computer software (LC Probe Design; Qiagen Operon, Alameda, CA, USA). Primer sequences are listed as follows: CRYAA, forward 5′-CCTGCTGCCCTTCCTGTCGT-3′, reverse 5′-TCCTGGCGCTCGTTGTGCT-3′; GAPDH, forward 5′-GAAGGTGAAGGTCGGAGTC- 3′, reverse 5′-GAAGATGGTGATGGGATTTC- 3′.
Western blot assay
Selected samples were lysed with pre-cooled extraction buffer (RIPA lysis buffer, P0013B; Beyotime, Jiangsu, China) and centrifuged at 3,200 × g for 10 min under 4 °C to obtain supernatants. After determining protein concentration by Quick Start Bradford Protein Assay Kit (Bio-Rad, Hercules, CA, USA), 5X SDS-PAGE loading buffer was added to sample extracts and denatured under 100 °C for 5 min. Then sample extracts were separated by 12 % gradient SDS-PAGE. Protein bands were transferred onto a PVDF blotting membrane (Millipore, Bedford, MA, USA), and subjected to immunolabeling using primary antibodies for CRYAA (1:1000 dilution, Abcam, Cambridge, MA, USA) and GAPDH (1:1000 dilution, Abcam, Cambridge, MA, USA). Then incubated the membrane with a horseradish peroxidase (HRP)-conjugated secondary antibody (1:2000 dilution; Abcam, Cambridge, MA, USA) for 30 min at room temperature. Immunoblotted bands were revealed by ECL chemiluminescence detection kit (Amersham Pharmacia Biotech, Cleveland, OH, USA). The intensity of bands was measured using ImageJ 1.45 s (USA).
Statistical analysis
All statistical analyses were performed using SPSS v20.0 (SPSS Inc., Chicago, IL, US). Experimental data were presented as mean ± SD. A P value less than 0.05 was considered statistically significant in all cases. The significance of the differences among three groups was determined by ANOVA. Least significant difference (LSD) test was used for multiple comparisons of means between groups. Spearman correlation analysis was used to analyze the relationship between the selected variables.
Results
Baseline characteristics of the sample
In the post-PPV group, there were five (12.5 %) patients with posterior subcapsular opacity and four (10 %) patients with cortical opacity. As all the control cases had mild nuclear sclerosis, only 31 (77.5 %) patients with nuclear sclerosis in the study group were included for further analysis. These 31 cases were further divided into two groups according to the type of postoperative tamponade, 19 in the C3F8 group and 12 in the SiO group. The 19 cases in C3F8 group received one PPV procedure, while of the SiO group, 11 had two procedures, one had three. The baseline data of 20 control and 31 PPV cases is listed in Table 1. No statistically significant differences were found for age, gender, axial length, or intraocular pressure among each group (all P > 0.05, according to ANOVA analysis and LSD test).
Progression of cataract after pars plana vitrectomy
The mean interval time between phacoemulsification and last vitreous procedure was 44 months (median: 24 months, range: 4–156 months) in the C3F8 group, and 23 months (median: 14 months, range: 6–70 months) in the SiO group. The SiO group had significantly shorter time interval between the PPV and cataract surgeries than the C3F8 group (P = 0.041).
The grading of cataracts before and after PPV in the two tamponade groups and in the control group are displayed in Table 2. Average grading of control cases was NC (1.4 ± 0.5), including 13 cases graded as NC1 and seven cases graded as NC2. Before cataract surgery, the LOCSIII grading of the two tamponade groups were similar to the control group (all P > 0.05; according to ANOVA analysis and LSD test). All cases were found to have an elevated NC grading after PPV. On average, before and after PPV, LOCSIII grades were (1.2 ± 0.4) and (3.3 ± 0 the.5) in C3F8 group, and (1.4 ± 0.5) and (3.9 ± 0.8) in the SiO group.
Hypermethylation of CRYAA promoter in the lens epithelial cells of cataracts secondary to pars plana vitrectiomy
Classic pyrograms of three cases are presented in Fig. 1. Average methylation data of each CpG island was calculated in each group. Of all the six selected CpG islands, both PPV groups presented higher methylation status compared to the control group (Fig. 2a). In the C3F8 group, 55.7 ± 5.0 % of the selected CpG islands was hypermethylated, and the in SiO group, the value was 59.6 ± 4.0 %, both of which were significantly higher than the control group (39.2 ± 7.5 %) (Fig. 2b, both P < 0.0,1; according to ANOVA analysis and LSD test). This strongly indicates that CpG islands in the CRYAA promoter of lens epithelial cells in the anterior capsular membrane from lenses of postvitrectomized eyes were hypermethylated.
CpG islands hypermethylation of the CRYAA promoter in cataract cases secondary to pars plana vitrectomy. a CpG islands are hypermethylated in two PPV groups. * C3F8 vs. control: P < 0.001. † SiO vs. control: P < 0.001. b Compared to the control group (39.2 ± 7.5 %), the six selected CpG islands in two PPV groups displayed hypermethylation: 55.7 ± 5.0 % and 59.6 ± 4.0 % for C3F8 and SiO groups, respectively. *P < 0.05 vs. control
Although the overall data seemed numerically elevated in SiO cases, no statistically significant difference in CRYAA methylation status was detected between the two PPV groups. The only one patient who underwent three procedures had a methylation rate as high as 65.3 %, while the average for those who underwent two surgeries was 59.2 ± 3.8 %.
The correlation between CpG islands methylation levels and age, gender, median and average interval time between PPV and cataract surgery, axial lengths, or intraocular pressure were also analyzed. However, no statistically significant correlations were observed in either PPV groups (all P > 0.05, according to Spearman correlation test; data not shown).
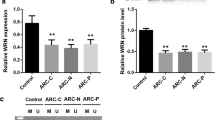
Decreased CRYAA mRNA and protein expression in the lens epithelial cells of cataract secondary to pars plana vitrectiomy
Comparing to the control, a significantly decrease in CRYAA mRNA expression in both PPV groups was confirmed by real-time PCR (both P < 0.05; Fig. 3). Meanwhile, the two PPV groups had significantly decreased levels of αA-crystallin expression (both P < 0.;5, Fig. 4). However, no statistically significant differences were found between the two PPV groups either for the CRYAA mRNA or protein expression.
Decreased expression of CRYAA mRNA in anterior capsular membranes of cataract cases secondary to pars plana vitrectomy. CRYAA mRNA expression was detected by real-time PCR. Results are shown as normalized fold expression levels of CRYAA mRNA. GAPDH was coamplified as an internal control. Data represent three independent experiments. *P < 0.05 vs. control
Decreased expression of αA-crystallins in anterior capsular membranes of cataract cases secondary to pars plana vitrectomy. CRYAA protein expression was detected by western blot. GAPDH was used as an internal control. Representative blots (left panels) and densitometric analysis with standard deviation (SD; right columns) of three independent experiments are shown. *P < 0.05 vs. control
Discussion
With the development of modern technology and our knowledge, pars plana vitrectomy is now a routine procedure in the clinic. It brings benefit to the patients by restoring their visual acuity. However, cataracts secondary to PPV, with incidence as high as 80 % in 2 years after PPV [2, 3, 5, 18], remains an unavoidable problem during the vision recovery process. It is also known that cataract surgery in vitrectomized eyes is more challenging than in nonvitrectomized eyes, most probably due to anatomical alterations imposed by lacking of vitreous support with primary vitreoretinal disorder and PPV surgery itself [19]. Therefore, it is important to look into the pathogenesis of cataract secondary to pars plana vitrectomy for better nonsurgical prevention and treatment. In this study, we found that CpG islands in the CRYAA promoter are hypermethylated in the lens epithelial cells of cataract patients after PPV. Real-time PCR and western blot confirmed that αA-crystallin expression is also significantly downregulated in these patients than in the control group. Overall, our findings suggest that downregulation of CRYAA associated with DNA hypermethylation might be involved in the development of cataract secondary to PPV.
In the two PPV groups with nuclear cataract, the average NC grading was significantly elevated after surgery, all of which indicated the formation and progression of cataract secondary to PPV [20, 21]. Prior studies showed various incidence rates in different cataract types after PPV. In the study of Pinter et al., PSC were seen more frequently in postvitrectomized eyes in 2 months to 6 years of follow-up [5]. In Thompson et al’s study, PSC was also found to be increased after vitrectomy, but this increase is relatively small and is not visually significant in most eyes [4]. Actually, most studies suggest that nuclear cataract might be the more common type of cataract after PPV [22, 23]. Hsuan et al. reported a characteristic, transient posterior subcapsular cataract (PSC) present in 89 % of tamponade patients within 24 h of surgery. However, according to the follow-up during the late postoperative period, such acute changes could generally resolve and then were followed by accelerated nuclear opacification with 67 % of patients developing nuclear cataracts in the tamponade cases after PPV [23]. Moreover, recent study of Gupta et al. showed an incidence rate of nuclear cataract as high as 91.89 % in high myopic eyes with previous vitrectomy, during a follow-up period of 3–6 weeks [22]. Our data (77.5 %) are also suggestive of nuclear cataracts as the major form of cataract secondary to PPV.
Regarding the median interval time between PPV and cataract surgeries, it was 24 months in patients with C3F8 tamponade and 14 months in those with SiO tamponade. The differences in time to cataract surgery between the two groups of post-PPV patients may be for the following two reasons. Firstly, as to the PPV with any kind of tamponades, the change of oxygen pressure in the vitreous cavity may be a crucial factor leading to cataracts. The partial oxygen pressure might be additionally elevated by ventilation with oxygen and a high oxygen pressure in the infusion fluid during surgery [24]. This elevated partial oxygen pressure may lead to increased oxidative stress to the lens proteins, and finally lead to the occurrence of a nuclear cataract [24]. Secondly, compared to C3F8, SiO can provide prolonged intraocular tamponade, and its prolonged direct contact with the posterior part of the lens may also be an important cause accelerating the development of cataracts after PPV [25]. However, further studies are required before the exact mechanism can be understood.
As for the pathogenesis of the post-PPV cataract, significantly increased oxygen tension in the vitreous and the anterior chamber of these eyes might be an important reason [20, 26–28]. Low levels of oxygen in the mid-vitreous and near the lens of the eye in humans may be important to the health of the lens, including maintenance of lens transparency [29]. When the gel vitreous, likely to function as a “sink” for O2 [27], is intact before vitrectomy, normal oxygen gradients within the eye can be maintained. Conversely, after surgical removal of vitreous or age-related liquefaction, liquid vitreous has a lower concentration of ascorbate and a slower rate of oxygen consumption [26]. In other words, the function of the vitreous body as a “sink” for O2 is likely to be seriously weakened after PPV. It is reported that vitrectomy surgery is associated with a significant increase in partial oxygen pressure (pO2) in the eye, according to observations in human beings and rabbit models [30, 31]. Such increased oxygen tension around the lens exposes its DNA and proteins to marked oxidative stress, and is recognized as an important risk factor for nuclear cataract formation [4, 32]. And the low incidence of cataracts in post-PPV patients with ischemic diabetic retinopathy also supports this [33].
Hypermethylation of the CRYAA promoter, and consequently decreased expression of αA-crystallin, might be another reason for the pathogenesis of the post-PPV cataract. Alpha-crystallin is the most important structural protein of the human lens, acting as a chaperone under conditions of oxidative stress and maintaining the transparency of the lens [11, 34–36]. Previous studies have shown that α-crystallin plays an essential role in protecting the lens against oxidative stress to delay the onset of cataract [37–39]. In this study, however, we observed a significant decrease in αA-crystallin expression in those post-PPV patients owing to the hypermethylation of its promoter compared with the control group. Hence, without protection of αA-crystallin, proteins in the hLECs, especially those antioxidative enzymes including glutathione peroxidase and superoxide dismutase, will undergo denaturation and aggregation under enhanced oxidative stress after PPV. Consequently, decreased activity of antioxidants and oxidation defense enzymes to maintain crystallins, is likely to leave the lens unprotected against oxidative stress, increasing the risk of cataract formation.
Epigenetic regulation of gene expression was recently shown to be associated with many eye diseases, such as age-related macular degeneration [40], glaucoma [41], and retinoblastoma [42]. Additionally, we reported that hypermethylation of CpG islands in the CRYAA promoter downregulates αA-crystallin expression either in age-related cataracts or high myopic cataracts [8, 14]. Our current study shows that hypermethylation of the six selected CpG islands in the CRYAA promoter may be also involved in nuclear cataract formation after PPV. The transcription of CRYAA gene is downregulated by hypermethylation of the promoter, followed by a decrease of mRNA and protein expression of CRYAA in hLECs of anterior capsular membranes (Figs. 3 and 4).
In conclusion, we observed an accelerated cataract formation and progression after PPV, and hypermethylation of CpG islands of the CRYAA gene might be involved in the formation of nuclear cataracts after PPV.
References
Melberg NS, Williams DF (1994) More on macular holes. Ophthalmology 101(11):1764–1765
Haller JA, Kerrison JB (1997) Cataract extraction after retinal detachment. Curr Opin Ophthalmol 8(3):39–43
Rivas-Aguino P, Garcia-Amaris RA, Berrocal MH, Sanchez JG, Rivas A, Arevalo JF (2009) Pars plana vitrectomy, phacoemulsification and intraocular lens implantation for the management of cataract and proliferative diabetic retinopathy: comparison of a combined versus two-step surgical approach. Arch Soc Esp Oftalmol 84(1):31–38
Thompson JT (2004) The role of patient age and intraocular gas use in cataract progression after vitrectomy for macular holes and epiretinal membranes. Am J Ophthalmol 137(2):250–257. doi:10.1016/j.ajo.2003.09.020
Pinter SM, Sugar A (1999) Phacoemulsification in eyes with past pars plana vitrectomy: case–control study. J Cataract Refract Surg 25(4):556–561
Su S, Liu P, Zhang H, Li Z, Song Z, Zhang L, Chen S (2011) Proteomic analysis of human age-related nuclear cataracts and normal lens nuclei. Invest Ophthalmol Vis Sci 52(7):4182–4191. doi:10.1167/iovs. 10-7094
Goishi K, Shimizu A, Najarro G, Watanabe S, Rogers R, Zon LI, Klagsbrun M (2006) AlphaA-crystallin expression prevents gamma-crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development 133(13):2585–2593. doi:10.1242/dev.02424
Zhou P, Luo Y, Liu X, Fan L, Lu Y (2012) Down-regulation and CpG island hypermethylation of CRYAA in age-related nuclear cataract. FASEB J: Off Publ Fed Am Soc Exp Biol 26(12):4897–4902. doi:10.1096/fj.12-213702
Zhu X, Korlimbinis A, Truscott RJ (2010) Age-dependent denaturation of enzymes in the human lens: a paradigm for organismic aging? Rejuvenation Res 13(5):553–560. doi:10.1089/rej.2009.1009
Horwitz J (2003) Alpha-crystallin. Exp Eye Res 76(2):145–153
Zhu X, Gaus K, Lu Y, Magenau A, Truscott RJ, Mitchell TW (2010) alpha- and beta-crystallins modulate the head group order of human lens membranes during aging. Invest Ophthalmol Vis Sci 51(10):5162–5167. doi:10.1167/iovs. 09-4947
Clark AR, Lubsen NH, Slingsby C (2012) sHSP in the eye lens: crystallin mutations, cataract and proteostasis. Int J Biochem Cell Biol 44(10):1687–1697. doi:10.1016/j.biocel.2012.02.015
Robertson KD (2005) DNA methylation and human disease. Nat Rev Genet 6(8):597–610. doi:10.1038/nrg1655
Zhu XJ, Zhou P, Zhang KK, Yang J, Luo Y, Lu Y (2013) Epigenetic regulation of alphaA-crystallin in high myopia-induced dark nuclear cataract. PLoS One 8(12):e81900. doi:10.1371/journal.pone.0081900
Chylack LT Jr, Wolfe JK, Friend J, Tung W, Singer DM, Brown NP, Hurst MA, Kopcke W, Schalch W (1995) Validation of methods for the assessment of cataract progression in the roche european-american anticataract trial (REACT). Ophthalmic Epidemiol 2(2):59–75
Royo JL, Hidalgo M, Ruiz A (2007) Pyrosequencing protocol using a universal biotinylated primer for mutation detection and SNP genotyping. Nat Protoc 2(7):1734–1739. doi:10.1038/nprot.2007.244
McCue BA, Park YH, Brazzell RK, Boltralik JJ (1991) Capillary gas chromatographic-electron-capture assay for the aldose reductase inhibitor imirestat in lens and plasma. J Chromatogr 565(1–2):255–264
Braunstein RE, Airiani S (2003) Cataract surgery results after pars plana vitrectomy. Curr Opin Ophthalmol 14(3):150–154
Shousha MA, Yoo SH (2010) Cataract surgery after pars plana vitrectomy. Curr Opin Ophthalmol 21(1):45–49. doi:10.1097/ICU.0b013e32833303bf
Cheng L, Azen SP, El-Bradey MH, Scholz BM, Chaidhawangul S, Toyoguchi M, Freeman WR (2001) Duration of vitrectomy and postoperative cataract in the vitrectomy for macular hole study. Am J Ophthalmol 132(6):881–887
Melberg NS, Thomas MA (1995) Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology 102(10):1466–1471
Gupta M, Lascaratos G, Syrogiannis A, Verma S (2011) Outcome of phacoemulsification in previously vitrectomized myopic eyes with axial length greater than 26 mm. Eur J Ophthalmol 21(4):379–384. doi:10.5301/EJO.2010.6096
Hsuan JD, Brown NA, Bron AJ, Patel CK, Rosen PH (2001) Posterior subcapsular and nuclear cataract after vitrectomy. J Cataract Refract Surg 27(3):437–444
Petermeier K, Szurman P, Bartz-Schmidt UK, Gekeler F (2010) Pathophysiology of cataract formation after vitrectomy. Klin Monatsbl Augenheilkd 227(3):175–180. doi:10.1055/s-0029-1245271
Milazzo S (2014) Pathogenesis of cataract after vitrectomy. J Fr Ophtalmol 37(3):243–244. doi:10.1016/j.jfo.2013.12.002
Shui YB, Holekamp NM, Kramer BC, Crowley JR, Wilkins MA, Chu F, Malone PE, Mangers SJ, Hou JH, Siegfried CJ, Beebe DC (2009) The gel state of the vitreous and ascorbate-dependent oxygen consumption: relationship to the etiology of nuclear cataracts. Arch Ophthalmol 127(4):475–482. doi:10.1001/archophthalmol.2008.621
Holekamp NM, Shui YB, Beebe DC (2005) Vitrectomy surgery increases oxygen exposure to the lens: a possible mechanism for nuclear cataract formation. Am J Ophthalmol 139(2):302–310. doi:10.1016/j.ajo.2004.09.046
Cherfan GM, Michels RG, de Bustros S, Enger C, Glaser BM (1991) Nuclear sclerotic cataract after vitrectomy for idiopathic epiretinal membranes causing macular pucker. Am J Ophthalmol 111(4):434–438
Dahan E, Allarakhia L (1991) Irrigation, aspiration, and polishing cannula. J Cataract Refract Surg 17(1):97–98
Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC (2010) Oxygen distribution in the human eye: relevance to the etiology of open-angle glaucoma after vitrectomy. Invest Ophthalmol Vis Sci 51(11):5731–5738. doi:10.1167/iovs. 10-5666
Barbazetto IA, Liang J, Chang S, Zheng L, Spector A, Dillon JP (2004) Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res 78(5):917–924. doi:10.1016/j.exer.2004.01.003
Beebe DC, Holekamp NM, Shui YB (2010) Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res 44(3):155–165. doi:10.1159/000316481
Holekamp NM, Bai F, Shui YB, Almony A, Beebe DC (2010) Ischemic diabetic retinopathy may protect against nuclear sclerotic cataract. Am J Ophthalmol 150(4):543–550 e541. doi:10.1016/j.ajo.2010.05.013
Hooi MY, Raftery MJ, Truscott RJ (2013) Accelerated aging of Asp 58 in alphaA crystallin and human cataract formation. Exp Eye Res 106:34–39. doi:10.1016/j.exer.2012.10.013
Truscott RJ, Zhu X (2010) Presbyopia and cataract: a question of heat and time. Prog Retin Eye Res 29(6):487–499. doi:10.1016/j.preteyeres.2010.05.002
Wang K, Spector A (1995) Alpha-crystallin can act as a chaperone under conditions of oxidative stress. Invest Ophthalmol Vis Sci 36(2):311–321
Spector A (1995) Oxidative stress-induced cataract: mechanism of action. FASEB J: Off Publ Fed Am Soc Exp Biol 9(12):1173–1182
Lou MF, Dickerson JE Jr (1992) Protein-thiol mixed disulfides in human lens. Exp Eye Res 55(6):889–896
Beswick HT, Harding JJ (1984) Conformational changes induced in bovine lens alpha-crystallin by carbamylation. Relevance to cataract. Biochem J 223(1):221–227
Hunter A, Spechler PA, Cwanger A, Song Y, Zhang Z, Ying GS, Hunter AK, Dezoeten E, Dunaief JL (2012) DNA methylation is associated with altered gene expression in AMD. Invest Ophthalmol Vis Sci 53(4):2089–2105. doi:10.1167/iovs. 11-8449
Wiggs JL (2012) The cell and molecular biology of complex forms of glaucoma: updates on genetic, environmental, and epigenetic risk factors. Invest Ophthalmol Vis Sci 53(5):2467–2469. doi:10.1167/iovs.12-9483e
McCarthy N (2012) Retinoblastoma: epigenetic outcome. Nat Rev Cancer 12(2):80. doi:10.1038/nrc3222
Acknowledgments
This work was supported by research grants from National Natural Science Foundation of China (Grant No. 81100653 and 81470613), the New Hundred Talents Program of Shanghai Municipal Health Bureau (Grant No. XBR2011056), the National Major Scientific Equipment program (Grant No. 2012YQ120080), and the National Health and Family Planning Commission of the People’s Republic of China (Grant No.201302015).
Authors’ contributions
Research design: XJZ, YL and CHJ. Performed the experiments: PZ and KKZ. Analyzed the data: KKZ. Contributed reagents/materials/analysis tools: PZ and CHJ. Obtained the funding: XJZ, YL and CHJ. Wrote the manuscript: XJZ, KKZ and CHJ. The authors wish to thank support from Ruiping Gu for assistance with the anterior capsular membrane collection.
Conflicts of interest
The authors declare that they have no competing interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiang-Jia Zhu and Ke-Ke Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhu, XJ., Zhang, KK., Zhou, P. et al. αA-crystallin gene CpG islands hypermethylation in nuclear cataract after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol 253, 1043–1051 (2015). https://doi.org/10.1007/s00417-015-2949-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-2949-7