Abstract
Background
To compare the anatomic and functional results between optical coherence tomography (OCT)-guided selective focal laser photocoagulation (OCT–laser) and conventional modified Early Treatment Diabetic Retinopathy Study (mETDRS) laser treatment for diabetic macular edema (DME).
Methods
We analyzed treatment outcomes in 47 consecutive eyes treated with OCT–laser compared to 31 matched eyes treated with mETDRS. In the OCT–laser group, we identified ‘significant actively-leaking microaneurysms on OCT’ (SALMO) which are responsible for edema in OCT B-scan images, and thoroughly ablated them with photocoagulation. Best-corrected visual acuity (BCVA) and retinal thickness by OCT were compared at baseline and 12 months after treatment between two groups.
Results
OCT–laser treatment resulted in significant improvements in BCVA, central subfield thickness (CST), and maximum retinal thickness (MRT) from baseline at 12 months from the time of therapy (+2.5 letter score, p = 0.04; −45.56 μm in CST, p < 0.001; −91.6 μm in MRT, p < 0.001). The mean number of treated ‘SALMO’ was 5.6 ± 4.0 (range 1–26), while the number of MAs in ‘treatable lesions’ by fluorescein angiography (FA) in the same eye was 16.3 ± 11.8 (range 2–42). There was no difference between OCT–laser and mETDRS groups in changes of these parameters from baseline at 12 months (p = 0.56, p = 0.89, p = 0.43 respectively). Fundus autofluorescence (FAF) and OCT revealed less tissue damage in OCT–laser-treated eyes, compared to eyes treated with mETDRS (p < 0.001).
Conclusions
OCT–laser shows similar anatomic and functional outcomes compared to conventional laser (modified ETDRS), with significantly less retinal damages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Focal laser photocoagulation has been used as the standard treatment for diabetic macular edema (DME) [1, 2]. The Early Treatment Diabetic Retinopathy Study (ETDRS) showed that focal/grid photocoagulation for DME reduced the 3-year risk of losing 3 lines or more of vision by approximately 50 % [1]. To obtain a similar therapeutic effect with reduced tissue damage and treatment-related complications, a modified ETDRS (mETDRS) protocol, which employs a reduced-intensity laser and partial coverage of ‘treatable lesions’, has been used. It was formalized by the Diabetic Retinopathy Clinical Research Network (DRCR.net) to reflect the prevailing treatment approach by most ophthalmologists in the USA [3].
However, there still exist divergences in technique for macular laser treatment. ‘Treatable lesions’ in the ETDRS were originally defined by findings on FA [1]. However, the leakage area on FA and retinal thickening on optical coherence tomography (OCT) do not coincide completely [4], and currently, focal/grid laser photocoagulation is often performed without FA [2, 5]. Moreover, a previous study suggested divergence in the placement and dosage of laser spots among retina specialists [6]. This variability in photocoagulation technique for DME may lead to variability in treatment results, including visual outcomes. Standardization of the treatment protocol is essential for clinical trials evaluating or comparing new treatment modalities such as anti- vascular endothelial growth factor (VEGF) [7–10]. Thus, there is a need for a consensus definition of a ‘treatable lesion’ in DME, and consensus regarding standard techniques of photocoagulation for DME treatment.
Recent advances in spectral-domain OCT (SD-OCT) technology have allowed visualization of a topographic map of the thickened retina as well as direct visualization of diabetic microaneurysms (MA) and intraretinal cystic changes, which enhances our understanding of the pathoanatomy of DME [11, 12]. In our previous study, we proposed a mechanism of retinal thickening in DME whereby MAs, which disrupt the integrity of the synaptic portion of the outer plexiform layer (OPL) (fluid conductivity barrier), induce fluid accumulation predominantly in the loose fiber portion of the outer plexiform layer (OPL) and outer nuclear layer (ONL) [13]. Based on this concept, direct visualization of the source of leakage on SD-OCT and direct photocoagulation would make it possible to standardize protocols for focal laser treatment and to reduce interpersonal variability.
In this study, we introduce a novel photocoagulation protocol, named OCT-guided selective focal laser photocoagulation. Using SD-OCT, we were able to directly visualize ‘significant actively-leaking MAs on OCT’ (SALMO) and apply direct photocoagulation to them. We compared the visual and anatomic outcomes with eyes treated with conventional mETDRS laser therapy.
Methods
Study design
This study included 47 consecutive eyes with DME treated with OCT-guided selective focal laser photocoagulation (OCT–laser group) and 31 eyes treated with the mETDRS focal/grid photocoagulation technique (mETDRS group) at Yonsei University Severance Hospital between September 2009 and May 2012. All research and measurements adhered to the tenets of the Declaration of Helsinki. The study was approved by the Institutional Review Board/Ethics Committee of Yonsei University.
Eligible individuals were at least 18 years old with a diagnosis of type 2 diabetes mellitus. We enrolled patients with clinically significant diabetic macular edema (CSME) and predominantly focal-type DME. CSME was defined as was described in ETDRS [14]. Predominantly focal-type DME was defined by: 1) ophthalmoscopy, 2) dye leakage from an MA in late phase on fluorescein angiography (FA), and 3) predominant swelling in the outer plexiform layer (OPL)/outer nuclear layer (ONL) near MAs and focal elevation in retinal topography on OCT. Eyes with the following conditions were excluded: significant media opacities, coexisting macular conditions (e.g., severe epiretinal membrane or definite vitreo-macular traction, extensive macular ischemic changes, or age-related macular degeneration), history of pan-retinal scatter photocoagulation within 6 months, history of focal laser photocoagulation for DME within 12 months, history of intravitreal or peribulbar injections of triamcinolone or anti-VEGF agent within 6 months, or history of vitrectomy or any other surgery within 6 months. Eyes treated with traditional mETDRS focal/grid photocoagulation (mETDRS group) were included for comparison. Among 120 eyes treated for CSME with mETDRS photocoagulation during the study period, 31 eyes that met the above criteria were included in the analysis, after excluding patients who were lost to follow-up or who received DME therapy other than laser (e.g., vitrectomy or intravitreal injection) within 12 months.
Before the initial laser treatment, patients received ophthalmic examinations including best-corrected visual acuity (BCVA), fundus exam, FA, and SD-OCT. Using SD-OCT, we measured the retinal thickness, both central subfield thickness (CST) and maximum retinal thickness in the OCT–laser and mETDRS treatment groups. After the initial laser treatment, follow-up evaluations and re-treatments were performed every 16 weeks over a 12-month period. Laser photocoagulation was repeated using the same protocol at each visit, unless DME resolved or the investigator noted substantial improvement in retinal thickness. At 12 months after treatment, BCVA, fundus examination, and SD-OCT were performed and outcomes were compared between OCT–laser and mETDRS-treated eyes.
Treatment protocols
OCT-guided selective focal laser photocoagulation
High-resolution SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany) images were acquired prior to treatment. Using closely spaced (30 μm) OCT B-scan images with raster scan covering the thickened retinal area (more than 30 × 15°), we identified actively leaking MAs which were responsible for the focal edema, predominantly the outer retina (OPL/ONL) swelling. On SD-OCT images, ‘SALMO’ were defined as circular structures which encroach upon and disrupt the integrity of the synaptic (inner) portion of the OPL, and induce focal fluid collection, predominantly in the OPL/ONL [13]. The location of SALMO was marked on corresponding areas in fundus photography images. For marking of the location of SALMO on fundus photographs, a scan reference image which showed the location of the SALMO was exported to an external computer as a separate file. Images were superimposed and aligned to fundus photographs according to retinal landmarks, such as the optic disc and retinal vessels, using Adobe Photoshop (CS2, Adobe Systems Incorporated, San Jose, CA, USA). The locations of SALMO were marked on fundus photographs as circles (Fig. 1). A SALMO-marked fundus photograph was projected on a screen beside the patient and was used as a guide while performing laser treatment.
Image processing of treatment plans for OCT-guided selective focal laser photocoagulation. Step 1: Find ‘significant actively-leaking microaneurysms on OCT’ (SALMO) (white arrows) using a 30-μm raster scan. SALMO were defined as circular structures which disrupt the integrity of the synaptic portion of the outer plexiform layer and which induced focal fluid collections, predominantly in the outer plexiform layer/outer nuclear layer. Step 2: Superimpose scan reference images of OCT to the fundus photograph, according to retinal landmarks (e.g., optic disc and retinal vessels) using Adobe® Photoshop®, and mark the location of SALMO on the fundus photograph with circles. Step 3: Left Treatment plan of OCT-guided selective focal laser photocoagulation. Right ‘Treatable lesion’ by ETDRS. Fewer microaneurysms required treatment with OCT-guided selective focal laser photocoagulation
Eyes were treated following pupillary dilatation and instillation of topical anesthesia using an ocular Mainster (standard) focal/grid contact lens (Ocular Instruments, Bellevue, WA, USA). Laser photocoagulation was performed by a single retina specialist (S.H.B) using a PASCAL laser (Pattern Scan Laser, OptiMedica Corporation, Santa Clara, CA, USA), with a 532-nm short pulse. The aiming laser beam was directly focused on the anterior surface of SALMO. SALMO were directly and repeatedly ablated by small single-spot laser beam with the following parameters: spot size = 60 μm, duration = 30 msec, power = 100–150 mW. When SALMO were located more than 500 μm outside the center of the fovea, the lesions were treated until the ophthalmologist noted whitening or darkening of the MA itself. Repeated photocoagulation was usually necessary, especially for large lesions. When small MAs were located within 500 μm of the foveal center, we attempted to photocoagulate directly. However, ablation was stopped when faint RPE burns were visible underneath.
Modified ETDRS photocoagulation
Modified ETDRS focal/grid photocoagulation (mETDRS) was performed by experienced retinal specialists with the same instrument, based on the DRCR.net guidelines. Treatment was comprised of focal direct laser for all leaking MAs in area of retinal thickening and grid pattern laser for area of thickened retina or retinal non-perfusion area in ‘treatable lesions’, as defined by the ETDRS [14]. Photocoagulation-induced color change of MA was not required, but laser treatment usually induced a light grayish color change in retina. Treatment was also performed with a PASCAL laser using a 60–100 μm diameter spot size and 30 msec duration.
Grading for damages after laser photocoagulation in FAF and OCT
Degree of tissue damage was evaluated using fundus autofluorescence (FAF) and SD-OCT, and the results were compared between the two different laser-treated groups. FAF was evaluated 12 months after treatment. Extent of tissue damage was characterized by the percentage of treated sites with new, visible hyper- or hypo-autofluorescent spots on FAF: mild to none (<10 %), moderate (10–50 %), or severe (>50 %). Low-quality FAF images were excluded from analysis. SD-OCT images were obtained 4 months after treatment, and were acquired at the same location as the original location of a MA. By SD-OCT, the extent of new tissue damage following laser treatment was characterized as ‘mild to none’ when damage was limited to the inner retinal layer, ‘moderate’ when there was partial damage of the outer retinal layer, or ‘severe’ when there was near-total disruption of the outer retinal layer. The degree of damage by OCT was averaged in the same eye and was characterized as ‘mild to none’, ’moderate’, or ‘severe’. All FAF and OCT images were graded by two medical retina specialists (J.Y.S., YK Chu). When their analysis differed significantly, a third medical retina specialist (S.H.B.) was asked to independently grade FAF and OCT images.
Statistical methods
Statistical analysis was performed with SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). For intergroup comparison, categorical data were assessed using the Chi-square test and Fisher’s exact test, and continuous variables were compared with the independent two-sample t-test and Mann–Whitney U test. We used the mixed model (covariance pattern, CS) to evaluate the course of response to treatment. The level of statistical significance was set at p < 0.05.
Results
Comparison of the baseline characteristics and treatment outcomes between OCT–laser and mETDRS groups
We analyzed 47 consecutive eyes of 36 patients in the OCT-guided selective focal laser group and 31 eyes of 22 patients in the mETDRS laser group. Baseline characteristics were not significantly different between the two groups in terms of age (p = 0.21), sex (p = 0.82), duration of diabetes mellitus (p = 0.64), history of prior treatment for DME (p = 0.82), history of pan-retinal photocoagulation (PRP, p = 0.61), or severity of diabetic retinopathy (DR, p = 0.82). There were also no significant differences in baseline measurements of visual acuity letter score (p = 0.27), CST (p = 0.21), or maximum retinal thickness (p = 0.62).
The mean change in visual acuity from baseline was +2.57 letters in the OCT–laser group and +1.45 letters in the mETDRS group (p = 0.56). The mean change in CST was −45.56 μm in the OCT–laser group and −43.48 μm in the mETDRS group (p = 0.89). And, the mean change in maximum retinal thickness was −91.60 μm in the OCT–laser group, versus −79.48 μm in the mETDRS group (p = 0.43). Outcomes did not differ significantly between the two treatment groups. The number of treatment sessions within 12 months was 1.75 ± 0.92 sessions in the OCT–laser group and 1.48 ± 0.93 in the mETDRS group (p = 0.23) (Table 1).
Treatment planning of OCT-guided selective focal laser photocoagulation
The mean number of SALMO treated was 5.6 ± 4.0 (range 1–26) during the 1st session of laser therapy, while the number of MAs in ‘treatable lesions’ by FA in the same eye was 16.3 ± 11.8 (range 2–42). The mean number of treated SALMO was 5.6 ± 4.0 in the 1st session, 2.5 ± 0.8 in the 2nd session, and 2.1 ± 0.9 in the 3rd session.
Eighty-three percent of SALMO were observed as prominent leakage sites by FA, and 63 % of SALMO were visible as red or white dots on FP.
Treatment response to OCT-guided selective focal laser photocoagulation
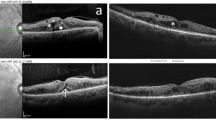
In the OCT–laser group, the mean baseline visual acuity letter score was 62.0 ± 15.7. Mean visual acuity letter score was 62.7 at 4 months, 61.21 at 8 months, and 64.59 at 12 months following therapy (p = 0.04, mixed model). The mean CST improved from 362.6 ± 85.17 μm to a mean thickness of 339.7 μm at 4 months, 333.2 μm at 8 months, and 317.53 μm at 12 months (p < 0.001). In addition, the mean maximum retinal thickness decreased from 503.4 ± 83.25 μm to 450.7 μm at 4 months, 439.2 μm at 8 months, and 412.2 μm at 12 months (p < 0.001). The CST and maximum retinal thickness were maximally reduced within 4 months after treatment, while the visual acuity letter score was most improved between 8 and 12 months (Fig. 2). Serial morphological changes by OCT, after OCT-guided selective focal laser photocoagulation, are shown in Fig. 3 and Online Resource 1.
Serial morphological changes on OCT after OCT-guided selective focal laser photocoagulation. (Top and middle) Serial regression pattern of ‘significant actively-leaking microaneurysms on OCT’ (SALMO) (arrows) with resolution of adjacent edema after OCT-guided selective focal laser photocoagulation. (Bottom) Gradual resolution of diabetic macular edema in the OCT thickness map
Damage to retinal pigment epithelium (RPE) and outer retina after laser treatment
Damage to the retinal pigment epithelium (RPE) and outer retina was evaluated using FAF and SD-OCT (Table 2 and Fig. 4). FAF imaging 12 months after laser treatment demonstrated that all cases in the OCT–laser group showed <10 % of new spots at sites of prior laser therapy. In contrast, the mETDRS group showed moderate to severe tissue damage following laser therapy, except for one eye (p < 0.001, Fisher’s exact test). SD-OCT images at 4 months after laser treatment revealed that the majority of eyes (94 %) in the OCT–laser group showed minimal damage confined to the inner retina, and only 6 % of eyes showed damage of the outer retina. In comparison, 84 % of eyes in the mETDRS group showed obvious outer retinal damage on OCT (p < 0.001).
Fundus autofluorescence and spectral domain OCT after laser photocoagulation. a,d Treatment plan of OCT-guided selective focal laser photocoagulation (a) and mETDRS (d). Red circles indicate treatment-targeted microaneurysms. Green line on d indicates ‘treatable lesion’ defined by ETDRS. b,e On fundus autofluorescence 12 months after treatment, no definite damage is observed on the treated site with OCT-guided selective focal laser photocoagulation (b) while multiple hyper- and hypo- autofluorescence spots are observed in the mETDRS treated group (e). c,f On the spectral-domain OCT 4 months after treatment, regressing microaneurysms (arrows) are noted with no definite changes in the outer retina and retinal pigment epithelium (c). In contrast, disruption of the outer retina and retinal pigment epithelium (triangles) are shown (f)
Discussion
In this study, we introduce a novel macular photocoagulation protocol, OCT-guided selective focal laser photocoagulation, for the treatment of DME. This novel protocol treats only the causative MA, not all MAs located in the area of retinal thickening. Therefore, we were able to treat fewer MA with less retinal damage, but obtain comparable treatment outcome to the conventional mETDRS treatment.
The ‘treatable lesions’ in the ETDRS were originally defined based on FA findings [1]. However, not all MAs are responsible for retinal thickening, even when they are found to be located in an area of leakage in FA [4]. Previous studies have demonstrated that substantial areas of the retina do not exhibit definite retinal thickening, even when associated with profound leakage by FA [15, 16]. Regions with dye leakage by FA and retinal thickening by OCT do not appear to overlap perfectly. Dye leakage patterns visible in FA cannot predict the clinical outcome of focal laser photocoagulation [17]. Therefore, some MAs, although they may show leakage in FA, may not be responsible for retinal swelling [4]. Nowadays, many ophthalmologists perform laser photocoagulation without FA guidance [2, 5].
SD-OCT is widely used in diagnosis and treatment of DME. SD-OCT provides objective geographic information on retinal swelling with retinal thickness maps, but also makes it possible to visualize MAs directly with B-scan images [12, 18]. MAs are visible as well-demarcated round or oval intra-retinal lesions, mainly located in the inner nuclear layer (INL) [12, 13]. However, not all MAs within thickened areas of the retina induce nearby focal fluid collection. In our previous study [13], leakage from MAs which disrupt the integrity of the synaptic portion of the OPL resulted in focal accumulation of fluid in loose fiber portions of the OPL and ONL, because the synaptic portion of the OPL acts as a barrier (fluid conductivity barrier) against fluid movement from the vascularized inner retina into the avascular outer retina [19, 20]. Based on this concept, MAs which disrupt or encroach upon the synaptic (inner) portion of the OPL, with collection of fluid in the adjacent outer retina, were defined as ‘SALMO’ and were targeted for focal laser treatment. Accordingly, laser treatment protocols were not just determined by a retinal thickness map. While FA findings showed discordance between dye leakage and thickening area on OCT [4], the distribution of these SALMO corresponded well with the thickened area on an thickness map on OCT.
Not all SALMO were definitely visible in FA or fundus photography (FP). Only 83 % of SALMO were observed as prominent leaking dots in FA, and only 63 % of SALMO were visible as red or white dots on fundus photography in our study. We think that there are several plausible explanations for this incongruity. Active MAs are usually located in the deep capillary plexuses, and deep capillary plexuses are less visible than a superficial capillary plexus in FA [21]. Also, active MAs are often localized near areas of hemorrhage. These intra-retinal hemorrhages may hamper the visualization of MAs in FA. In addition, incompletely-perfused MAs may not be well-visualized by FA. These results point to some of the limitations of FA and FP in finding the causative MAs in DME. Thus, determining of SALMO using OCT B-scan images would make it possible to find and treat the accurate leakage sources based on the pathogenesis of focal DME.
Recently, pharmacological therapies, including anti-VEGF agents, have been investigated for the treatment of DME [7, 8]. The potential for combined therapy with laser photocoagulation and pharmacological agents is currently being debated [3, 9, 10]. In the DRCR net study, combined prompt treatment with laser produced suboptimal visual outcomes, but required fewer injections and clinic visits. It is possible that inhomogeneity of treatment protocols used by investigators in this large clinical trial affected treatment results. In addition, unlike pharmacological therapies, the laser photocoagulation protocol and methods are highly variable among surgeons. One study showed significant differences among retina specialists in the number and placement of planned laser spots, even when the retina specialists were given identical information about DMR and treatable lesions [6]. Therefore, standardization of laser photocoagulation protocols in DME is necessary, particularly in the setting of a clinical trial. Use of OCT-guided selective focal laser photocoagulation may be one approach for overcoming ophthalmologist-related variability, because it permits objective determination of causative MAs and treatment planning using OCT.
OCT–laser can be performed using a conventional laser system. However, treating a precise location, according to the treatment plan is important for reducing interpersonal variability of laser treatment. Although laser photocoagulation was applied manually in our study, the navigated laser (NAVILAS; OD-OS GmbH, Teltow, Germany) photocoagulator may be useful for improving accuracy and overcoming interpersonal variability [22].
Our novel treatment protocol could reduce retinal damage in terms of the number of treated MAs, and the degree of outer retinal and RPE damage. Our novel protocol allowed photocoagulation of fewer MAs than the conventional treatment. That is, in the same eye, the number of SALMO found in OCT were about a third of the number of MAs in ‘treatable lesions’ which were identified by FA. In addition, FAF and SD-OCT images after treatment showed mild changes in the OCT–laser group of eyes, while most eyes in the mETDRS group showed moderate to severe damage, even though the investigators used the same laser delivery systems and similar laser power settings. Previous studies have reported that photocoagulation can result in enlargement of the area of RPE atrophy by up to 300 %, and may occasionally result in development of secondary choroidal neovascular membranes [23, 24]. Although the original ETDRS was modified into the mETDRS protocol to minimize such damages, it still has potential risks. The minimal damage to the RPE and outer retina that we observed in the OCT–laser group of eyes may be related to our laser delivery techniques. We tried to focus the aiming beam on the anterior surface of MAs and to ablate it directly, and whitening of MAs themselves was observed. Thus, laser beams seemed to be defocused in the RPE layer, and most of the laser’s energy was absorbed by the roof of the MAs. Absorption of laser energy by the MA diminished damage to the RPE and outer retina. Theoretically, the slightly longer wavelength of yellow laser (krypton, 568 nm; dye and diode, 577 nm) may be more effective in producing retinal vessel (MAs) occlusion in comparison to the green laser.
This study has some limitations including its retrospective nature. Because we have excluded patients who received additional treatment other than laser (vitrectomy or intravitreal injection), severe or refractory DME cases have been excluded. This selection-bias may have lead to a relatively favorable assessment of our treatment outcomes. However, our results were promising and suggest the need for further prospective investigations with a larger patient cohort and longer follow-up times.
Although recently published results of pharmacologic therapies have changed the management approach to DME, laser photocoagulation still has a role. Patients who cannot tolerate monthly injections or visits, or who have a high risk for cardiovascular or cerebrovascular complication, or who do not respond well to ranibizumab therapy may benefit from laser treatment. About 3–5 % of patients treated with ranibizumab have been reported to lose more than 10 BCVA letters over 12 months [3, 7, 9]. Also, in real-world situations, treatment results of intraviteal injection are not as favorable as those in published clinical trials, because of the limited number of visits and injections. The question that still needs to be answered is whether combined laser treatment confers additional benefit in terms of VA and quality of life, or in terms of a reduced number of anti-VEGF injections [3, 25]. Since improvements in vision following laser treatment usually occur very slowly [25], long-term follow-up is required to determine treatment efficacy.
In conclusion, OCT-guided selective focal laser photocoagulation shows comparable treatment outcome to that of conventional treatment, but significantly reduces retinal damage This novel laser protocol would be a promising method as a laser alone or as an adjuvant modality for pharmacologic agents. Future advances in laser protocols and techniques will help to optimize customized treatments for DME.
References
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Arch Ophthalmol 103:1796–1806
Aiello LP, Edwards AR, Beck RW et al (2010) Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 117:946–953
Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP et al (2010) Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 117:1064–1077
Blair NP, Shahidi M, Lai WW, Zelkha R (2008) Correlation between microaneurysms and retinal thickness in diabetic macular edema. Retina 28:1097–1103
Writing Committee for the Diabetic Retinopathy Clinical Research Network, Fong DS, Strauber SF et al (2007) Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol 125:469–480
Van Dijk HW, Verbraak FD, Kok PHB et al (2013) Variability in photocoagulation treatment of diabetic macular oedema. Acta Ophthalmol 91(8):722–727
Massin P, Bandello F, Garweg JG et al (2010) Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 33:2399–2405
Nguyen QD, Brown DM, Marcus DM et al (2012) Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119:789–801
Mitchell P, Bandello F, Schmidt-Erfurth U et al (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118:615–625
Do DV, Nguyen QD, Khwaja AA et al (2013) Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol 131:139–145
Wang H, Chhablani J, Freeman WR et al (2012) Characterization of diabetic microaneurysms by simultaneous fluorescein angiography and spectral-domain optical coherence tomography. Am J Ophthalmol 153:861–867
Horii T, Murakami T, Nishijima K et al (2010) Optical coherence tomographic characteristics of microaneurysms in diabetic retinopathy. Am J Ophthalmol 150:840–848
Byeon SH, Chu YK, Hong YT et al (2012) New insights into the pathoanatomy of diabetic macular edema: angiographic patterns and optical coherence tomography. Retina 32:1087–1099
Early Treatment Diabetic Retinopathy Study Research Group (1987) Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology 94:761–774
Shahidi M, Ogura Y, Blair NP et al (1991) Retinal thickness analysis for quantitative assessment of diabetic macular edema. Arch Ophthalmol 109:1115–1119
Lobo CL, Bernardes RC, Cunha-Vaz JG (2000) Alterations of the blood-retinal barrier and retinal thickness in preclinical retinopathy in subjects with type 2 diabetes. Arch Ophthalmol 118:1364–1369
Early Treatment Diabetic Retinopathy Study Research Group (1995) Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Arch Ophthalmol 113:1144–1155
Lee SN, Chhablani J, Chan CK et al (2013) Characterization of microaneurysm closure after focal laser photocoagulation in diabetic macular edema. Am J Ophthalmol 155:905–912
Tso MO (1982) Pathology of cystoid macular edema. Ophthalmology 89:902–915
Wolter JR (1981) The histopathology of cystoid macular edema. Graefes Arch Clin Exp Ophthalmol 216:85–101
Snodderly DM, Weinhaus RS (1990) Retinal vasculature of the fovea of the squirrel monkey, Saimiri sciureus: three-dimensional architecture, visual screening, and relationships to the neuronal layers. J Comp Neurol 297:145–163
Kozak I, Oster SF, Cortes MA et al (2011) Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology 118:1119–1124
Maeshima K, Utsugi-Sutoh N, Otani T, Kishi S (2004) Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina 24:507–511
Han DP, Mieler WF, Burton TC (1992) Submacular fibrosis after photocoagulation for diabetic macular edema. Am J Ophthalmol 113:513–521
Nguyen QD, Shah SM, Khwaja AA et al (2010) Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 117:2146–2151
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2007865)
Meeting Presentation: Partly presented at the 36th annual meeting of the Macular Society, Feb 2013, Dana Point, CA, USA.
The authors want to thank Hye Sun Lee (Biostatistics Collaboration Unit, Yonsei University College of Medicine) for statistical support.
Conflict of interest
The authors have no proprietary or commercial interest in any materials discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Serial morphological changes on OCT after OCT-guided selective focal laser photocoagulation. Serial regression pattern of ‘significant actively leaking microaneurysms on OCT’ (SALMO) with resolution of adjacent edema after OCT-guided selective focal laser photocoagulation (MPG 4448 kb)
Rights and permissions
About this article
Cite this article
Shin, J.Y., Byeon, S.H. & Kwon, O.W. Optical coherence tomography-guided selective focal laser photocoagulation: a novel laser protocol for diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 253, 527–535 (2015). https://doi.org/10.1007/s00417-014-2729-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2729-9