Abstract
Purpose
To investigate the effect of the overexpression of miRNA-9 to the ratio of pro- and anti-angiogenic isoforms of vascular endothelial growth factor (VEGF) in human retinal pigment cells (ARPE-19).
Methods
Oxidative stress was induced to ARPE-19 cells by 4-hydroxynonenal (4-HNE), tert-butyl hydroperoxide (t-BH), and hypoxia chamber with 1 % O2. Expression patterns of miRNAs were validated by qPCR. Relative mRNA levels of VEGF and PEDF were measured by semi-quantitative PCR. After the transfection of miR-9 mimic and inhibitor, transcriptional levels of VEGF165a, VEGF 165b, and SRPK-1 were measured by qPCR.
Results
We demonstrated that miR-9 expression is decreased in ARPE-19 human retinal pigment cells under hypoxic stress induced by 4-HNE, a lipid peroxidation end-product. We observed that miR-9 mimic transfection of ARPE-19 inhibited one of its targets, serine-arginine protein kinase-1 (SRPK-1), modulating the transcriptional level of VEGF165b. Transfection of miR-9 reduced the alternative splicing of VEGF165a mRNA in ARPE-19 cells under hypoxic conditions, suggesting that miR-mediated regulation of alternative splicing could be a potential therapeutic target in neovascular pathologies.
Conclusions
Hypoxic stress decreased the miR-9 level in ARPE-19 cells, which increased the transcriptional level of SRPK-1, resulting in alternative splicing shift to pro-angiogenic isoforms of VEGF165 in human retinal pigment epithelial cells
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is a chronic degenerative disease that causes irreversible loss of central vision function in elderly people over 65 years old [1–3]. There are two types of this disease, dry (atrophic) AMD and wet (neovascular) AMD, which can be characterized by the accumulation of drusen between Bruch’s membrane and the retinal pigment epithelium (RPE) during the early stages of the disease [4, 5]. Drusen consist of deposits of extracellular debris, primarily composed of lipids and proteins that are known risk factors of this disease [6, 7]. With chronic oxidative stress, dry AMD can progress to wet AMD, forming chronic neovascularization (CNV) from choroidal blood vessels [8–11]. 4-Hydroxy-2-nonenal (4-HNE) is a peroxidation product of polyunsaturated fatty acids (PUFAs) and a component of lipofuscin in RPE [12–15]. 4-HNE has been reported to induce oxidative stress, forming 4-HNE protein adducts by covalently binding to cysteine, lysine, or histidine residues [16].
Vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen, is involved in angiogenesis, vascular permeability in hypoxic conditions of wound healing, tumor formation, and retinal neovascularization [17–19]. The VEGF family consists of VEGF-A, −B, −C, and –D as well as placenta growth factor (PIGF)-1 and −2 [20]. The VEGF receptors (VEGFRs) include VEGFR-1, −2, and −3, and VEGF-A is highly associated with VEGFR-2 in endothelial cell recruitment, proliferation, and migration in developing new blood vessels [21]. Pigment epithelium-derived factor (PEDF), a 50-kDa glycoprotein, belongs to the serine protease inhibitor (serpin) family, which is encoded by the SERPINF1 gene on chromosome 17p13 [22, 23]. PEDF down-regulates hypoxia-inducible factor (HIF)-1α and enhances the gamma secretase-dependent cleavage of the VEGFR-1 transmembrane domain, interfering with VEGFR-2-induced angiogenesis [24, 25]. An increase in the VEGF-to-PEDF ratio induced by hypoxic stress may play a key role in CNV [26].
Of the VEGF-A isoforms, VEGF165 plays a critical role in the development of CNV [27]. VEGF165 is alternatively spliced into VEGF165a and VEGF165b by serine-arginine-rich splicing factor 1 (SRSF1) [28]. VEGF165a is generated by proximal splice-site including terminal exon 8a, while VEGF165b is generated by distal splice-site including terminal exon 8b [29]. VEGF165a stimulates angiogenesis while VEGF165b is anti-angiogenic [30]. SRSF1 is phosphorylated by serine-arginine protein kinase-1 (SRPK-1) in retinal pigment epithelial cells [31]. Selcuklu et al. reported that they identified SRPK-1 as a direct target of miR-9 from microarray profiling of MCF-7, a human breast cancer cell line [32].
MicroRNAs, single-stranded noncoding small (∼22 nucleotides) RNA molecules, post-transcriptionally regulate expression of target genes at the 3′-untranslated regions (UTRs) of mRNAs [33, 34]. Although miR-9 has been reported to exert diverse effects in neuronal development and tumor formation related to the cellular oxidative state, it is difficult to classify a clear role of miR-9 in human retinal pigment cells [35, 36]. In the present study, we screened several miRNA patterns in human retinal pigment epithelial (RPE) cells under oxidative stress. We demonstrated that miR-9 could increase the transcriptional level of VEGF165b by down-regulating SRPK-1 in RPE cells.
Materials and methods
Cell culture
ARPE-19 cells were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were maintained in Dulbecco’s modified Eagle’s medium/F12 (DME/F12 1:1, Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum and 1 % penicillin-streptomycin. Cells were incubated at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air and the culture medium was changed every 3 days.
Cell viability
Cell viability was measured with the sulfonated tetrazolium salt WST-1 (Takara, Japan) according to the manufacturer’s instructions. Cells were seeded in a 96-well microplate at a density of 2 × 104 cells/well and incubated at 37 °C for 24 h before assay. The cells were then treated with various concentrations of 4-HNE and t-BH. After the addition of WST-1 solution (10 μl/well), cell viability was determined at 450-nm absorbance.
Hypoxia induction
For hypoxia induction, cells were placed in a MIC-101 modular incubator chamber (Billups-Rothenberg, Inc., CA, USA) with a mixture of 1 % O2, 5 % CO2, and 94 % N2 at 37 °C.
Determination of mRNA and miRNA Expression
After RNA was isolated from cultured cells using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA), cDNA was synthesized using AccuPower PreMix (Bioneer, Daejeon, Republic of Korea) in the presence of oligo (dT) primer. PCR was performed using a gradient thermal cycler (Takara, Japan). The reaction cycle consisted of incubation at 94 °C for 5 min, then 30 cycles of denaturation at 94 °C for 30 s, annealing at 55–62 °C for 30 s, and extension at 72 °C for 30 s. The primer sequences were as follows: VEGF-A: 5′-CCA TGA ACT TTC TGC TGT CTT-3′ and 5′-TCG ATC GTT CTG TAT CAG TCT-3′, PEDF-1: 5′-CAG AAG AAC CTC AAG AGT GCC-3′ and 5′-CTT CAT CCA AGT AGA AAT CC-3′, HIF-1α: 5´-AAG AAA CCG CCT ATG ACG TG-3´ and 5´-CCA CCT CTT TTT GCA AGC AT-3´, SRPK1: 5´-CAC GGC ATG CAT GGC CTT TGA-3´and 5´-CGG CGG CAG TGG CTC TCT TC-3´, VEGF165a: 5´-TGT TTG TAC AAG ATC CGC AGA CGT G-3´ and 5´-TCA CCG CCT CGG CTT GTC ACA TCT GCA AGT ACG TT-3´, VEGF165b: 5´-TGT TTG TAC AAG ATC CGC AGA CGT G-3´ and 5´-GTT CTG TAT CAG TCT TTC CTG GTG AGA GAT CTG CA-3´, Ang-2: 5´-ATC AGC CAA CCA GGA AAT GA-3´ and 5´-AGG ACC ACA TGC ATC AAA CC-3´, FGF-2: 5´-TCA AGC AGA AGA GAG AGG AGT TGT-3´ and 5´-AAA GAA ACA CTC ATC CGT AAC ACA-3´, β-actin: 5′-ATC CAC GAA ACT ACC TTC AA-3′ and 5′-ATC CAC ACG GAG TAC TTG-3′. Experiments were performed in triplicate in three independent experiments.
For miRNA detection, reverse transcription of 100 ng small RNA using the EasyScript cDNA Synthesis Kit (Applied Biological Materials, Richmond, BC, Canada) and qPCR using TOPreal SYBR qPCR System (Enzynomics, Republic of Korea) were performed according to the manufacturer’s instructions. The primer sequences were as follows; miRNA-9: 5´-TCT TTG GTT ATC TAG CTG TAT GA-3´, miRNA-210: 5´-CTG TGC GTG TGA CAG CGG CTG A-3´, miRNA-21: 5´-TAG CTT ATC AGA CTG ATG TTG A-3´, miRNA-126: 5´-TCG TAC CGT GAG TAA TAA TGC G-3´, miRNA-146a: 5´-TGA GAA CTG AAT TCC ATG GGT T-3´, U6: 5´-CTC GCT TCG GCA GCA CA-3´ and 5´- AAC GCT TCA CGA ATT TGC GT- 3´. Thermal cycle conditions were as follows: 95 °C for 30 s; 40 cycles of 95 °C for 5 s and 60 °C for 32 s. The expression levels of target genes were determined by using the 2−ΔΔCt method. U6 was used as an endogenous control.
Transfection of miRNA mimic and inhibitor
ARPE-19 cells were seeded at 2 × 105 cells/well in a six-well plate (BD Biosciences). The next day, using 6.25 μl Lipofectamine 2 000 reagent (Invitrogen) per well according to the manufacturer’s instructions, the cells were transfected with one of the following RNA oligonucleotides at a 100nM final concentration: miRNA-9 duplex (Bioneer, Republic of Korea) or miRNA-9 antisense (Bioneer). After 4 h, the transfection medium was replaced with fresh growth medium with 10 % FBS.
Immuno blotting
Whole cell lysate from ARPE-19 cells was prepared in extraction buffer (Invitrogen) containing protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA). The lysate (10 μg) was denatured by boiling in SDS sample buffer (Bio-Rad, Hercules, CA, USA), resolved by SDS–PAGE and then transferred to PVDF membrane by electroblotting. Blots were then incubated with a mouse anti-human VEGF165a antibody (Abcam, Cambridge, UK), a mouse anti-human VEGF-165b (Abcam), antibody or a mouse anti-human β-actin antibody (Thermo, Rockford, IL, USA) plus a horseradish peroxidase-linked secondary antibody, and detected by chemiluminescence using a Fujifilm LAS-3000 system (Fujifilm, Tokyo, Japan). The bands were quantified by densitometric analysis using ImageJ software(version 1.48, NIH, Bethesda, MD, USA).
Statistical analysis
Statistical analysis was performed using SPSS version 12.0 (SPSS, Chicago, IL, USA). Data are expressed as means ± SEM. Student’s t test was used to examine the correlation analysis. p values less than 0.05 were considered significant.
Results
MiR-9 is down-regulated by hypoxic stress in ARPE-19 cells
We found that miR-9 was down-regulated in a dose-dependent manner by the addition of 4-HNE or t-BH to ARPE-19 cells (Fig. 1a and b). MiR-9 expression was significantly decreased after hypoxic stress in a time-dependent manner compared with normoxia controls (Fig. 1c). With 5-aza-2'-deoxycytidine, a DNA methylation inhibitor, miR-9 expression increased up to about 16-fold in ARPE-19 cells. We then confirmed that miR-9 was significantly decreased following the addition of 4-HNE (Fig. 1d).
Oxidative stress induces miR-9 decrease in ARPE-19 cells. The relative levels of miRNA-9 expression were measured by quantitative real-time PCR after ARPE-19 cells were subjected to 4-HNE (a), t-BH (b), and hypoxic chamber (c) with 1 % O2. d Expression levels of miR-9 in cells treated with 5-Aza-dC (3 days) were analyzed using U6 snRNA as an internal control. Values are presented as mean ± SD from triplicate wells. *, p < 0.05, **, p < 0.01 compared to control groups
4-Hydroxynonenal increased the VEGF-A/PEDF ratio in ARPE-19 cells
Human retinal pigment epithelial cells (ARPE-19) were treated with various concentrations (20, 40, 60, and 80 μM) of 4-HNE for 1 h, and cell viability was determined by WST-1 assay. 4-HNE at 20–30 μM yielded over 50 % cell viability (data not shown). Treatment of ARPE-19 cells with 30 μM 4-HNE for 1 h significantly increased the mRNA levels of VEGF-A isoforms and also increased the mRNA level of NLRP-3 compared with normoxia- and LPS-(10 μg/ml) induced hypoxia controls (Fig. 2a). 4-HNE stimulation of ARPE-19 cells had no effect on the mRNA level of PEDF. Prolonged stimulation of ARPE-19 cells with 4-HNE for 4 days dose-dependently increased the mRNA level of VEGF-A isoforms (Fig. 2c). However, no significant differences in the mRNA levels of VEGFR-1, 2, and PEDF were observed (Fig. 2b). With various concentrations of 4-HNE (10–50 μM), ARPE-19 cells showed increased VEGF-A/PEDF mRNA ratios (threefold) compared with the normoxia control (Fig. 2d).
Effects of 4-HNE on the VEGF/PEDF mRNA ratio in ARPE-19 cells. Reverse transcriptase-polymerase chain reaction (RT-PCR) analyses of VEGF-A isoforms. The resulting PCR products were analyzed on a 1.2 % agarose gel. a Treatment of ARPE-19 cells with 30 μM 4-HNE for 1 h significantly increased the mRNA levels of VEGF-165 and 121 isoforms and also increased the mRNA level of NLRP-3 compared with normoxia- and LPS-(10 μg/ml) induced hypoxia controls. Total RNA was extracted after incubation with 4-HNE for 4 days (b-d). b The relative mRNA expressions of VEGF-165, VEGF-121, PEDF, NLRP-3, VEGFR-1, and VEGFR-2 were analyzed using RT-PCR. c Quantitative analysis for VEGF-A mRNA was performed using real-time PCR. d 4-HNE increased VEGF/PEDF mRNA ratio. The results were normalized to β-actin and expressed as fold increase over control (*, p < 0.05 and **, p < 0.01)
4-HNE up-regulated pro-angiogenic VEGF165a and SRPK-1
Hypoxic stimulation by 4-HNE increased the mRNA levels of HIF-1α and SRPK-1 in ARPE-19 cells (Fig. 3a). The relative mRNA ratio of VEGF165a (a pro-angiogenic isoform of VEGF165) and VEGF 165b (an anti-angiogenic isoform of VEGF165) increased as higher doses of 4-HNE (>25 μM) were used to stimulate ARPE-19 cells (Fig. 3b).
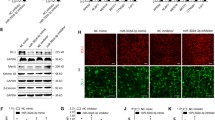
mRNA expression of HIF-1α, SRPK-1 (a), and VEGF165 isoforms (b) in ARPE-19 cells. Total RNA from ARPE-19 cells was analyzed for mRNA expression of HIF-1α, VEGF165a, VEGF165b, SRPK-1, and β-actin. Semi-quantitative RT-PCR products were separated on a 1.2 % agarose gels and stained with ethidium bromide. Values represent densitometry analysis (ImageJ software) in the expression of the selected genes
Effect of miR-9 on the expression of SRPK-1, VEGF165a, and VEGF165b
To elucidate the role of miR-9 in regulating the mRNA levels of SRPK-1, VEGF165a, and VEGF165b in hypoxia, ARPE-19 cells were transfected with miR-9 mimic and inhibitor to affect the endogenous miR-9 level. The transfection with miR-negative control had no specific effect on the SRPK-1 level in cells stimulated by 4-HNE (Fig. 4a). Under 4-HNE-induced hypoxia, ARPE-19 cells transfected with miR-9 mimic did not show hypoxic elevation of SRPK-1 and VEGF165a compared with the hypoxic control and exhibited a similar sensitivity to hypoxia compared with the hypoxic control. Treatment with miR-9 mimic decreased the mRNA level of VEGF165a in hypoxia (Fig. 4b, c). The transfection of miR-9 inhibitor led to an approximately two-fold increase in the SRPK-1 mRNA level in normoxia. Both in normoxia and hypoxia, miR-9 mimic transfection increased the mRNA level of VEGF165b in ARPE-19 cells, while the miR-9 inhibitor decreased the VEGF165b mRNA level (Fig. 4d). The protein expression of VEGF-165 isoform (VEGF-165a and VEGF-165b) was mildly increased in the presence of 4-HNE (10 μM) for 24 h. The expression rate of VEGF165a to VEGF165b was inversed by miR-9 overexpression in the presence of 4-HNE (Fig. 4e).
miR-9 as a negative regulator of SRPK-1 in ARPE-19 cells under oxidative stress. ARPE-19 cells were transfected with 50 nM miR-negative control or mimics. At 48 h after transfection, cells were incubated with 30 μM 4-HNE for 1 h. The levels of SRPK-1, VEGF165a, and VEGF165b were determined by real-time quantitative PCR (a–d). Values are presented as mean ± SD from triplicate wells. *, p < 0.05 compared with the control. e Western blot analysis of VEGF165a and VEGF165b. ARPE-19 cells were treated with 4-HNE (10 μM) for 24 h with or without miR-9 transfection. Relative ratio of VEGF-165a and VEGF-165b expression was determined by densitometry (ImageJ 1.48 software, NIH image)
Discussion
The purpose of this study was to identify differentially expressed miRNAs that affect the expression of pro-angiogenic VEGF165a and to investigate the potential of retinal miRNA as a biomarker for predicting chronic neovascularization in the RPE. Increases of miR-9 in ARPE-19 cells can be measured by using oxidative stress molecules such as LPS, N-(4-hydroxyphenyl) retinamide (4-HPR), hydrogen peroxide, and molecular hydrogen [37–39]. We observed a decreased level of intracellular miR-9 in ARPE-19 cells under hypoxic conditions by sub-lethal concentrations (25–30 μM) of 4-HNE. The cellular concentrations of 4-HNE vary from 0.1–0.3 μM under normoxic conditions, while it accumulates up to concentrations of 10 μM to 5 mM under oxidative stress [40].
The transcriptional activity of miR-9 seems to be related to gene silencing by DNA methylation. Previous studies have demonstrated that the high frequency of hypermethylated CpG islands at miR-9 genes resulted in down-regulation of miR-9 in human cancer [41]. Epigenetic inactivation of miR-9 level may be associated with DNA hypermethylation in human RPE. We demonstrated that the expression level of miR-9 could be restored by hypomethylation of genomic DNA in ARPE-19 cells.
The VEGF gene is comprised of eight exons and seven introns approximately 14 kilobases in length. Members of the VEGF family are alternatively spliced to form pro-angiogenic VEGFxxx and anti-angiogenic VEGFxxxb, where xxx indicates the number of amino acids on the polypeptide chain. Several studies demonstrated that VEGFxxxb expression is lower than VEGFxxx in tumor cells. The selection of a distal splicing site of VEGF exon 8 (exon 8a and exon 8b) results in expression of VEGFxxxb, which differs from VEGFxxx at the C-terminal region of the pre-mRNA. The receptor-binding C-terminal domain of VEGF165b acts as a competitive inhibitor of VEGF165a and allows the inhibition of endothelial proliferation, migration, and vasodilatation [29].
Alternative splicing of VEGF165 at exons 8a/8b depends on the balance of the serine/arginine rich (SR) proteins that recognize splicing enhancers or silencers at serine-arginine rich domains of the spliceosome. SR proteins can be directly regulated by SRPK-1-mediated phosphorylation, which causes SRs to translocate to the nucleus [42]. Gammons et al. reported that SRIN340, a selective SRPK inhibitor, reduced pro-angiogenic VEGF165a expression in the 50/10 oxygen-induced retinopathy (50/10 OIR) model which exposes newborn rats to repeated cycles of 24 h of 50 % and 10 % oxygen [43]. Selcuklu et al. tested SRPK1 for a direct target of miR-9 using luciferase reporter system containing full-length 3'-UTR of SRPK-1 in a human breast cancer cell line (MCF-7). We hypothesized that the overexpression of miR-9 may influence the ratio of pro- and anti-VEGF isoforms in human retinal pigment epithelial cells under oxidative stress by transcriptional regulation of SRPK-1 mRNA. Western-blot analysis showed that miR-9 could selectively reduce the expression of pro-angiogenic VEGF165a isoform in oxidative milieu caused by 4-HNE, while anti-angiogenic VEGF165b remained unaffected.
In summary, we demonstrated that miR-9 is down-regulated in ARPE-19 cells under 4-HNE-induced oxidative stress. Increased miR-9 expression enhanced the transcriptional level of anti-angiogenic VEGF165b in ARPE-19 cells (Fig. 5). Reduced miR-9 expression in human retinal pigment cells under hypoxia might serve as a biomarker for chronic neovascularization.
References
Coleman HR, Chan CC, Ferris FL 3rd, Chew EY (2008) Age-related macular degeneration. Lancet 372:1835–1845
Fine SL, Berger JW, Maguire MG, Ho AC (2000) Age-related macular degeneration. N Engl J Med 342:483–492
Voleti VB, Hubschman JP (2013) Age-related eye disease. Maturitas 75:29–33
Bowes Rickman C, Farsiu S, Toth CA, Klingeborn M (2013) Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest Ophthalmol Vis Sci 54: ORSF68-80
Buschini E, Piras A, Nuzzi R, Vercelli A (2011) Age-related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol 95:14–25
Wang L, Clark ME, Crossman DK, Kojima K, Messinger JD, Mobley JA, Curcio CA (2010) Abundant lipid and protein components of drusen. PLoS One 5: e10329
Rabin DM, Rabin RL, Blenkinsop TA, Temple S, Stern JH (2013) Chronic oxidative stress upregulates Drusen-related protein expression in adult human RPE stem cell-derived RPE cells: a novel culture model for dry AMD. Aging 5:51–66
Ardeljan D, Chan CC (2013) Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res 37:68–89
Salminen A, Kauppinen A, Hyttinen JM, Toropainen E, Kaarniranta K (2010) Endoplasmic reticulum stress in age-related macular degeneration: trigger for neovascularization. Mol Med 16:535–542
Tuo J, Grob S, Zhang K, Chan CC (2012) Genetics of immunological and inflammatory components in age-related macular degeneration. Ocul Immunol Inflamm 20:27–36
Zarbin MA (2004) Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122:598–614
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128
Poli G, Schaur RJ (2000) 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life 50:315–321
Shimokawa I, Higami Y, Horiuchi S, Iwasaki M, Ikeda T (1998) Advanced glycosylation end products in adrenal lipofuscin. J Gerontol A Biol Sci Med Sci 53:B49–51
Kim HC, Bing G, Jhoo WK, Kim WK, Shin EJ, Park ES, Choi YS, Lee DW, Shin CY, Ryu JR, Ko KH (2002) Oxidative damage causes formation of lipofuscin-like substances in the hippocampus of the senescence-accelerated mouse after kainate treatment. Behav Brain Res 131:211–220
Carbone DL, Doorn JA, Kiebler Z, Petersen DR (2005) Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem Res Toxicol 18:1324–1331
Svendsen MN, Werther K, Nielsen HJ, Kristjansen PE (2002) VEGF and tumour angiogenesis. Impact of surgery, wound healing, inflammation and blood transfusion. Scand J Gastroenterol 37:373–379
Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M (2010) Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell 140:268–279
Kwak N, Okamoto N, Wood JM, Campochiaro PA (2000) VEGF is major stimulator in model of choroidal neovascularization. Invest Ophthalmol Vis Sci 41:3158–3164
Gordon MS, Mendelson DS, Kato G (2010) Tumor angiogenesis and novel antiangiogenic strategies. Int J Cancer 126:1777–1787
Koolwijk P, Peters E, van der Vecht B, Hornig C, Weich HA, Alitalo K, Hicklin DJ, Wu Y, Witte L, van Hinsbergh VW (2001) Involvement of VEGFR-2 (kdr/flk-1) but not VEGFR-1 (flt-1) in VEGF-A and VEGF-C-induced tube formation by human microvascular endothelial cells in fibrin matrices in vitro. Angiogenesis 4:53–60
Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX (2006) Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. Faseb J 20:323–325
Tombran-Tink J, Pawar H, Swaroop A, Rodriguez I, Chader GJ (1994) Localization of the gene for pigment epithelium-derived factor (PEDF) to chromosome 17p13.1 and expression in cultured human retinoblastoma cells. Genomics 19:266–272
Cai J, Jiang WG, Grant MB, Boulton M (2006) Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J Biol Chem 281:3604–3613
Nozaki M, Sakurai E, Raisler BJ, Baffi JZ, Witta J, Ogura Y, Brekken RA, Sage EH, Ambati BK, Ambati J (2006) Loss of SPARC-mediated VEGFR-1 suppression after injury reveals a novel antiangiogenic activity of VEGF-A. J Clin Invest 116:422–429
Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J (2001) Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett 489:270–276
Lee JH, Canny MD, De Erkenez A, Krilleke D, Ng YS, Shima DT, Pardi A, Jucker F (2005) A therapeutic aptamer inhibits angiogenesis by specifically targeting the heparin binding domain of VEGF165. Proc Natl Acad Sci U S A 102:18902–18907
Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, Bates DO (2010) Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to anti-angiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem 285:5532–5540
Biselli-Chicote PM, Oliveira AR, Pavarino EC, Goloni-Bertollo EM (2012) VEGF gene alternative splicing: pro- and anti-angiogenic isoforms in cancer. J Cancer Res Clin Oncol 138:363–370
Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA (2006) VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis 12:626–632
Ghosh G, Adams JA (2011) Phosphorylation mechanism and structure of serine-arginine protein kinases. Febs J 278:587–597
Selcuklu SD, Donoghue MT, Rehmet K, de Souza GM, Fort A, Kovvuru P, Muniyappa MK, Kerin MJ, Enright AJ, Spillane C (2012) MicroRNA-9 inhibition of cell proliferation and identification of novel miR-9 targets by transcriptome profiling in breast cancer cells. J Biol Chem 287:29516–29528
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Coolen M, Katz S, Bally-Cuif L (2013) miR-9: a versatile regulator of neurogenesis. Front Cell Neurosci 7:220
Chen P, Price C, Li Z, Li Y, Cao D, Wiley A, He C, Gurbuxani S, Kunjamma RB, Huang H et al (2013) miR-9 is an essential oncogenic microRNA specifically overexpressed in mixed lineage leukemia-rearranged leukemia. Proc Natl Acad Sci U S A 110:11511–11516
Kutty RK, Samuel W, Jaworski C, Duncan T, Nagineni CN, Raghavachari N, Wiggert B, Redmond TM (2010) MicroRNA expression in human retinal pigment epithelial (ARPE-19) cells: increased expression of microRNA-9 by N-(4-hydroxyphenyl) retinamide. Mol Vis 16:1475–1486
Howell JC, Chun E, Farrell AN, Hur EY, Caroti CM, Iuvone PM, Haque R (2013) Global microRNA expression profiling: curcumin (diferuloylmethane) alters oxidative stress-responsive microRNAs in human ARPE-19 cells. Mol Vis 19:544–560
Liu GD, Zhang H, Wang L, Han Q, Zhou SF, Liu P (2013) Molecular hydrogen regulates the expression of miR-9, miR-21 and miR-199 in LPS-activated retinal microglia cells. Int J Ophthalmol 6:280–285
Uchida K (2003) 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 42:318–343
Tsai KW, Liao YL, Wu CW, Hu LY, Li SC, Chan WC, Ho MR, Lai CH, Kao HW, Fang WL et al (2011) Aberrant hypermethylation of miR-9 genes in gastric cancer. Epigenetics 6:1189–1197
Dong Z, Noda K, Kanda A, Fukuhara J, Ando R, Murata M, Saito W, Hagiwara M, Ishida S (2013) Specific inhibition of serine/arginine-rich protein kinase attenuates choroidal neovascularization. Mol Vis 19:536–543
Gammons MV, Dick AD, Harper SJ, Bates DO (2013) SRPK1 inhibition modulates VEGF splicing to reduce pathological neovascularization in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci 54:5797–5806
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project of the Ministry of Health and Welfare Affairs, Republic of Korea (grant#: HI12C0005) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2012R1A1A2006909).
Declaration of interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yoon, C., Kim, D., Kim, S. et al. MiR-9 regulates the post-transcriptional level of VEGF165a by targeting SRPK-1 in ARPE-19 cells. Graefes Arch Clin Exp Ophthalmol 252, 1369–1376 (2014). https://doi.org/10.1007/s00417-014-2698-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2698-z