Abstract
Purpose
Inner retinal cleavage can be misdiagnosed as a glaucomatous retinal nerve fiber layer (RNFL) defect. This study was performed to characterize eyes with inner retinal cleavage.
Methods
Inner retinal cleavage is defined as the appearance of a dark spindle-shaped space between the nerve fibers. Patients who presented at our institution with inner retinal cleavage were enrolled in the study. All participants were evaluated by fundus examination, visual field testing with standard automated perimetry, and optical coherence tomography (OCT) imaging.
Results
A total of 15 eyes of 11 subjects with inner retinal cleavage were included in the study. The median age of the subjects was 57 years (age range, 30–67 years). In each case, inner retinal cleavage was located adjacent to retinal blood vessels. Tissue bridging the cleavage area was observed in ten eyes. Six eyes had epiretinal membranes (ERMs), two eyes had glaucoma, and one eye had ERM in addition to glaucoma. Six eyes with inner retinal cleavage without combined ocular abnormalities had highly myopic refractive error (−6.50 to −8.50 diopters). Cross-sectional OCT images of the areas of inner retinal cleavage demonstrated defects with irregular margins and empty spaces in the inner layers of the retina. During the follow-up period, no eye showed changes in inner retinal layer cleavage or visual field sensitivity.
Conclusions
Inner retinal cleavage was found in eyes with high myopia or ERMs. Inner retinal cleavage was associated with structural changes distinct from those associated with glaucomatous RNFL defects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1992, Chihara and Chihara reported three cases of retinal nerve fiber layer (RNFL) cleavage in myopic eyes. RNFL cleavage was defined as the appearance of a dark, spindle-shaped space between the nerve fibers without any reduction in thickness or width of the nerve fiber layer or any associated visual field defect [1]. They used the term “cleavage” to discriminate this phenomenon from a “defect,” which is defined as a dark groove or non-spindle-shaped slit in the RNFL [1]. Komeima et al. also reported one case of paravascular inner retinal cleavage in a myopic eye [2] and one case in an eye with an epiretinal membrane (ERM) [3]. To date, these three case reports are the only published descriptions of inner retinal cleavage. In our practice, we have also observed inner retinal cleavage (Figs. 1 and 2), and have observed that cleavage of the inner retina can be misdiagnosed as a glaucomatous RNFL defect. In fact, most inner retinal cleavage cases that presented at our clinic were referred to us because of suspected glaucoma. Herein, we sought to highlight the differences between inner retinal cleavage and glaucomatous RNFL defects.
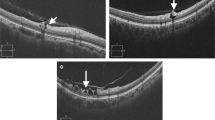
Representative red-free fundus photographs and cross-sectional images of inner retinal cleavage (areas between the white arrows) in eyes with high myopia (left panel) and epiretinal membrane (right panel). Inner retinal cleavage presents as a dark, spindle-shaped space between nerve fibers in red-free fundus photographs. In the cross-sectional images at the level of the white lines, inner retinal cleavage is associated with empty spaces with abrupt, irregular margins in the inner retina (red arrows)
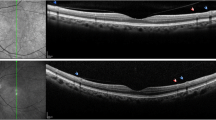
Representative red-free fundus photographs (upper row) and cross-sectional images (lower row) of an eye with inner retinal cleavage (area between the white arrows, left panel) and an eye with a glaucomatous retinal nerve fiber layer (RNFL) defect (area between the white arrows, right panel). The white arrowhead indicates tissue bridging the cleaved area. In the cross-sectional images at the level of white lines, inner retinal cleavage is associated with empty spaces with abrupt, irregular margins in the inner retina (red arrows). In contrast, in a glaucomatous RNFL defect, the margin of RNFL thinning is not abrupt, and other inner retinal layers are relatively intact without empty spaces (green arrow)
Methods
This retrospective study was approved by the Institutional Review Board at Kim’s Eye Hospital, Seoul, Korea, and all patient procedures conformed to the Declaration of Helsinki guidelines. As this study is based on retrospective chart review, informed consent from the participants was not required; the Institutional Review Board of our institution approved the exemption of informed consent. Among patients who presented to a glaucoma specialist (Y.H.H.) during the period from May 2012 to November 2013, those with inner retinal cleavage were enrolled in the present study. Each subject underwent an ophthalmic examination that included the following assessments: (1) visual acuity and refractive error using a model TX-20P autorefractor keratometer (Canon, Tokyo, Japan), (2) intraocular pressure using a Goldmann applanation tonometer, (3) anterior segment examination by slit-lamp biomicroscopy, and (4) optic nerve head (ONH) evaluation and fundus examination using a 90-diopter lens. The diagnosis of inner retinal cleavage was based on the clinical features observed during the fundus examination. Once inner retinal cleavage was detected by fundus examination, further examinations, including (1) the 24–2 Swedish Interactive Threshold Algorithm standard automated visual field test using a Humphrey Visual Field Analyzer (Carl Zeiss Meditec, Dublin, CA, USA), (2) red-free fundus photography using a Kowa Nonmyd7 fundus camera (Kowa, Tokyo, Japan), and (3) cross-sectional image acquisition in the inner retinal cleavage area using a Cirrus high-definition optical coherence tomography device (Cirrus HD-OCT; Carl Zeiss Meditec, Dublin, CA, USA) or spectral OCT/scanning laser ophthalmoscopy (SLO; OPKO/OTI, Miami, FL, USA), were performed. At our institution, a Cirrus HD-OCT is used in the glaucoma clinic, whereas an OCT/SLO is used at the retina hospital. Therefore, patients who were diagnosed with inner retinal cleavage after visiting the glaucoma clinic were evaluated using the Cirrus HD-OCT, and those referred from the retina hospital for an RNFL abnormality were evaluated using the OCT/SLO. In the present study, we only assessed the cross-sectional configurations, and did not analyze any quantitative parameters obtained by OCT. Although the quantitative parameters obtained by Cirrus HD-OCT and OCT/SLO are not interchangeable [4], these two devices have similar resolutions [5]. Thus, we think that the type of OCT may not have a significant effect on the qualitative analysis.
Refractive error was presented as a spherical equivalent (spherical refractive error +1/2 cylindrical refractive error in the negative form). In eyes that had previously undergone refractive or cataract surgery, refractive error prior to the surgery was analyzed. The presence of visual field defects corresponding to the area of the cleaved retina was assessed using Garway–Heath maps [6]. We focused strictly on the presence of inner retinal cleavage regardless of comorbid ocular or systemic conditions. Therefore, previous history of other ocular disorders, ocular surgery, or systemic disorders was not considered exclusion criteria.
Glaucoma was defined as ONH with glaucomatous changes, i.e., increased cup:disc ratio and narrowing of the neuroretinal rim; RNFL defect on red-free fundus photography, i.e., a dark wedge-shaped area with its apex touching the optic disc border in the brightly striated pattern of the surrounding RNFL [7] or generalized loss of RNFL visibility in the upper or lower retina [8]); and/or glaucomatous visual field defects, i.e., (1) a cluster of three points with probabilities of <5 % on the pattern deviation map in at least one hemifield, including at least one point with a probability of <1 %, or a cluster of two points with a probability of <1 %, (2) glaucomatous hemifield test results outside normal limits, or (3) a pattern standard deviation beyond 95 % of normal limits) as confirmed by at least two reliable examinations (false positives and negatives <15 %, fixation losses <15 %).
ERMs were first observed during the fundus examination and confirmed by fundus photographs as well as cross-sectional images of the macula obtained by OCT. If a cleaved area was included in the conventional macular scan area, no additional scan was obtained. If not, an additional scan was obtained of the cleaved area to inspect its cross-sectional configuration.
The structural and functional changes in the inner retinal cleavage area were assessed using serial fundus photographs and visual field test results by two investigators (Y.H.H. and Y.Y.K.), each of whom was masked to the other’s judgment. Structural changes in the area of inner retinal cleavage were defined as a widening or narrowing of the preexisting cleavage, the development of a new area of cleavage, or changes in the configuration of bridging tissues within the cleaved area. Visual field changes associated with the cleaved area were defined as the development of a new scotoma or the widening/deepening of a preexisting scotoma. Any disagreements regarding the structural and functional changes in the inner retinal cleavage area between the investigators were planned to be resolved by a third adjudicator (H.K.K.).
Results
During the study period, 15 eyes from 11 subjects showed inner retinal cleavage. The clinical characteristics of these 15 eyes are listed in Table 1. The median age of the subjects was 57 years (age range, 30–67 years). Of these 15 eyes, three had a history of cataract surgery, while the other eyes did not have any history of ocular trauma or surgery. Inner retinal cleavage was present in the superior hemisphere in nine eyes (60.0 %), in the inferior hemisphere in two eyes (13.3 %), and both superior and inferior hemispheres in four eyes (26.7 %). In each case, the area of inner retinal cleavage was adjacent to retinal vessels (Figs. 1 and 2). Tissue bridging the area of cleavage was noted in ten eyes (66.7 %, Fig. 2).
Of the 15 eyes, six (40.0 %) had ERMs, two (13.3 %) had glaucoma, and one (6.7 %) had an ERM as well as glaucoma. In eyes with glaucoma, the location of inner retinal cleavage was separate from the glaucomatous RNFL defect in two eyes, and overlapped with the glaucomatous defect in one eye. In all eyes with glaucoma, intraocular pressure was well-controlled with topical medication (intraocular pressure range, 12–17 mmHg). All eyes had early-stage visual field defects (mean deviation range, −0.19 to −4.04 dB) [9]. Six eyes with inner retinal cleavage without accompanying ocular abnormalities had highly myopic refractive error (−6.50 to −8.50 diopters).
In all eyes, no visual field defect was found in the area corresponding to the cleaved retina. Cross-sectional OCT images of areas with inner retinal cleavage demonstrated defects with irregular margins and empty spaces in the inner retinal layers (Figs. 1 and 2). No eye showed any outer retinal layer abnormalities.
Longitudinal follow-up was available for ten eyes. During the follow-up period (3–23 months, median 9 months), no eye showed any structural changes in the inner retinal cleavage area or any change in visual field sensitivity. There was no disagreement with regard to structural or functional changes in the inner retinal cleavage area between the two investigators.
Discussion
In the present study, we report the clinical characteristics of eyes with inner retinal cleavage. The presence of inner retinal cleavage was associated with the presence of high myopia or ERM. The development of inner retinal cleavage did not correlate with standard automated visual field test abnormalities. Cross-sectional images obtained by OCT confirmed the presence of inner retinal defects with empty spaces.
All of the eyes included in this study had high myopia or ERM, which suggests that myopic change or ERM formation may contribute to the development of inner retinal cleavage. In myopic eyes, axial elongation may stretch the retina, thereby leading to cleavage. Chiraha and Chihara suggested that RNFL cleavage develops in young myopic eyes when the direction of growth runs perpendicular to the course of the axon [1]. Similarly, the development of ERM in the macular area and associated tangential traction could result in stretching and cleavage of the adjacent retina.
In the present study, cross-sectional OCT images demonstrated defects in cleaved inner retinal layers but relatively intact outer retinal layers. This pattern could potentially be attributed to the lesser flexibility of retinal vessels [10]. Given that retinal vessels are located in the inner retinal layers, reduced flexibility in that location could lead to a higher chance of cleavage. Furthermore, ERMs are located on the inner surface of the retina. Thus, the traction induced by ERM primarily affects inner retinal layers, which further supports our hypothesis.
It is important to identify inner retinal cleavage to prevent misdiagnosis of a glaucomatous RNFL defect. Our clinical observations identified several characteristics of inner retinal cleavage that can be used to discriminate this phenomenon from a glaucomatous RNFL defect. First, inner retinal cleavage and glaucomatous RNFL defects differ in overall morphology. For instance, in a glaucomatous RNFL defect, RNFL thinning develops along the trajectory of circumpapillary retinal nerve fibers. This sort of defect results in a wedge-shaped area that increases in width as it travels further from the ONH. In contrast, inner retinal cleavage develops in areas of retinal stretching. Cleavage width, therefore, depends on the degree of stretch and the strength of the inner retina, which can result in a spindle-shaped or serpentine appearance.
Given that the neuroretinal rim consists mainly of nerve fiber axons, the tip of the wedge-shaped defect characteristic of glaucoma is connected to the ONH margin with adjacent RNFL thinning. In contrast, the observed instances of inner retinal cleavage did not contact the ONH margin. While inner retinal cleavage was observed in proximity to the ONH, there was no associated neuroretinal rim thinning.
Each instance of inner retinal cleavage was identified adjacent to retinal blood vessels. The lower flexibility of retinal vessels could render the surrounding tissue vulnerable to cleavage when stretched [10]. In contrast, glaucomatous RNFL defects are not limited to vascularized areas of the RNFL.
Bridging tissue has been commonly observed over the area of inner retinal cleavage. Jeoung et al. [11] reported observations of retinal nerve fiber overlap in glaucomatous RNFL defects. However, the estimated incidence of such overlap was only 2.3 %. We therefore believe that the presence of bridging tissue is a key to differentiating inner retinal cleavage from glaucomatous RNFL defects. Although we do not currently know why bridging tissues do not collapse into the cleaved area, we speculate that the tangential traction forces from both ends of the tissue may prevent collapse. To verify our hypothesis, longitudinal observations of bridging tissues with changes in the cleavage area may be needed.
In the cross-sectional images obtained by OCT, inner retinal cleavage was associated with empty spaces in the inner retinal layers. These spaces were observed throughout the RNFL and other inner retinal layers, and tended to have abrupt, irregular margins. In contrast, the margin of a glaucomatous RNFL defect is not abrupt, and adjacent layers of the inner retina are relatively intact, without empty spaces (Fig. 2).
Glaucomatous RNFL defects are often associated with visual field defects. However, in the present study, standard automated visual field testing with the Humphrey Visual Field Analyzer did not show abnormal visual field test results in areas of the visual field corresponding to the inner retinal cleavage location. In a case report by Komeima et al. [2], a relative scotoma in the area of the retinal cleavage was noted when microperimetry was performed using scanning laser ophthalmoscopy. Nonetheless, the results of standard automated visual field testing were normal, which is in agreement with our study results. These findings suggest that the presence of inner retinal cleavage may not cause a severe scotoma. However, given that RNFL defects in the early stages of glaucoma do not induce visual field defects, this finding may have limited clinical value for the distinction of glaucomatous RNFL defects from inner retinal cleavage.
Finally, glaucomatous RNFL defects are characterized by progressive thinning, which can manifest as widening, deepening, or the formation of a new RNFL defect. To date, it is not clear whether inner retinal cleavage is a progressive phenomenon. The area of cleavage did not enlarge in any of the eyes included in this study. If inner retinal cleavage is caused by axial elongation, the process may not undergo profound change after the completion of axial elongation. However, in cases with inner retinal cleavage due to the traction induced by ERM, the area of cleavage could be expected to change in either direction with ERM growth or surgical removal [3]. Further studies regarding longitudinal changes in inner retinal cleavage may be needed.
An OCT presents average, quadrant, and clock-hour sector circumpapillary RNFL thicknesses within the area of the scan circle, which is 3.46 mm in diameter. This measurement may be helpful for the evaluation of glaucomatous RNFL defects that cross the scan circle. However, in many cases, the area of inner retinal cleavage did not overlap with the area circumscribed by the scan circle. We therefore believe that measuring circumpapillary RNFL thickness by OCT may not be useful for the assessment of inner retinal cleavage. In the same context, macular retinal thickness was not analyzed because the conventional macular scan area did not include the area of cleavage in some cases. Further, macular analysis can be affected by high myopia or the presence of an ERM [12, 13]. Therefore, in the present study, circumpapillary RNFL thickness and macular retinal thickness (including ganglion cell) analysis was not performed.
In conclusion, inner retinal cleavage was found to be associated with high myopia and/or the presence of ERM. Moreover, specific characteristics can be used to differentiate inner retinal cleavage from glaucomatous RNFL defects. These findings may help clinicians make the correct diagnosis.
References
Chihara E, Chihara K (1992) Apparent cleavage of the retinal nerve fiber layer in asymptomatic eyes with high myopia. Graefes Arch Clin Exp Ophthalmol 230:416–420
Komeima K, Kikuchi M, Ito Y, Terasaki H, Miyake Y (2005) Paravascular inner retinal cleavage in a highly myopic eye. Arch Ophthalmol 123:1449–1450
Komeima K, Ito Y, Nakamura M, Terasaki H (2009) Inner retinal cleavage associated with idiopathic epiretinal membrane. Retin Cases Brief Rep 4:132–134
Koh KM, Jin S, Hwang YH (2014) Cirrus high-definition optical coherence tomography versus spectral optical coherence tomography/scanning laser ophthalmoscopy in the diagnosis of glaucoma. Curr Eye Res 39:62–68
Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, Enzmann V, Wolf S (2009) Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci 50:3432–3437
Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA (2000) Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology 107:1809–1815
Hoyt WF, Frisen L, Newman NM (1973) Fundoscopy of nerve fiber layer defects in glaucoma. Invest Ophthalmol Vis Sci 12:814–829
Jeoung JW, Kim SH, Park KH, Kim TW, Kim DM (2010) Quantitative assessment of diffuse retinal nerve fiber layer atrophy using optical coherence tomography: diffuse atrophy imaging study. Ophthalmology 117:1946–1952
Budenz DL (1997) Atlas of visual fields. Lippincott–Raven, Philadelphia, pp 143–145
Sayanagi K, Ikuno Y, Gomi F, Tano Y (2005) Retinal vascular microfolds in highly myopic eyes. Am J Ophthalmol 139:658–663
Jeoung JW, Kim TW, Kang KB, Lee JJ, Park KH, Kim DM (2008) Overlapping of retinal nerve fibers in the horizontal plane. Invest Ophthalmol Vis Sci 49:1753–1757
Hwang YH, Jeong YC, Kim HK, Sohn YH (2014) Macular ganglion cell analysis for early detection of glaucoma. Ophthalmology [Epub ahead of print]. doi:10.1016/j.ophtha.2014.02.019
Hwang YH (2014) Patterns of macular ganglion cell abnormalities in various ocular conditions. Invest Ophthalmol Vis Sci
Financial interest
The authors have no financial or proprietary interest in any of the materials or methods discussed in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, Y.H., Kim, Y.Y., Kim, H.K. et al. Characteristics of eyes with inner retinal cleavage. Graefes Arch Clin Exp Ophthalmol 253, 215–220 (2015). https://doi.org/10.1007/s00417-014-2685-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2685-4