Abstract
Background
Dry eye disease (DED) is one of the most common ocular disorders worldwide. The pathophysiological mechanisms involved in the development of DED are not well-understood, and thus treating DED has been a significant challenge for ophthalmologists. Most of the currently available diagnostic tests demonstrate low correlation to patient symptoms and have low reproducibility.
Methods
Recently, sophisticated in vivo imaging modalities have become available for patient care, namely, in vivo confocal microscopy (IVCM) and optical coherence tomography (OCT). These emerging modalities are powerful and non-invasive, allowing real-time visualization of cellular and anatomical structures of the cornea and ocular surface. Here we discuss how, by providing both qualitative and quantitative assessment, these techniques can be used to demonstrate early subclinical disease, grade layer-by-layer severity, and allow monitoring of disease severity by cellular alterations. Imaging-guided stratification of patients may also be possible in conjunction with clinical examination methods.
Conclusions
Visualization of subclinical changes and stratification of patients in vivo allows objective image-guided evaluation of tailored treatment response based on cellular morphological alterations specific to each patient. This image-guided approach to DED may ultimately improve patient outcomes and make it possible to study the efficacy of novel therapies in clinical trials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Dry Eye Workshop (DEWS) conducted in 2007 defined dry eye disease (DED) as, “a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface. It is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface” [1]. DED is a global health problem with several large population-based studies estimating the prevalence ranging from approximately 30 % to 50 % of the population at various ages [2–8]. In the United States alone, DED is estimated to affect around 5 million people, aged 50 years or more, with the disease being more prevalent in the elderly and female population [9–14]. However, with increasing contact lens wear and use of electronic digital devices, an increased prevalence of DED is now seen among the younger population as well. Recent epidemiological studies report dry eye symptoms in about 25 % of high school students and 30–65 % of office workers [15–21].
The quality of life (QOL) in patients with DED is significantly affected due to their altered visual acuity and discomfort, resulting in the inability to carry out activities of daily living and hampering social functioning [22–28]. Several utility assessment models have shown that the impact of moderate to severe DED is comparable to moderate to severe angina [29, 30]. Moreover, the economic impact of DED is also significant, due to both direct medical and healthcare-related costs, as well as due to indirect productivity losses [31–34]. A recent study, for example, estimated a US prevalence-adjusted average annual cost for managing DED patients at $3.84 billion, underscoring the accompanying economic burden of disease [35].
The reported therapeutic success of current regimens in DED is variable [36–38]. In part, this has been attributed to the heterogeneity within a patient cohort in terms of severity, multitude of confounding factors, and underlying etiology in DED, as well as unreliable evaluation of inflammation pre- and post-treatment, and the overall variability in measurements of clinical signs. Thus, it has been very difficult for clinicians to assess objective improvement in disease and the efficacy of a particular treatment regimen. Similarly, the pharmaceutical industry has faced significant challenges in obtaining approval of new therapeutic drugs over the past decade, as the improvement of clinical signs has not been possible with the current clinical endpoints available [39–41]. Thus there is an urgent need for the development of new biomarkers for DED that are reliable, reproducible, consistent with symptoms, and reflect the underlying pathogenesis and severity of disease [42]. Herein we discuss a novel, non-invasive in vivo imaging approach that can potentially emerge as a tool for quantitative evaluation of disease and monitoring of treatment efficacy in DED, supplementing the current standard diagnostic tests. Through quantitative impressions of in vivo confocal micrographs based on densities of the sub-epithelial corneal immune cells, palpebral conjunctival immune cells and sub-basal nerves, when taken together with clinical diagnostic tests, in vivo imaging may enhance the clinical management of dry eye disease.
Methods
A PubMed literature search was conducted for papers, using the keywords: in vivo confocal microscopy, dry eye, in vivo confocal microscopy in cornea, meibomian gland dysfunction, optical coherence tomography, and ocular imaging. Bibliography of selected manuscripts were also reviewed and referenced in our manuscript. Limits for our literature search filters included papers in English from 1980 to 2013, including both human and animal studies published as case reports, review papers, or original research. We also referenced product information brochures and manufacturers’ webpages for technical information regarding medical devices discussed in this review paper.
Background, classification, and grading of dry eye
Background
The tear film is composed of three layers, the innermost mucin, middle aqueous, and outermost lipid layers. The mucin layer, composed of gel-forming mucins, soluble mucins, electrolytes, and water, is largely secreted by conjunctival goblet cells with some contribution of soluble mucins from the lacrimal gland, corneal, and conjunctival cells [43–45]. This hydrophilic mucin layer of the tear film rests upon the mucin of the glycocalyx, which is formed by the corneal and conjunctival epithelial membrane-spanning mucins [43, 46]. While both the mucin of the glycocalyx and the mucin layer of the tear film serve to provide hydration and lubrication to the ocular surface, they also help retard colonization of pathogens through restricting cell adhesion (mucin of the glycocalyx) and acting as a lavage to wash out pathogens, debris, and inflammatory molecules in the tear film (mucin layer) [47]. Both the mucin of the glycocalyx and mucin layer of the tear film can be affected in DED [48, 49]. Lacrimal glands are the main contributors to the aqueous layer of the tear film that provides hydration and lubrication of the ocular surface [50], while meibomian glands maintain the outermost lipid layer to retard tear evaporation [51].
Classification
Based on abnormalities of the tear film composition, DED has been broadly classified into two categories, aqueous-deficient dry eye (ADDE) and evaporative dry eye (EDE), corresponding to disorders of the lacrimal and meibomian glands respectively. ADDE can further be classified into Sjögren’s syndrome (SS) and non-Sjögren’s syndrome (NSS) dry eye. EDE can be classified into intrinsic and extrinsic causes, with meibomian gland dysfunction (MGD) being the most common cause of EDE. As ADDE and EDE are not mutually exclusive and may act together to produce signs and symptoms [52], it may be challenging to identify pure forms of each disease due to mixed forms of DED using the current system of classification. In addition, the initial underlying dry eye mechanism may lead to involvement of additional mechanisms, thus resulting in misclassification at different stages of disease. Therefore, with time and progression of disease severity, the clinical phenotype of DED may be altered as well, often leading to mixed clinical phenotypes [53].
Grading
Currently, dry eye disease severity is graded into four categories based on a combination of patient symptoms and clinical signs [1]. However, several studies have demonstrated the lack of correlation between the severity of symptoms and signs [54–59]. For example, patients in early stages of DED may demonstrate symptoms, without clear clinical signs [60–62]. Conversely, patients may show mild or severe objective clinical signs of DED, but may be symptom-free [6, 11, 63]. The disparity between signs and symptoms is multifactorial, but may in part be explained by the reduction of corneal nerve density with dry eye disease; reduced or loss of corneal sensation secondary to reduced corneal sub-basal nerve density from nerve damage in DED may explain the presence of clinical signs of ocular surface damage in the absence of concordant symptoms of ocular discomfort [64].
Methods and limitations in the diagnosis and treatment of aqueous-deficient dry eye disease
The current diagnostic protocol for DED is based on the recommendations of the 2007 DEWS [65]. This includes symptomatology questionnaires, such as the OSDI, in combination with the various clinical diagnostic tests. The traditional clinical diagnostic tests include corneal fluorescein staining, conjunctival Lissamine Green or Rose Bengal staining, tear film break-up time (TBUT), Schirmer’s tests, and more recently tear osmolarity. Each of these tests addresses a specific and necessary component of the complex nature of DED. However, given their varied reproducibility, they have limitations in meeting the prerequisites of ideal diagnostic tests, which include high specificity, sensitivity, reliability, and validity [66–68]. Of the existing clinical examinations, studies have shown better reproducibility with tear hyperosmolarity testing [69–72], even though significant overlap exists between the scores of normal subjects and patients with DED. This overlap of scores between normal subjects and dry eye patients is seen with other dry eye tests as well. Moreover, while tear osmolarity has been shown to be reproducible as a diagnostic test, the utility of this test for following dry eye patients may be more limited [73]. A recent multicenter study evaluated the most commonly used clinical tools to grade severity of DED, and concluded that there is a significant overlap in symptom severity scores amongst the prospectively defined normal subjects and patients with DED [70]. In addition, technical variations in measurement, variable conditions while conducting the tests [74], and potential diurnal variation of tear parameters [75–77], may all lead to increased variability of these tests.
The current approach to treatment of DED is based on the recommendations of the Management and Therapy subcommittee of the International Dry Eye Workshop 2007. Based on review of literature, preferred practice patterns by the American Academy of Ophthalmology and the International Task Force (ITF) Delphi Panel on DED, the subcommittee proposed treatment guidelines based on the severity of disease [78].
Methods and limitations in the diagnosis and treatment of meibomian gland dysfuction
MGD lends a significant burden of disease given its high prevalence, making for up to half or more of all patients that present with symptoms of dry eye [63, 79–82]. Population-based studies indicate that Asians have higher prevalence of MGD, ranging from 60.8 % to 69.3 % in those aged over 40 years [83]. However, two large population-based studies on Caucasians aged 40 to 97 years revealed prevalence rates from 3.5 % to 19.9 % [83]. Unfortunately, despite its widespread global prevalence, treatment of MGD is complicated by issues ranging from lack of sensitive diagnostic tests and a poorly understood natural history of disease to paucity of quantifiable methods for assessment of therapeutic efficacy, thereby stalling the development of effective and sustainable therapeutic strategies.
The recent 2011 International Workshop on Meibomian Gland Dysfunction (IWMGD 2011) defined MGD as “a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease” [61, 83]. This definition illustrates the complexity of mechanisms underlying MGD, much of which are still poorly understood. The precise pathogenesis of MGD is yet to be determined; however, there are several hypotheses that implicate the role of gonadal steroids [84, 85], bacteria [86–88], and androgens [89–92]. These etiological factors lead to hyperkeratinization of the terminal ducts and an increased viscosity of expressed meibum. Hyperkeratinization may then lead to glandular obstruction, and these changes may result in subsequent alterations in tear film stability, resulting in increased tear evaporation. Obstructive MGD is by far the most common presentation of MGD [60, 93]. Moreover, while previously controversial, the importance of inflammation in the pathogenesis of MGD has now been recognized by the 2011 IWMGD.
There remain two key issues when tackling MGD: (a) presence of an early, more prevalent, asymptomatic stage of disease, making early diagnosis toward effective treatment difficult using the existing standard clinical examination methods [61, 63], and (b) a disconnect between symptoms and signs, making both diagnosis and evaluation of therapy challenging for the physician, given the reliance on solely traditional clinical methods of examination [62, 63, 94, 95]. Traditional methods of diagnosis and assessment of MGD involve a clinical slit-lamp examination with a focus on the health and appearance of the lid margin (telangiectasia, orifice plugging or obstruction, lid swelling, lid tenderness), lissamine green staining of the conjunctiva and eyelid margin indicative of compromised cells, meibum expressibility and quality to assess gland obstruction and inflammation respectively, meibography to assess glandular dropout and appearance of the acini, and assessment of tear film stability and production through TBUT and Schirmer’s test to help categorize disease. Most of these clinical methods are prone to subjectivity and lack of reliability due to inter-user variation in grading and assessment [96]. Powell and colleagues determined kappa values ranging from 0.23 to 0.5 at best for inter-user reliability using meibography and slit-lamp bio-microscopy in the assessment of MGD [96]. Nichols and group also found similarly low kappa values for inter-observer reliability using meibography (simple κ = 0.38, weighted κ = 0.57) [97]. With the advances in meibography employing computerized grading (100-grade scale), there has been some improvement in inter-observer reliability as compared to subjective methods, but the inter-observer variability still remains prominent (mean difference in score of 19.3 between observers) [98]. Koh and colleagues developed a semi-automated method to digitally quantify infra-red meibographs that can objectively measure meibomian gland length and width [99]. This software advancement is exciting in that it has high sensitivity and specificity, but data regarding inter-observer reliability are not available [99]. Perhaps with the recent development of 3D reconstruction using FD-OCT meibography, further quantifiable parameters are likely to be developed toward improved assessment and grading systems [100]. Despite advances made to date, as a function of the limited magnification offered by these devices, current methods fail to detect cellular detail and consequently identification of patients with subclinical inflammation, which may account for symptoms with signs which are undetectable using traditional examination methods [101, 102].
Developing sensitive parameters using cutting-edge tools to detect subclinical inflammation in a clinical setting is critical for the early diagnosis, tailored management, and prevention of irreversible damage to the ocular surface in patients with MGD. The role of inflammation in the pathogenesis of MGD may be intertwined with glandular obstruction and bacterial colonization of the eyelid margin. While the evolution of MGD remains elusive, it has been postulated that obstructive MGD increases intraglandular pressure, resulting in cell stress of ductal and acinar epithelia, which can subsequently trigger activity of cell proteins, leading to the release of inflammatory mediators, and subsequent local inflammation. Moreover, it is possible that hyperkeratinization of terminal ducts could be triggered by inflammatory events. Likewise, increased bacterial growth is linked with subclinical inflammatory events through the release of free fatty acids that may irritate the tissue [86, 87, 93, 103, 104]. Inflammatory cytokines such as interleukin (IL)-1α may then activate epithelial cells [105], which in turn produce further inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-1α and -β, thereby maintaining a chronic subclinical inflammatory milieu, resulting in obstructive glandular disease [106, 107]. Currently, slit-lamp bio-microscopy, which provides a maximal magnification of ×40, is the gold standard tool in the assessment of the ocular surface. However, subclinical inflammation, which may be present at the initial stages of disease, cannot be detected using this method, potentially leading to disparity between patient symptoms and clinical signs [62, 95]. Given these techniques and the associated subjectivity in diagnostic assessment, there is lack of a consensus about the definition of MGD, and consequently its diagnosis [93, 108–116]. Therefore, there is an unmet need to develop alternative sensitive, more reproducible and reliable clinical parameters for the diagnosis and evaluation of disease severity in MGD. In addition, the time lag between the detection of early signs and onset of symptomatic disease is currently unknown, as are the usefulness of particular early signs as biomarkers of established symptomatic disease.
Treatment of MGD varies greatly among eye care professionals, ranging from conservative management to medical therapy or a combination of both. Conservative measures have a proven role in the management of MGD, such as lubrication for symptomatic relief from mechanical friction [117, 118], hot compresses with lid massage for gentle but firm expression of inspissated meibum from the ducts [113, 119], which can be performed at home or in clinic using recent devices that work on the same principle of heating followed by compression [120–126]. While lid hygiene is indicated primarily for anterior blepharitis, it does serve a role in the management of MGD (posterior blepharitis), possibly by exfoliation of lid debris and reducing lid margin bacterial load [117, 127, 128]. Anti-microbials such as systemic tetracyclines (doxycyline, minocycline) [129–133], and topical macrolides (1 % azithromycin), which also have anti-inflammatory properties, are the most commonly prescribed treatments of MGD [131, 134, 135]. Systemic tetracyclines work by inhibiting microbial lipase production, thereby reducing release of proinflammatory free fatty acids and diglycerides onto the lid margin [87, 136–139]. They also inhibit the activity of tissue matrix metalloproteinases (MMP) such as MMP-2 and MMP-9 [140]. Macrolides serve as immunomodulatory and anti-inflamamtory drugs through a myriad of mechanisms including increase in phagocytosis, downregulation of adhesion molecules [141–143], and reduction of inflammatory cytokines such as IL-6 [144]. Current topical treatments include off-label azithromycin [135, 145, 146], or an antibiotic–steroid combination [147] for acute exacerbations [148]. Thus, inflammation has emerged as a target in the management of MGD. Despite the evolving role of inflammation in MGD, only 12 randomized controlled trials have been published studying interventions in this disease [149]. The results are inconclusive with regard to anti-inflammatory therapy (topical corticosteroid, oral antibiotics) in MGD [149], explaining the paucity of evidence-based treatment guidelines and continued controversy regarding the role of topical corticosteroids in the treatment of MGD, with or without adjuvant antibiotic therapy. This controversy arises from the lack of clinically apparent inflammation in the early stages of disease [60–62], necessitating the development of tools that can detect and reliably quantify subclinical inflammation or activated immune response in the face of an unremarkable clinical examination; yet a symptomatic patient. The 2011 MGD workshop concluded that “new methods to assess MGD, both clinically and biologically, are needed to further the field, alone and in conjunction with dry eye disease”, pointing out the lack of objective evaluation parameters [83, 94]. Towards that goal, with the advent of a high-magnification (×800), non-invasive, fast, laser-scanning in vivo confocal microscope, (IVCM), detection of subclinical inflammation in MGD has become possible [150]. Given the speed with which it can be performed in an out-patient setting, and the near histological details acquired in a non-invasive manner, IVCM has very quickly begun to provide new, quantifiable insights into the evaluation of MGD [115, 148, 151–155].
Diagnostic imaging in dry eye disease
Sophisticated in vivo imaging techniques such as in vivo confocal microscopy and anterior segment optical coherence tomography (OCT) have recently made headway in the evaluation of DED. Their diagnostic utility is discussed in the following sections in a tissue-specific manner, with a focus on the cornea, palpebral conjunctiva and meibomian glands. Figures 1, 2, and 3 illustrate the superior resolution, high magnification, and detailed structural information gathered using these in vivo imaging techniques in DED and MGD.
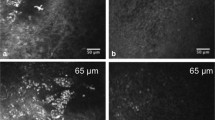
Corneal imaging in health and dry eye disease. a Horizontal OCT section of the normal human cornea showing the epithelium, Bowman’s membrane, stroma, Descemet’s membrane and endothelium. In vivo confocal micrographs (IVCM) of the cornea in normal and dry eye subjects (b–g). b Normal superficial epithelium showing regularly arranged cells with dark nuclei. c Superficial epithelium in dry eye disease showing squamous metaplasia, hyperreflectivity, and lower cell density as compared to normal. d Subbasal layer showing normal corneal nerve plexus and dendritic cells. e Subbasal layer in dry eye with increased density of dendritic cells. f Reduced density of nerves. g Hyperreflective and tortuous nerves. IVCM image: magnification: ×800
Principles of laser-scanning in vivo confocal microscopy (IVCM)
The principle of confocal microscopy is its conjugate alignment of light rays focused on the tissue with those reflected by the tissue and transmitted to the observer, hence the term "confocal" [156]. The Heidelberg Retina Tomograph with the Rostock Cornea Module (HRT/RCM, Heidelberg Engineering, Dossenheim, Germany) is a laser-scanning IVCM, using a 670 nm diode laser [157]. It allows quasi-histological, real-time imaging of the cornea, conjunctiva, and meibomian glands, generating a 400 × 400 μm image (384 pixels × 384 pixels) with a magnification of close to 800-fold and a lateral resolution of 1 μm/pixel [115, 154, 155, 158]. The high-resolution HRT/RCM has become a powerful tool in understanding the microstructural anatomy in both ocular health and disease.
In vivo confocal microscopy-guided evaluation of dry eye
Cornea
In vivo confocal microscopy is emerging as a promising tool in the study of corneal ultrastructure, both in health and disease. The ability of IVCM to examine each layer of the cornea in detail and to identify pathological changes at the cellular level is particularly useful in DED, where the changes are often subclinical and are not picked up by the standard slit-lamp biomicroscopy. IVCM provides high-resolution images of the individual layers of the cornea, i.e., the epithelium, Bowman’s layer, stroma, Descemet’s membrane and the endothelium. Further, the subbasal corneal nerve plexus, stromal nerves [159–162], and dendritiform and non-dendritic immune cells can be clearly identified.
Several studies using IVCM in DED have described the changes induced in the corneal layers, and provide an insight to the underlying pathophysiological mechanisms.
The outermost corneal layer, the epithelium consists of the superficial epithelial layer, an intermediate and the innermost basal cell layer. Being an ocular surface disease, DED affects the superficial epithelial cells. IVCM reveals reduced density and morphological alterations of superficial epithelial cells [163–168]. These corneal superficial epithelial cells increase in size, and cell borders become hyperreflective and irregular. Further, the nuclei become prominent and hyperreflective, and increase in size [163]. These changes are attributed to desquamation and squamous metaplasia. Erdelyi et al. also reported a decrease in the intermediate cell layer [165]. Moreover, increased epithelial basal cell density has been reported in a number of studies [164–166], which has been postulated to be due to the fact that there is an increased turnover of epithelial cells as a result of the epitheliopathy.
Epithelial dendritic cells are the major type of immune antigen-presenting cells in the cornea, and are responsible for generating an immune response or maintaining tolerance [169, 170]. These dendritic cells are primarily located in the subbasal layer in close proximity to the subbasal nerve plexus, with their density declining from the periphery towards the center [170–172]. Using confocal microscopy, Lin et al. have shown an increased number of dendritic cells in DED, both in the center and the periphery [173]. They also suggest that that the numbers of dendrites represent an active stage of these cells. Similarly, Tuisko et al. showed an increased infiltration of the subbasal nerve plexus with purportedly mature antigen-presenting cells [171]. A similar increase in central corneal dendritic cells has also been reported in conditions such as infectious keratitis, with the area covered by these cells (cell field) and their number of dendrites demonstrating the mature and active stage of these cells [174]. Finally, an increase in the number of non-dendritic leukocytes has also been noted in the cornea in DED [173, 174]. Both quantitative and qualitative changes in the nerve plexus have been reported in DED. Examination of the corneal nerve morphology using IVCM has shown various abnormalities such as increased tortuosity, reflectivity, and beading [164, 166, 175, 176]. These morphological changes are believed to be due to the damage and subsequent attempted regeneration of subbasal nerves. Different studies have demonstrated somewhat conflicting results with regard to nerve density. Benitez et al. and Villani et al. in 2007 have reported a decrease in the density of the nerves [164, 175, 177] and this reduction in density is in concordance with decreased clinical corneal sensitivity [164, 171, 175, 177–179]. However, some studies have shown no change or even an increase in the nerve density [163, 176, 180], and hypersensitivity of the cornea has been reported clinically [171, 181]. These changes may be explained by the fact that different stages and severity of DED induce different degeneration/regeneration patterns of nerves.
The basic underlying pathophysiological mechanism responsible for the above noted changes in DED is probably increased inflammation [182]. Several pro-inflammatory cytokines are increased, the type and concentration of which vary with the underlying etiology of DED [183–188]. These cytokines could lead to damage of the epithelial cells and corneal nerves, which in turn can increase pro-inflammatory mediators. The release of pro-inflammatory mediators can subsequently activate or enhance an immune or inflammatory response [189]. The observed decrease in corneal nerves can induce further damage to the epithelium, due to the diminishment of neurotrophic mediators [190, 191]. In addition, neuronal damage may serve as a stimulus for inflammatory cells, called neurogenic inflammation, leading to a vicious cycle of increased inflammation with simultaneous nerve and epithelial cell damage [192, 193].
IVCM has been reported to correlate well with patient-reported symptoms as well as other diagnostic tests [194, 195]. Thus, by being able to demonstrate alterations in the epithelial layers and corneal nerves, and immune cell changes at a cellular level that correlate with clinical signs and symptoms of DED, IVCM may serve as a useful assessment tool supplementary to clinical diagnostic modalities. The density and morphology of dendritic immune cells and superficial epithelial cells, as well as the status of subbasal corneal nerves, have the potential to serve as imaging biomarkers for inflammation in DED. However, additional studies are required to validate these imaging markers and their utility in clinical practice, which could be used for treatment stratification and measurement of therapeutic efficacy when taken together with clinical tests.
Conjunctiva and meibomian glands
In 2005, Kobayashi and colleagues pioneered the application of laser-scanning IVCM to study normal human bulbar and palpebral conjunctiva in a set of four healthy volunteers [155]. For the first time, a layer-by-layer description of the conjunctival epithelium was provided, and glandular structures beneath the palpebral conjunctiva were postulated to be meibomian glands [155]. Since then, the application of IVCM to conjunctival and meibomian gland imaging has started to expand, generating insightful and novel data from conditions such as Sjögren’s syndrome [152], contact lens wear [151], and aging, [154]. Later, Matsumoto et al. defined the first set of diagnostic quantitative imaging parameters in MGD, namely acinar unit density and diameter, and tested them against normal controls [115]. They demonstrated that both acinar unit density and diameter were significantly altered in MGD [115].
Ibrahim et al. tested additional quantifiable acinar parameters in the evaluation of MGD, and logically progressed work in the field by determining the sensitivity and specificity of glandular IVCM parameters in the diagnosis of MGD [196]. They demonstrated that meibomian gland acinar imaging parameters not only correlated strongly with clinical tests for tear film stability and ocular surface integrity, but that when taken together, they yielded high specificity and sensitivity in diagnosing MGD [196]. The first, and to date, the only qualitative diagnostic IVCM grading system of meibomian glands was devised and described by Villani et al. in 2011 [152]. They described a 4-point scoring system based on a subjective assessment of the hyperreflectivity of meibum, and hyperreflective speckling of the acinar epithelium and interstitial tissue; the higher the score, the more likely the presence of MGD [152]. The observed hyperreflectivity and speckling of the meibum, acinar epithelial and interstitium may be explained by the recent detection of subclinical inflammation in MGD [150, 197, 198]. Qazi and colleagues identified and described a unique layer-by-layer distribution of palpebral conjunctival inflammatory cells in MGD; dense populations of inflammatory cells were seen in the palpebral conjunctival epithelium, as well as the conjunctival substantia propria. Occasionally, inflammatory cells can be seen encircling meibomian gland ducts, and lying embedded in the acinar epithelium. However, most remarkably, inflammatory cells can be seen residing within meibomian glands and are associated with ductal dilatation and in some cases, frank glandular obstruction [197]. Following these glands post-treatment can be made possible by following a systematic imaging protocol, scanning multiple serial regions and maintaining consistency of method and regions imaged. The sequences of images acquired can then be compared between visits. Given the high magnification of IVCM (×800), which permits imaging to cellular and microstructural detail, it becomes pivotal to scan all meridians (central, nasal, temporal, superior, inferior) of the palpebral conjunctiva in order to reach a clinical impression based on mean values of multiple representative images. These findings bear significance in potentially being able to guide physicians towards tailored therapy. Observing concurrent epithelial and stromal inflammation may provide for evidence-based decisions with regard to topical versus systemic therapy for MGD, where adjuvant systemic therapy would be critical to eradicating the deeper-seated inflammation and bringing symptomatic relief. Treating patients topically without an assessment of the subclinical severity of disease often results in poor patient satisfaction and refractory symptoms, suggestive of continued stromal or intraductal inflammation [150]. Similarly, visualization of intraductal inflammatory cells could prove to be an indicator of the need for intraductal meibomian gland probing to relieve symptoms attributable to glandular obstruction. IVCM is thus emerging as an informative and objective supplementary tool in the evaluation of MGD and associated dry eye disease. Nevertheless, additional studies are required to validate these parameters and to demonstrate their clinical utility.
IVCM-aided assessment of therapeutic efficacy
Cornea
Anti-inflammatory therapy is now a major component of treatment of DED. However, inflammation and/or changes in immune cells may not be appreciated in the initial stages or in mild disease using slit-lamp examination. Confocal microscopy can assess subclinical immune cell changes, and hence may be used as a tool to determine the need for anti-inflammatory therapy. Moreover, serial imaging may be used for objective assessment of therapeutic success of anti-inflammatory therapy. Based on the dysplastic changes of the superficial epithelial cells in DED [163], improvement in density and morphology can be used to follow the epithelial healing with treatment. Further, as dry eye disease has been associated with decreased corneal nerve density [164, 175], nerve regeneration can be monitored by IVCM during the course of treatment and if required can be accelerated using therapies that focus on providing neurotrophic support. Finally, improvement in superficial epithelial health, nerve parameters and reduction in inflammation by IVCM may be used as objective end points in clinical trials and to validate and standardize treatment protocols for DED.
Conjunctiva and meibomian glands
Translation of therapeutic success of medical and conservative therapy in MGD into subjective improvement by patients is not immediate, and typically a lag of symptoms and signs by slit-lamp biomicroscopy are observed [197, 198]. This lag of clinical improvement, while on treatment, can be distressing for the patient. Patient dissatisfaction with the inability to feel any change in symptoms can result in poor compliance with the therapeutic regimen. In our experience, medical therapy requires approximately 2 to 5 months of stringent compliance before therapeutic effects may be appreciated by the patient [197]. IVCM allows sensitive detection of changes in subclinical immune cell changes in all layers of the palpebral conjunctiva and within meibomian glands as early as 4 to 6 weeks post-therapy [197]. These findings of reduction in epithelial inflammation are consistent with those determined by Matsumoto and colleagues [148], and aid in the determination of success of therapy. To ensure patient compliance in the early stages of therapy when symptomatic improvement lags behind clinical response [198], and patients may therefore be prone to discontinuing medical treatment, our anecdotal experience suggests that using visual aids of the patient’s response to treatment through in vivo confocal micrographs of conjunctival immune cells permits continued compliance with therapy until symptomatic relief is also achieved.
One of the more invasive therapeutic strategies that is gaining popularity, is intraductal probing of meibomian glands using the Maskin intraductal probe [199]. In a pilot study of 25 patients, Maskin demonstrated that intraductal probing has provided immediate symptomatic relief in 96 % of patients, 80 % of whom only required one treatment in a follow-up period of 11.5 months [199]. Similar results were seen by an independent investigator who followed ten patients for a period of 6 months post-probing [200]. These patients have thus far only been assessed using symptom questionnaires, making IVCM-aided quantitative evaluation meibomian gland morphology a necessary next step in validating this promising therapeutic intervention.
Principles of anterior segment optical coherence tomography (AS-OCT)
Optical coherence tomography (OCT) is a non-contact in vivo imaging method that incorporates the principle of low-coherence interferometry to generate cross-sectional images of ocular tissues and its microstructures [201]. AS-OCT permits near-histological visualization and biometry of the anatomical structures starting from the tear film, corneal epithelium, stroma, endothelium, corneo-scleral junction, sclera, anterior chamber, trabecular meshwork, irido-corneal angle, anterior pigment of the iris, and anterior capsule of the lens, making it an indispensable tool for clinical and surgical management of anterior segment pathology [202–211].
Methods of signal acquisition and processing determine features of the OCT such as speed of imaging and resolution of the images. Based on these technical differences in design, the two most popular types of commercially available OCT for anterior segment imaging are time-domain OCT (TD-OCT), such as the Visante omni and Visante OCT (Carl Zeiss, Meditec Inc.) [212, 213], and spectral-domain OCT (SD-OCT), available as slit-lamp OCT (SL-OCT, Heidelberg Engineering, Vista, CA, USA), and more recently, the Envisu C-class (Bioptigen, Durham, NC, USA) [214–216]. Moreover, the RTVue OCT (Optovue) with the anterior segment cornea module provides high-resolution anterior segment imaging. Technical features of these two techniques with respect to AS-OCT are listed in Table 1.
The ultra-high resolution OCT (UHROCT), which has a lateral resolution of 10 μm, is also used extensively for in vivo corneal and anterior chamber imaging in ophthalmic practice [217–219]. It provides good tissue penetration, resolution for diagnostic assessment, and the ability to use images for 3D reconstruction [220]. Quantitative parametric and volumetric analysis of the cornea and lacrimal functional unit becomes possible with the UHROCT.
AS-OCT in the diagnosis and assessment of therapy in dry eye
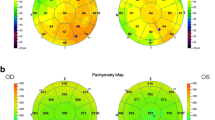
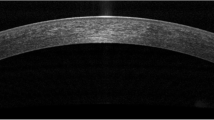
It is only in the past 2 years that AS-OCT has emerged as a quick, non-invasive, and quantitative diagnostic tool in the assessment of dry eye disease, particularly ADDE [212]. In the assessment of DED, tear film biometry is of particular interest. The measurements taken include the tear meniscus height (TMH), the tear meniscus depth (TMD), and tear meniscus area (TMA) [212, 221]. These parameters are significantly decreased in patients with dry eye disease, especially secondary to Sjögren’s syndrome, as compared to controls (Fig. 2), thereby serving as reliable, accurate, non-invasive, and quick diagnostic markers of Sjögren’s disease in the evaluation of DED [212, 222]. Based on stronger correlation with other ocular surface tests, the lower tear menisci measurements seem to be more clinically relevant than upper menisci in the diagnosis of dry eye [223]. Ibrahim et al. showed that TMH, with a sensitivity and specificity of 67 % and 81 % respectively, is reduced in dry eye disease, along with other tests measuring the tear film such as TMH on slit-lamp examination, tear meniscometry, tear break-up time, and Schirmer’s test [212]. Furthermore, they proceeded to test the role of OCT-derived TMH measurements in assessing response to punctal plug therapy for dry eye, which confirmed TMH as a quick and sensitive parameter in the diagnosis and therapeutic assessment of dry eye [224]. Other groups have confirmed the correlation of AS-OCT tear film biometry with Schirmer’s test [225], while retaining high reliability of central tear film measurements (intraclass correlation coefficient = 0.97), making AS-OCT a reliable, precise and accurate tool in tear film biometry [226]. However, it is worthwhile to note that tear film biometry may not be useful in the assessment of MGD-dependent EDE.
Further, Chen et al. found that tear meniscus volume measurements using AS-OCT are reduced in Sjögren’s patients, correlating strongly with corneal staining and TBUT [227]. It has been determined that OCT-derived tear menisci measurements are most useful in diagnosing and following patients with Sjögren’s syndrome-related dry eye than patients with non-Sjögren’s dry eye or evaporative dry eye [221]. Dry eye symptoms may also be associated with conjunctivochalasis. Several groups have successfully adapted OCT-derived tear menisci measurements to evaluate and classify these lid-parallel conjunctival folds toward developing further objective parameters in the assessment of DED [211, 228, 229].
Conclusions
Diagnostic assessment methods for DED have burgeoned towards objective and quantitative measures that reduce inter-user variability and encourage a consensus among physicians in the assessment and management of dry eye disorders. Early detection of ongoing subclinical immune cell changes and inflammation may explain the lack of clinical signs in the presence of symptoms, making it imperative to treat the underlying pathology aggressively, with the goal of preventing tissue damage and retarding subclinical inflammation. IVCM may allow clinicians to make rapid assessments of the patient’s tissue immune response in out-patient settings, and prescribe tailored management early in the disease in an image-guided fashion, gearing towards potentially better clinical outcomes. IVCM also provides objective parameters for evaluating and monitoring disease severity. Prospective randomized clinical trials studies using in vivo imaging tools such as IVCM and AS-OCT are needed, to not only determine their absolute utility, but also to test their true worth as objective in vivo tissue biomarkers of disease. While AS-OCT provides high-resolution, magnified imaging of the anterior segment with fast acquisition times, this technology is limited by the structural details provided. AS-OCT is unable to provide visualization of the microstructural anatomy and cellular detail, unlike IVCM. Furthermore, depth of imaging is contingent upon the wavelength of the light source, with reduced tissue penetration for shorter wavelengths. Some of the issues currently facing IVCM are: (a) the level of expertise and training required to efficiently and adequately acquire high-quality images of the region of interest, (b) the user’s ability to re-register at exactly the same location each time, especially with regards to the lid margin and palpebral conjunctiva, which would be important in monitoring response to therapy for specific procedures such as meibomian duct probing, and (c) identification of the phenotype of cells visualized using IVCM. While the former two issues can be addressed through rigorous training, experience, and following a systematic protocol, unequivocal identification of cell phenotype makes for an inviting avenue to be explored by interested groups. While the phenotype of immune cells is largely consistent and distinctive on IVCM of the cornea, given the cellular architecture of the palpebral conjunctiva with hyper-reflective goblet and epithelial cells, it becomes more challenging to identify immune cells that may not conform to commonly accepted morphology. Therefore, this makes adherence to a strict and meticulous image analysis protocol even more important. Nevertheless, in order to derive definitive conclusions about the immune response from IVCM studies, exploratory immunohistochemistry studies are needed towards establishing corneal and conjunctival cell phenotypes in correlation to IVCM.
References
(2007) The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5:75–92
Uchino M, Nishiwaki Y, Michikawa T, Shirakawa K, Kuwahara E, Yamada M, Dogru M, Schaumberg DA, Kawakita T, Takebayashi T, Tsubota K (2011) Prevalence and risk factors of dry eye disease in Japan: Koumi Study. Ophthalmology 118:2361–2367
Han SB, Hyon JY, Woo SJ, Lee JJ, Kim TH, Kim KW (2011) Prevalence of dry eye disease in an elderly Korean population. Arch Ophthalmol 129:633–638
Guo B, Lu P, Chen X, Zhang W, Chen R (2010) Prevalence of dry eye disease in Mongolians at high altitude in China: the Henan Eye Study. Ophthalmic Epidemiol 17:234–241
Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ (2003) Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol 31:229–232
McCarty CA, Bansal AK, Livingston PM, Stanislavsky YL, Taylor HR (1998) The epidemiology of dry eye in Melbourne, Australia. Ophthalmology 105:1114–1119
Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM (2003) Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology 110:1096–1101
Lee AJ, Lee J, Saw SM, Gazzard G, Koh D, Widjaja D, Tan DT (2002) Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol 86:1347–1351
(2007) The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 93–107
Schaumberg DA, Dana R, Buring JE, Sullivan DA (2009) Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol 127:763–768
Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S (1997) Prevalence of dry eye among the elderly. Am J Ophthalmol 124:723–728
Moss SE, Klein R, Klein BE (2000) Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 118:1264–1268
Schaumberg DA, Sullivan DA, Buring JE, Dana MR (2003) Prevalence of dry eye syndrome among US women. Am J Ophthalmol 136:318–326
Galor A, Feuer W, Lee DJ, Florez H, Carter D, Pouyeh B, Prunty WJ, Perez VL (2011) Prevalence and risk factors of dry eye syndrome in a United States Veterans Affairs population. Am J Ophthalmol 152:377.e2–384.e2
Moschos MM, Chatziralli IP, Siasou G, Papazisis L (2012) Visual problems in young adults due to computer use. Klin Monatsbl Augenheilkd 229:379–381
Thomson WD (1998) Eye problems and visual display terminals–the facts and the fallacies. Ophthalmic Physiol Opt 18:111–119
Uchino M, Schaumberg DA, Dogru M, Uchino Y, Fukagawa K, Shimmura S, Satoh T, Takebayashi T, Tsubota K (2008) Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology 115:1982–1988
Blehm C, Vishnu S, Khattak A, Mitra S, Yee RW (2005) Computer vision syndrome: a review. Surv Ophthalmol 50:253–262
Tsubota K, Nakamori K (1993) Dry eyes and video display terminals. N Engl J Med 328:584
Uchino M, Dogru M, Uchino Y, Fukagawa K, Shimmura S, Takebayashi T, Schaumberg DA, Tsubota K (2008) Japan Ministry of Health study on prevalence of dry eye disease among Japanese high school students. Am J Ophthalmol 146:925.e2–929.e2
Zhang Y, Chen H, Wu X (2012) Prevalence and risk factors associated with dry eye syndrome among senior high school students in a county of Shandong Province, China. Ophthalmic Epidemiol 19:226–230
Pouyeh B, Viteri E, Feuer W, Lee DJ, Florez H, Fabian JA, Perez VL, Galor A (2012) Impact of ocular surface symptoms on quality of life in a United States Veterans Affairs population. Am J Ophthalmol 153:1061.e3–1066.e3
Li M, Gong L, Chapin WJ, Zhu M (2012) Assessment of vision-related quality of life in dry eye patients. Invest Ophthalmol Vis Sci 53:5722–5727
Mertzanis P, Abetz L, Rajagopalan K, Espindle D, Chalmers R, Snyder C, Caffery B, Edrington T, Simpson T, Nelson JD, Begley C (2005) The relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci 46:46–50
Goto E, Yagi Y, Matsumoto Y, Tsubota K (2002) Impaired functional visual acuity of dry eye patients. Am J Ophthalmol 133:181–186
Miljanovic B, Dana R, Sullivan DA, Schaumberg DA (2007) Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol 143:409–415
Tong L, Waduthantri S, Wong TY, Saw SM, Wang JJ, Rosman M, Lamoureux E (2010) Impact of symptomatic dry eye on vision-related daily activities: the Singapore Malay Eye Study. Eye (Lond) 24:1486–1491
Le Q, Zhou X, Ge L, Wu L, Hong J, Xu J (2012) Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol 12:22
Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W (2003) Utility assessment among patients with dry eye disease. Ophthalmology 110:1412–1419
Buchholz P, Steeds CS, Stern LS, Wiederkehr DP, Doyle JJ, Katz LM, Figueiredo FC (2006) Utility assessment to measure the impact of dry eye disease. Ocul Surf 4:155–161
Galor A, Zheng DD, Arheart KL, Lam BL, Perez VL, McCollister KE, Ocasio M, McClure LA, Lee DJ (2012) Dry eye medication use and expenditures: data from the medical expenditure panel survey 2001 to 2006. Cornea 31(12):1403–1407
Reddy P, Grad O, Rajagopalan K (2004) The economic burden of dry eye: a conceptual framework and preliminary assessment. Cornea 23:751–761
Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, Klein R, Tielsch JM, Vijan S, Saaddine J (2006) The economic burden of major adult visual disorders in the United States. Arch Ophthalmol 124:1754–1760
Patel VD, Watanabe JH, Strauss JA, Dubey AT (2011) Work productivity loss in patients with dry eye disease: an online survey. Curr Med Res Opin 27:1041–1048
Yu J, Asche CV, Fairchild CJ (2011) The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea 30:379–387
Akpek EK, Lindsley KB, Adyanthaya RS, Swamy R, Baer AN, McDonnell PJ (2011) Treatment of Sjogren’s syndrome-associated dry eye an evidence-based review. Ophthalmology 118:1242–1252
Friedman NJ (2010) Impact of dry eye disease and treatment on quality of life. Curr Opin Ophthalmol 21:310–316
Fiscella RG (2011) Understanding dry eye disease: a managed care perspective. Am J Manag Care 17(Suppl 16):S432–S439
Luthe R (2010) Dry eye drug development: when will the floodgates open? Ophthalmol Manag
Karpecki P (2013) Why dry eye trials often fail. Review Optom
Caceres V (2011) Treating dry eye. ASCRS Eye World
Epstein SP, Gadaria-Rathod N, Wei Y, Maguire MG, Asbell PA (2013) HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp Eye Res 111:95–104
Gipson IK, Argueso P (2003) Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol 231:1–49
Spurr-Michaud S, Argueso P, Gipson I (2007) Assay of mucins in human tear fluid. Exp Eye Res 84:939–950
Carraway KL, Perez A, Idris N, Jepson S, Arango M, Komatsu M, Haq B, Price-Schiavi SA, Zhang J, Carraway CA (2002) Muc4/sialomucin complex, the intramembrane ErbB2 ligand, in cancer and epithelia: to protect and to survive. Prog Nucleic Acid Res Mol Biol 71:149–185
Gipson IK (2004) Distribution of mucins at the ocular surface. Exp Eye Res 78:379–388
Hodges RR, Dartt DA (2013) Tear film mucins: Front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp Eye Res 117:62–78
Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK (2010) Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp Eye Res 90:444–451
Gipson IK, Hori Y, Argueso P (2004) Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf 2:131–148
Dartt DA (2004) Interaction of EGF family growth factors and neurotransmitters in regulating lacrimal gland secretion. Exp Eye Res 78:337–345
Knop E, Knop N, Millar T, Obata H, Sullivan DA (2011) The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci 52:1938–1978
Shimazaki J, Goto E, Ono M, Shimmura S, Tsubota K (1998) Meibomian gland dysfunction in patients with Sjogren syndrome. Ophthalmology 105:1485–1488
Bron AJ, Yokoi N, Gafney E, Tiffany JM (2009) Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocul Surf 7:78–92
Mizuno Y, Yamada M, Miyake Y (2010) Association between clinical diagnostic tests and health-related quality of life surveys in patients with dry eye syndrome. Jpn J Ophthalmol 54:259–265
Fuentes-Paez G, Herreras JM, Cordero Y, Almaraz A, Gonzalez MJ, Calonge M (2011) Lack of concordance between dry eye syndrome questionnaires and diagnostic tests. Arch Soc Esp Oftalmol 86:3–7
Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S (1997) Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology 104:1395–1401
Begley CG, Chalmers RL, Abetz L, Venkataraman K, Mertzanis P, Caffery BA, Snyder C, Edrington T, Nelson D, Simpson T (2003) The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci 44:4753–4761
Barboza MN, Barboza GN, de Melo GM, Sato E, Dantas MC, Dantas PE, Felberg S (2008) Correlation between signals and symptoms of dry eye in Sjogren’s syndrome patients. Arq Bras Oftalmol 71:547–552
Nichols KK, Nichols JJ, Mitchell GL (2004) The lack of association between signs and symptoms in patients with dry eye disease. Cornea 23:762–770
Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ (2010) Nonobvious obstructive meibomian gland dysfunction. Cornea 29:1333–1345
Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, Foulks GN (2011) The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci 52:1930–1937
Cuevas M, Gonzalez-Garcia MJ, Castellanos E, Quispaya R, Parra Pde L, Fernandez I, Calonge M (2012) Correlations among symptoms, signs, and clinical tests in evaporative-type dry eye disease caused by Meibomian gland dysfunction (MGD). Curr Eye Res 37:855–863
Viso E, Rodriguez-Ares MT, Abelenda D, Oubina B, Gude F (2012) Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Invest Ophthalmol Vis Sci 53:2601–2606
Adatia FA, Michaeli-Cohen A, Naor J, Caffery B, Bookman A, Slomovic A (2004) Correlation between corneal sensitivity, subjective dry eye symptoms and corneal staining in Sjogren’s syndrome. Can J Ophthalmol 39:767–771
(2007) Methodologies to diagnose and monitor dry eye disease: report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 108–152
Nichols KK, Zadnik K (2002) The repeatability of diagnostic tests and surveys in dry eye. Adv Exp Med Biol 506:1171–1175
Nichols KK, Mitchell GL, Zadnik K (2004) The repeatability of clinical measurements of dry eye. Cornea 23:272–285
Johnson ME, Murphy PJ (2005) The agreement and repeatability of tear meniscus height measurement methods. Optom Vis Sci 82:1030–1037
Lemp MA, Bron AJ, Baudouin C, Benitez Del Castillo JM, Geffen D, Tauber J, Foulks GN, Pepose JS, Sullivan BD (2011) Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol 151:792.e1–798.e1
Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, Pepose JS, Kosheleff V, Porreco A, Lemp MA (2010) An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci 51:6125–6130
Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A (2006) Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol Vis Sci 47:4309–4315
Versura P, Profazio V, Campos EC (2010) Performance of tear osmolarity compared to previous diagnostic tests for dry eye diseases. Curr Eye Res 35:553–564
Amparo F, Jin Y, Hamrah P, Schaumberg DA, Dana R (2014) What is the value of incorporating tear osmolarity measurement in assessing patient response to therapy in dry eye disease? Am J Ophthalmol 157:69.e2–77.e2
Kerimoglu H, Ozturk B, Gunduz K, Bozkurt B, Kamis U, Okka M (2010) Effect of altered eating habits and periods during Ramadan fasting on intraocular pressure, tear secretion, corneal and anterior chamber parameters. Eye (Lond) 24:97–100
Carney LG, Hill RM (1976) Human tear pH. Diurnal variations. Arch Ophthalmol 94:821–824
Patel S, Bevan R, Farrell JC (1988) Diurnal variation in precorneal tear film stability. Am J Optom Physiol Opt 65:151–154
Cedarstaff TH, Tomlinson A (1983) Human tear volume, quality and evaporation: a comparison of Schirmer, tear break-up time and resistance hygrometry techniques. Ophthalmic Physiol Opt 3:239–245
(2007) Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 5: 163–178
Siak JJ, Tong L, Wong WL, Cajucom-Uy H, Rosman M, Saw SM, Wong TY (2012) Prevalence and risk factors of Meibomian gland dysfunction: the Singapore Malay Eye Study. Cornea 31(11):1223–1228
Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK (2011) The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 52:1994–2005
Foulks GN, Borchman D (2010) Meibomian gland dysfunction: the past, present, and future. Eye Contact Lens 36:249–253
Hom MM, Martinson JR, Knapp LL, Paugh JR (1990) Prevalence of Meibomian gland dysfunction. Optom Vis Sci 67:710–712
Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K, Lemp MA, Sullivan DA (2011) The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci 52:1922–1929
Sullivan DA, Sullivan BD, Evans JE, Schirra F, Yamagami H, Liu M, Richards SM, Suzuki T, Schaumberg DA, Sullivan RM, Dana MR (2002) Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci 966:211–222
Sullivan DA, Schaumberg DA, Suzuki T, Schirra F, Liu M, Richards S, Sullivan RM, Dana MR, Sullivan BD (2002) Sex steroids, meibomian gland dysfunction and evaporative dry eye in Sjogren’s syndrome. Lupus 11:667
Dougherty JM, McCulley JP (1984) Comparative bacteriology of chronic blepharitis. Br J Ophthalmol 68:524–528
Dougherty JM, McCulley JP (1986) Bacterial lipases and chronic blepharitis. Invest Ophthalmol Vis Sci 27:486–491
McCulley JP, Dougherty JM (1986) Bacterial aspects of chronic blepharitis. Trans Ophthalmol Soc U K 105(Pt 3):314–318
Lee HJ, Chang C (2003) Recent advances in androgen receptor action. Cell Mol Life Sci 60:1613–1622
Sullivan DA, Sullivan BD, Ullman MD, Rocha EM, Krenzer KL, Cermak JM, Toda I, Doane MG, Evans JE, Wickham LA (2000) Androgen influence on the meibomian gland. Invest Ophthalmol Vis Sci 41:3732–3742
Sullivan BD, Evans JE, Cermak JM, Krenzer KL, Dana MR, Sullivan DA (2002) Complete androgen insensitivity syndrome: effect on human meibomian gland secretions. Arch Ophthalmol 120:1689–1699
Sullivan BD, Evans JE, Dana MR, Sullivan DA (2002) Impact of androgen deficiency on the lipid profiles in human meibomian gland secretions. Adv Exp Med Biol 506:449–458
Foulks GN, Bron AJ (2003) Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf 1:107–126
Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, Yee R, Yokoi N, Arita R, Dogru M (2011) The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci 52:2006–2049
Golebiowski B, Chim K, So J, Jalbert I (2012) Lid margins: sensitivity, staining, meibomian gland dysfunction, and symptoms. Optom Vis Sci 89:e1443–e1449
Powell DR, Nichols JJ, Nichols KK (2012) Inter-examiner reliability in meibomian gland dysfunction assessment. Invest Ophthalmol Vis Sci 53:3120–3125
Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK (2005) An assessment of grading scales for meibography images. Cornea 24:382–388
Pult H, Riede-Pult B (2013) Comparison of subjective grading and objective assessment in meibography. Contact Lens Anterior Eye J Br Contact Lens Assoc 36:22–27
Koh YW, Celik T, Lee HK, Petznick A, Tong L (2012) Detection of meibomian glands and classification of meibography images. J Biomed Opt 17:086008
Hwang HS, Shin JG, Lee BH, Eom TJ, Joo CK (2013) In vivo 3D meibography of the human eyelid using real time imaging fourier-domain OCT. PloS One 8:e67143
Goto E, Shimazaki J, Monden Y, Takano Y, Yagi Y, Shimmura S, Tsubota K (2002) Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology 109:2030–2035
Korb DR, Henriquez AS (1980) Meibomian gland dysfunction and contact lens intolerance. J Am Optom Assoc 51:243–251
Dougherty JM, Osgood JK, McCulley JP (1991) The role of wax and sterol ester fatty acids in chronic blepharitis. Invest Ophthalmol Vis Sci 32:1932–1937
Shine WE, McCulley JP (1993) Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci 34:3515–3521
McCulley JP, Shine WE (2004) The lipid layer of tears: dependent on meibomian gland function. Exp Eye Res 78:361–365
Suzuki T, Sano Y, Sasaki O, Kinoshita S (2002) Ocular surface inflammation induced by Propionibacterium acnes. Cornea 21:812–817
Freedberg IM, Tomic-Canic M, Komine M, Blumenberg M (2001) Keratins and the keratinocyte activation cycle. J Invest Dermatol 116:633–640
Mathers WD (1993) Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology 100:347–351
Mathers WD, Lane JA (1998) Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol 438:349–360
Shimazaki J, Sakata M, Tsubota K (1995) Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol 113:1266–1270
Lee SH, Tseng SC (1997) Rose bengal staining and cytologic characteristics associated with lipid tear deficiency. Am J Ophthalmol 124:736–750
Yokoi N, Mossa F, Tiffany JM, Bron AJ (1999) Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol 117:723–729
Goto E, Monden Y, Takano Y, Mori A, Shimmura S, Shimazaki J, Tsubota K (2002) Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol 86:1403–1407
Matsumoto Y, Dogru M, Goto E, Ishida R, Kojima T, Onguchi T, Yagi Y, Shimazaki J, Tsubota K (2006) Efficacy of a new warm moist air device on tear functions of patients with simple meibomian gland dysfunction. Cornea 25:644–650
Matsumoto Y, Sato EA, Ibrahim OM, Dogru M, Tsubota K (2008) The application of in vivo laser confocal microscopy to the diagnosis and evaluation of meibomian gland dysfunction. Mol Vis 14:1263–1271
Arita R, Itoh K, Maeda S, Maeda K, Furuta A, Fukuoka S, Tomidokoro A, Amano S (2009) Proposed diagnostic criteria for obstructive meibomian gland dysfunction. Ophthalmology 116:2058.e1–2063.e1
Romero JM, Biser SA, Perry HD, Levinson DH, Doshi SJ, Terraciano A, Donnenfeld ED (2004) Conservative treatment of meibomian gland dysfunction. Eye Contact Lens 30:14–19
Prabhasawat P, Tesavibul N, Mahawong W (2012) A randomized double-masked study of 0.05% cyclosporine ophthalmic emulsion in the treatment of meibomian gland dysfunction. Cornea 31:1386–1393
Goto E, Endo K, Suzuki A, Fujikura Y, Tsubota K (2002) Improvement of tear stability following warm compression in patients with meibomian gland dysfunction. Adv Exp Med Biol 506:1149–1152
Olson MC, Korb DR, Greiner JV (2003) Increase in tear film lipid layer thickness following treatment with warm compresses in patients with meibomian gland dysfunction. Eye Contact lens 29:96–99
Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, Holland EJ, Lemp MA, McDonald JE 2nd, Silbert DI, Blackie CA, Stevens CA, Bedi R (2012) A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea 31:396–404
Greiner JV (2013) Long-term (12-month) improvement in meibomian gland function and reduced dry eye symptoms with a single thermal pulsation treatment. Clin Exp Ophthalmol 41:524–530
Greiner JV (2012) A single LipiFlow(R) Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res 37:272–278
Friedland BR, Fleming CP, Blackie CA, Korb DR (2011) A novel thermodynamic treatment for meibomian gland dysfunction. Curr Eye Res 36:79–87
Korb DR, Blackie CA (2010) Restoration of meibomian gland functionality with novel thermodynamic treatment device—a case report. Cornea 29:930–933
Korb DR, Blackie CA (2013) Case report: a successful lipiflow treatment of a single case of Meibomian gland dysfunction and dropout. Eye Contact Lens 39(3):e1–e3
Guillon M, Maissa C, Wong S (2012) Eyelid margin modification associated with eyelid hygiene in anterior blepharitis and meibomian gland dysfunction. Eye Contact Lens 38:319–325
Guillon M, Maissa C, Wong S (2012) Symptomatic relief associated with eyelid hygiene in anterior blepharitis and MGD. Eye Contact Lens 38:306–312
Sanchez J, Somolinos AL, Almodovar PI, Webster G, Bradshaw M, Powala C (2005) A randomized, double-blind, placebo-controlled trial of the combined effect of doxycycline hyclate 20-mg tablets and metronidazole 0.75% topical lotion in the treatment of rosacea. J Am Acad Dermatol 53:791–797
Frucht-Pery J, Sagi E, Hemo I, Ever-Hadani P (1993) Efficacy of doxycycline and tetracycline in ocular rosacea. Am J Ophthalmol 116:88–92
Yoo SE, Lee DC, Chang MH (2005) The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J Ophthalmol 19:258–263
Theobald K, Bradshaw M, Leyden J (2007) Anti-inflammatory dose doxycycline (40 mg controlled-release) confers maximum anti-inflammatory efficacy in rosacea. Skinmed 6:221–226
Del Rosso JQ, Webster GF, Jackson M, Rendon M, Rich P, Torok H, Bradshaw M (2007) Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol 56:791–802
Lee H, Min K, Kim EK, Kim TI (2012) Minocycline controls clinical outcomes and inflammatory cytokines in moderate and severe Meibomian gland dysfunction. Am J Ophthalmol 154(6):949.e1–957.e1
Foulks GN, Borchman D, Yappert M, Kakar S (2013) Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea 32(1):44–53
Dougherty JM, McCulley JP, Silvany RE, Meyer DR (1991) The role of tetracycline in chronic blepharitis. Inhibition of lipase production in staphylococci. Invest Ophthalmol Vis Sci 32:2970–2975
Duerden JM, Tiffany JM (1990) Lipid synthesis in vitro by rabbit Meibomian gland tissue and its inhibition by tetracycline. Biochim Biophys Acta 1042:13–18
Shine WE, McCulley JP, Pandya AG (2003) Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res 76:417–420
Souchier M, Joffre C, Gregoire S, Bretillon L, Muselier A, Acar N, Beynat J, Bron A, D’Athis P, Creuzot-Garcher C (2008) Changes in meibomian fatty acids and clinical signs in patients with meibomian gland dysfunction after minocycline treatment. Br J Ophthalmol 92:819–822
Nelson M, Hillen W, Greenwald RA (eds) (2001) Tetracyclines in biology, chemistry and medicine. Springer, Germany, 336 p
Yamaryo T, Oishi K, Yoshimine H, Tsuchihashi Y, Matsushima K, Nagatake T (2003) Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrob Agents Chemother 47:48–53
Sanz MJ, Nabah YN, Cerda-Nicolas M, O’Connor JE, Issekutz AC, Cortijo J, Morcillo EJ (2005) Erythromycin exerts in vivo anti-inflammatory activity downregulating cell adhesion molecule expression. Br J Pharmacol 144:190–201
Xu G, Fujita J, Negayama K, Yuube K, Hojo S, Yamaji Y, Kawanishi K, Takahara J (1996) Effect of macrolide antibiotics on macrophage functions. Microbiol Immunol 40:473–479
Bouwman JJ, Visseren FL, Bouter PK, Diepersloot RJ (2004) Azithromycin inhibits interleukin-6 but not fibrinogen production in hepatocytes infected with cytomegalovirus and chlamydia pneumoniae. J Lab Clin Med 144:18–26
Foulks GN, Borchman D, Yappert M, Kim SH, McKay JW (2010) Topical azithromycin therapy for meibomian gland dysfunction: clinical response and lipid alterations. Cornea 29:781–788
Luchs J (2010) Azithromycin in DuraSite for the treatment of blepharitis. Clin Ophthalmol 4:681–688
Akyol-Salman I, Azizi S, Mumcu UY, Ates O, Baykal O (2011) Comparison of the efficacy of topical N-acetyl-cysteine and a topical steroid-antibiotic combination therapy in the treatment of meibomian gland dysfunction. J Ocul Pharmacol Ther 28:49–52
Matsumoto Y, Shigeno Y, Sato EA, Ibrahim OM, Saiki M, Negishi K, Ogawa Y, Dogru M, Tsubota K (2009) The evaluation of the treatment response in obstructive meibomian gland disease by in vivo laser confocal microscopy. Graefes Arch Clin Exp Ophthalmol 247:821–829
Lindsley K, Matsumura S, Hatef E, Akpek EK (2012) Interventions for chronic blepharitis. Cochrane Database Syst Rev 5, CD005556
Hamrah P, Qazi Y, Blackie CA, Korb DR (2012) Subclinical inflammation may explain the persistence of refractory dry eye symptoms after apparently successful treatment for meibomian gland dysfunction. ARVO Meeting Abstracts 53(6):594
Villani E, Ceresara G, Beretta S, Magnani F, Viola F, Ratiglia R (2011) In vivo confocal microscopy of meibomian glands in contact lens wearers. Invest Ophthalmol Vis Sci 52:5215–5219
Villani E, Beretta S, De Capitani M, Galimberti D, Viola F, Ratiglia R (2010) In vivo confocal microscopy of meibomian glands in Sjogren’s syndrome. Invest Ophthalmol Vis Sci 52:933–939
Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Shimazaki J, Fujishima H, Tsubota K (2012) In vivo confocal microscopy evaluation of meibomian gland dysfunction in atopic-keratoconjunctivitis patients. Ophthalmology 119:1961–1968
Wei A, Hong J, Sun X, Xu J (2011) Evaluation of age-related changes in human palpebral conjunctiva and meibomian glands by in vivo confocal microscopy. Cornea 30:1007–1012
Kobayashi A, Yoshita T, Sugiyama K (2005) In vivo findings of the bulbar/palpebral conjunctiva and presumed meibomian glands by laser scanning confocal microscopy. Cornea 24:985–988
Cavanagh HD, Jester JV, Essepian J, Shields W, Lemp MA (1990) Confocal microscopy of the living eye. CLAO J 16:65–73
Bohnke M, Masters BR (1999) Confocal microscopy of the cornea. Prog Retin Eye Res 18:553–628
Efron N, Al-Dossari M, Pritchard N (2009) In vivo confocal microscopy of the palpebral conjunctiva and tarsal plate. Optom Vis Sci 86:E1303–E1308
Muller LJ, Marfurt CF, Kruse F, Tervo TM (2003) Corneal nerves: structure, contents and function. Exp Eye Res 76:521–542
Grupcheva CN, Wong T, Riley AF, McGhee CN (2002) Assessing the sub-basal nerve plexus of the living healthy human cornea by in vivo confocal microscopy. Clin Exp Ophthalmol 30:187–190
Oliveira-Soto L, Efron N (2001) Morphology of corneal nerves using confocal microscopy. Cornea 20:374–384
Marfurt CF, Cox J, Deek S, Dvorscak L (2010) Anatomy of the human corneal innervation. Exp Eye Res 90:478–492
Tuominen IS, Konttinen YT, Vesaluoma MH, Moilanen JA, Helinto M, Tervo TM (2003) Corneal innervation and morphology in primary Sjogren’s syndrome. Invest Ophthalmol Vis Sci 44:2545–2549
Villani E, Galimberti D, Viola F, Mapelli C, Ratiglia R (2007) The cornea in Sjogren’s syndrome: an in vivo confocal study. Invest Ophthalmol Vis Sci 48:2017–2022
Erdelyi B, Kraak R, Zhivov A, Guthoff R, Nemeth J (2007) In vivo confocal laser scanning microscopy of the cornea in dry eye. Graefes Arch Clin Exp Ophthalmol 245:39–44
Zhang X, Chen Q, Chen W, Cui L, Ma H, Lu F (2011) Tear dynamics and corneal confocal microscopy of subjects with mild self-reported office dry eye. Ophthalmology 118:902–907
Benitez del Castillo JM, Wasfy MA, Fernandez C, Garcia-Sanchez J (2004) An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest Ophthalmol Vis Sci 45:3030–3035
Vera LS, Gueudry J, Delcampe A, Roujeau JC, Brasseur G, Muraine M (2009) In vivo confocal microscopic evaluation of corneal changes in chronic Stevens–Johnson syndrome and toxic epidermal necrolysis. Cornea 28:401–407
Hamrah P, Zhang Q, Liu Y, Dana MR (2002) Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci 43:639–646
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Tuisku IS, Konttinen YT, Konttinen LM, Tervo TM (2008) Alterations in corneal sensitivity and nerve morphology in patients with primary Sjogren’s syndrome. Exp Eye Res 86:879–885
Zhivov A, Stave J, Vollmar B, Guthoff R (2005) In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol 243:1056–1061
Lin H, Li W, Dong N, Chen W, Liu J, Chen L, Yuan H, Geng Z, Liu Z (2010) Changes in corneal epithelial layer inflammatory cells in aqueous tear-deficient dry eye. Invest Ophthalmol Vis Sci 51:122–128
Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, Chodosh J, Pavan-Langston D, Dana R, Hamrah P (2011) Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci 52:5136–5143
Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Diaz-Valle D, Gegundez JA, Fernandez C, Garcia-Sanchez J (2007) Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci 48:173–181
Zhang M, Chen J, Luo L, Xiao Q, Sun M, Liu Z (2005) Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea 24:818–824
Labbe A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C (2012) The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci 53:4926–4931
Bourcier T, Acosta MC, Borderie V, Borras F, Gallar J, Bury T, Laroche L, Belmonte C (2005) Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci 46:2341–2345
Xu KP, Yagi Y, Tsubota K (1996) Decrease in corneal sensitivity and change in tear function in dry eye. Cornea 15:235–239
Hosal BM, Ornek N, Zilelioglu G, Elhan AH (2005) Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond) 19:1276–1279
De Paiva CS, Pflugfelder SC (2004) Corneal epitheliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. Am J Ophthalmol 137:109–115
Stevenson W, Chauhan SK, Dana R (2012) Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol 130:90–100
Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC (2010) Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol Vis Sci 51:3083–3091
Li S, Sack R, Vijmasi T, Sathe S, Beaton A, Quigley D, Gallup M, McNamara NA (2008) Antibody protein array analysis of the tear film cytokines. Optom Vis Sci 85:653–660
Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC (2001) Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci 42:2283–2292
Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM (2009) Systemic and local interleukin-17 and linked cytokines associated with Sjogren’s syndrome immunopathogenesis. Am J Pathol 175:1167–1177
Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH (2011) Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci 26:938–944
Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA (2009) Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 28:1023–1027
Pflugfelder SC, de Paiva CS, Li DQ, Stern ME (2008) Epithelial-immune cell interaction in dry eye. Cornea 27(Suppl 1):S9–S11
Garcia-Hirschfeld J, Lopez-Briones LG, Belmonte C (1994) Neurotrophic influences on corneal epithelial cells. Exp Eye Res 59:597–605
Beuerman RW, Schimmelpfennig B (1980) Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol 69:196–201
Beuerman RW, Stern ME (2005) Neurogenic inflammation: a first line of defense for the ocular surface. Ocul Surf 3:S203–S206
Belmonte C, Acosta MC, Gallar J (2004) Neural basis of sensation in intact and injured corneas. Exp Eye Res 78:513–525
Avetisov SE, Borodina NV, Safonova TN, Fedorov AA, Lutsevich EE, Matevosova EA, Malozhen SA (2009) Potentialities of confocal microscopy in the evaluation of the cornea in the dry eye syndrome. Vestn Oftalmol 125:52–54
Labbe A, Liang Q, Wang Z, Zhang Y, Xu L, Baudouin C, Sun X (2013) Corneal nerve structure and function in patients with non-sjogren dry eye: clinical correlations. Invest Ophthalmol Vis Sci 54:5144–5150
Ibrahim OM, Matsumoto Y, Dogru M, Adan ES, Wakamatsu TH, Goto T, Negishi K, Tsubota K (2010) The efficacy, sensitivity, and specificity of in vivo laser confocal microscopy in the diagnosis of meibomian gland dysfunction. Ophthalmology 117:665–672
Qazi Y, Cavalcanti B, Cruzat A, Cheng S, Williams C, Trinidad M, Witkin D, Blackie CA, Korb DR, Hamrah P (2012) Immune response in meibomian gland dysfunction (MGD) and the effect of anti-inflammatory therapy: an in vivo confocal microscopy (IVCM) study. ARVO Meeting Abstracts 53
Qazi Y, Cavalcanti BC, Cruzat A, Trinidad M, Williams C, Blackie CA, Korb DR, Hamrah P (2012) Symptomatic, clinical and imaging response following anti-inflammatory therapy for Meibomian Gland Dysfunction (MGD); Chicago. pp. page 186
Maskin SL (2010) Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea 29:1145–1152
Wladis EJ (2012) Intraductal meibomian gland probing in the management of ocular rosacea. Ophthal Plast Reconstr Surg 28(6):416–418
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA et al (1991) Optical coherence tomography. Science 254:1178–1181
Zhou Y, Tian L, Wang N, Dougherty PJ (2011) Anterior segment optical coherence tomography measurement of LASIK flaps: femtosecond laser vs microkeratome. J Refract Surg 27:408–416
Werner L, Michelson J, Ollerton A, Leishman L, Bodnar Z (2012) Anterior segment optical coherence tomography in the assessment of postoperative intraocular lens optic changes. J Cataract Refract Surg 38:1077–1085
Zhang XX, Zhong XW, Wu JS, Wang Z, Yu KM, Liu Q, Yang B (2012) Corneal flap morphological analysis using anterior segment optical coherence tomography in laser in situ keratomileusis with femtosecond lasers versus mechanical microkeratome. Int J Ophthalmol 5:69–73
Shabana N, Aquino MC, See J, Ce Z, Tan AM, Nolan WP, Hitchings R, Young SM, Loon SC, Sng CC, Wong W, Chew PT (2012) Quantitative evaluation of anterior chamber parameters using anterior segment optical coherence tomography in primary angle closure mechanisms. Clin Exp Ophthalmol 40:792–801
Sayegh RR, Pineda R 2nd (2012) Practical applications of anterior segment optical coherence tomography imaging following corneal surgery. Semin Ophthalmol 27:125–132
Salim S (2012) The role of anterior segment optical coherence tomography in glaucoma. J Ophthalmol 2012:476801
Majander AS, Lindahl PM, Vasara LK, Krootila K (2012) Anterior segment optical coherence tomography in congenital corneal opacities. Ophthalmology 119:2450–2457
Jhanji V, Constantinou M, Beltz J, Vajpayee RB (2011) Evaluation of posterior wound profile after penetrating keratoplasty using anterior segment optical coherence tomography. Cornea 30:277–280
Aptel F, Beccat S, Fortoul V, Denis P (2011) Biometric analysis of pigment dispersion syndrome using anterior segment optical coherence tomography. Ophthalmology 118:1563–1570
Gumus K, Crockett CH, Pflugfelder SC (2010) Anterior segment optical coherence tomography: a diagnostic instrument for conjunctivochalasis. Am J Ophthalmol 150:798–806
Ibrahim OM, Dogru M, Takano Y, Satake Y, Wakamatsu TH, Fukagawa K, Tsubota K, Fujishima H (2010) Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology 117:1923–1929
Lim LS, Aung HT, Aung T, Tan DT (2008) Corneal imaging with anterior segment optical coherence tomography for lamellar keratoplasty procedures. Am J Ophthalmol 145:81–90
Sarunic MV, Asrani S, Izatt JA (2008) Imaging the ocular anterior segment with real-time, full-range Fourier-domain optical coherence tomography. Arch Ophthalmol 126:537–542
Asrani S, Sarunic M, Santiago C, Izatt J (2008) Detailed visualization of the anterior segment using fourier-domain optical coherence tomography. Arch Ophthalmol 126:765–771
Radhakrishnan S, Rollins AM, Roth JE, Yazdanfar S, Westphal V, Bardenstein DS, Izatt JA (2001) Real-time optical coherence tomography of the anterior segment at 1310 nm. Arch Ophthalmol 119:1179–1185
Gora M, Karnowski K, Szkulmowski M, Kaluzny BJ, Huber R, Kowalczyk A, Wojtkowski M (2009) Ultra high-speed swept source OCT imaging of the anterior segment of human eye at 200 kHz with adjustable imaging range. Opt Express 17:14880–14894
Jungwirth J, Baumann B, Pircher M, Gotzinger E, Hitzenberger CK (2009) Extended in vivo anterior eye-segment imaging with full-range complex spectral domain optical coherence tomography. J Biomed Opt 14:050501
Hutchings N, Simpson TL, Hyun C, Moayed AA, Hariri S, Sorbara L, Bizheva K (2010) Swelling of the human cornea revealed by high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci 51:4579–4584
Bizheva K, Lee P, Sorbara L, Hutchings N, Simpson T (2010) In vivo volumetric imaging of the human upper eyelid with ultrahigh-resolution optical coherence tomography. J Biomed Opt 15:040508
Qiu X, Gong L, Lu Y, Jin H, Robitaille M (2012) The diagnostic significance of Fourier-domain optical coherence tomography in Sjogren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol 90:e359–e366
Qiu X, Gong L, Sun X, Jin H (2010) Age-related variations of human tear meniscus and diagnosis of dry eye with Fourier-domain anterior segment optical coherence tomography. Cornea 30:543–549
Nguyen P, Huang D, Li Y, Sadda SR, Ramos S, Pappuru RR, Yiu SC (2012) Correlation between optical coherence tomography-derived assessments of lower tear meniscus parameters and clinical features of dry eye disease. Cornea 31:680–685
Ibrahim OM, Dogru M, Kojima T, Matsumoto Y, Wakamatsu TH, Tsubota K, Fujishima H (2012) OCT assessment of tear meniscus after punctal occlusion in dry eye disease. Optom Vis Sci 89:E770–E776
Altan-Yaycioglu R, Sizmaz S, Canan H, Coban-Karatas M (2013) Optical coherence tomography for measuring the tear film meniscus: correlation with Schirmer test and tear-film breakup time. Curr Eye Res 38:736–742
Werkmeister RM, Alex A, Kaya S, Unterhuber A, Hofer B, Riedl J, Bronhagl M, Vietauer M, Schmidl D, Schmoll T, Garhofer G, Drexler W, Leitgeb RA, Groeschl M, Schmetterer L (2013) Measurement of tear film thickness using ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci 54:5578–5583
Chen Q, Zhang X, Cui L, Huang Q, Chen W, Ma H, Lu F (2011) Upper and lower tear menisci in Sjogren’s syndrome dry eye. Invest Ophthalmol Vis Sci 52:9373–9378
Veres A, Tapaszto B, Kosina-Hagyo K, Somfai GM, Nemeth J (2011) Imaging lid-parallel conjunctival folds with OCT and comparing its grading with the slit lamp classification in dry eye patients and normal subjects. Invest Ophthalmol Vis Sci 52:2945–2951
Tapaszto B, Veres A, Kosina-Hagyo K, Somfai GM, Nemeth J (2011) OCT Imaging of lid-parallel conjunctival folds in soft contact lens wearers. Optom Vis Sci 88:1206–1213
Zeiss C (2009) Visante Omni — a new dimension in anterior segment evaluation (Carl Zeiss Meditech Inc.)
Dartt D, Bex P, D’Amore P, Dana R, Mcloon L, Niederkorn J (2011) Ocular periphery and disorders. Academic Press, Oxford, UK
Bioptigen (2013) Envisu C-Class System Comparison. Bioptigen Inc.
Li Y, Tang M, Zhang X, Salaroli CH, Ramos JL, Huang D (2010) Pachymetric mapping with Fourier-domain optical coherence tomography. J Cataract Refract Surg 36:826–831
Optovue (2008–2013) RTVue: power for your practice
Acknowledgments
We would like to thank our sponsors for their generous support through grants from the National Institute of Health (NIH K08-EY020575 to PH), Research to Prevent Blindness Career Development Award (PH) and Falk Medical Research Foundation (PH).
Conflict of interest
None for all authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yureeda Qazi and Shruti Aggarwal contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qazi, Y., Aggarwal, S. & Hamrah, P. Image-guided evaluation and monitoring of treatment response in patients with dry eye disease. Graefes Arch Clin Exp Ophthalmol 252, 857–872 (2014). https://doi.org/10.1007/s00417-014-2618-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2618-2