Abstract
Purpose
Minocycline, a second-generation tetracycline with anti-inflammatory and anti-apoptotic properties, was reported to be neuroprotective in experimental glaucoma and optic nerve transection as well as in other neurodegenerative diseases. The purpose of this study was to investigate the mechanism underlying that neuroprotective effect in murine glaucoma.
Methods
Elevated intraocular pressure was induced in 159 rats by the translimbal photocoagulation laser model. Minocycline 22 mg/kg or saline was injected intraperitoneally starting 3 days before the induction of glaucoma, and continued daily until the animals were sacrificed. The effect of minocycline on gene expression was evaluated using a quantitative polymerase chain reaction (PCR) array for apoptosis. The involvement of selected pro-apoptotic, pro-survival, and inflammatory genes was further analyzed by quantitative real-time PCR at multiple time points. Immunohistochemistry was used to study the effect of minocycline on microglial activation and to localize Bcl-2 changes.
Results
Minocycline significantly increased the anti-apoptotic gene Bcl-2 expression at day 8 and day 14 after the induction of glaucoma (p = 0.04 and p = 0.03 respectively), and decreased IL-18 expression in the retina at day 14 and day 30 (p = 0.04 and p < 0.001 respectively). PCR arrays suggested that additional genes were affected by minocycline, including Tp53bp2, TRAF4, osteoprotegerin, caspase 1 and 4, and members of the tumor necrosis factor superfamily. Additionally, minocycline decreased the amount of activated microglia in glaucomatous eyes.
Conclusions
These results suggest that minocycline upregulates pro-survival genes and downregulates apoptotic genes, thus shifting the balance toward the anti-apoptotic side in experimental glaucoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The second-generation tetracycline, minocycline, is commonly used in humans because of its beneficial antimicrobial and anti-inflammatory actions [1]. Minocycline has also been found to be neuroprotective in many animal models of neuronal injury [2–6], as well as in clinical trials of acute stroke and schizophrenia [7, 8]. We previously showed that minocycline significantly delays retinal ganglion cell (RGC) death in models of experimental glaucoma and optic nerve transection (ONT) [9], and that its neuroprotective effect is specific for secondary degeneration of the optic nerve (ON) and retina [10]. Minocycline was also found to be neuroprotective in models of photoreceptor death and retinal toxicity [11–13]. Its potential attractiveness as a drug lies in its easy absorption from the gastrointestinal tract, with a half-life of 18 hours. It is also highly lipid soluble and has a superior ability to penetrate the blood–brain and blood–retinal barriers [12, 14].
The mechanism underlying the neuroprotection capabilities of minocycline is still unclear, but recent evidence has suggested that it may arise from two mechanisms that are distinct from the drug’s antibiotic properties [6]. The first of these mechanisms is its direct anti-inflammatory effect. Minocycline has emerged as a potent inhibitor of microglial activation and migration [15, 16]. Microglia activity during neuropathologic states and their cytotoxic effect have implicated them as mediators of neuronal loss. Microglial activation has been shown to contribute to RGC loss associated with ON damage in glaucoma [17], retinal ischemia [18], and endothelin-1-induced optic neuropathy [19]. Baptiste et al. demonstrated that minocycline delays the death of axotomized RGCs by inhibiting microglia activation [16]. In addition, Bosco et al. reported that minocycline treatment reduced retinal microglia activation, thereby improving ON integrity in a DBA/2 J mouse model of glaucoma [20]. Minocycline reduces the expression and release of proinflammatory cytokines and nitric oxide from activated retinal microglia and suppresses inflammatory cytokine production. Based on these characteristics, we now investigated whether minocycline inhibits microglial activation and used the translimbal photocoagulation model for experimental glaucoma.
The second mechanism by which minocycline induces its protection has been described as it being mediated by the induction of anti-apoptotic intracellular signaling pathways and a decrease in glutamate toxicity [21–25].Those studies suggested that the anti-apoptotic action of minocycline targets both caspase-dependent and caspase-independent cell death processes [23, 26], both of which are known to be activated in primary and secondary degeneration of the ON and retina [27], including that caused by glaucoma. While many studies have investigated this mechanism in models of brain injury, there is limited comparable information in models of glaucoma and ONT. We used polymerase chain reaction (PCR) arrays, real-time PCR (rt-PCR), and immunohistochemistry to explore the mechanism underlying neuroprotection of minocycline in experimental glaucoma.
Materials and methods
Animals
Wistar rats (375-425 g) were used in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research in protocols approved and monitored by the Animal Care Committee of the Tel-Aviv University School of Medicine. The animals were housed with a 14-hour light and 10-hour dark cycle with standard chow and water ad libitum.
Experimental glaucoma
Elevated IOP was induced in one eye of 159 animals by treating the outflow channels of the eye through the peripheral cornea with a diode laser (Coherent Radiation, Clement-Ferrand, France) at 532 nm. Briefly, the animals were anesthetized with intraperitoneal ketamine (50 mg/kg) and xylazine (5 mg/kg), and given topical proparacaine 1 % eye drops. Laser energy (60–80 spots of 50 micron size, 0.5 watt power and 0.5 second duration) was delivered perpendicular to the trabecular meshwork. Treatment was repeated at 1 week. IOP was measured under anesthesia, recording the average of ten readings with the TonoLab tonometer (TioLat, Helsinki, Finland). IOP measurements were taken immediately before, 1 day after each treatment, and then weekly.
Systemic minocycline treatment
The rats were randomly divided into a minocycline 22 mg/kg per day (Sigma–Aldrich Corp, St Louis, MO, USA) treatment group and a saline treatment group (controls). Treatment (minocycline or saline) was given by IP injections initiated 3 days before the first laser and continued daily until sacrifice.
Immunohistochemistry
Immunohistochemistry was used to evaluate the effect of minocycline on activated microglia and to localize minocycline effect on expression of Bcl-2. Both eyes of each animal (n = 40) were enucleated and cryopreserved in sucrose/OCT (Sakura Finetek, USA Inc.; Torrance, CA, USA). Cryosections 10-ųm thick were collected onto Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at –80 °C before immunolabelling. At least three sections from each eye were examined. The effect of minocycline on microglial activation was evaluated at 14 and 30 days following the induction of glaucoma. Sections were incubated with mouse anti-rat primary antibodies CD68, an antibody known to stain activated microglia (1:200, AbD Serotec Ltd, MCA341R) [28], and rabbit anti-mouse Iba1, a general marker for microglia (Wako, 01919741) [29, 30]. The secondary antibody was Alexa fluor 568 anti-rabbit 1:500 or Alexa fluor 488 anti-mouse 1:500 (Invitrogene). Retina was assessed histologically with a UV fluorescent microscope.
For Bcl-2 and Thy 1, a marker of RGC, sections were incubated with goat- anti rat Bcl-2 (1:50, Santa Cruz Biotechnology) and mouse anti-rat Thy 1 (1:100, Millipore Corporation, Billerica, MA, USA). Secondary antibody was FITC-conjugated AffiniPure donkey anti-goat IgG (1:100 and Rhodamine red X-conjugated AffiniPure donkey anti-mouse IgG, Jackson ImmunoResearch).
Negative controls included nonimmune serum of the same species as the primary antibody at the same protein concentration and incubation buffer alone.
All measurements were performed in a masked way normalized to the length of the section.
Quantitative polymerase chain reaction (PCR) array for apoptosis
RT2 ProfilerTM PCR Arrays (Catalog # PARN-012 SABiosciences, Frederick, MD, USA) was performed to check for expression of genes involved in facets of apoptosis in glaucomatous eyes and control fellow eyes treated with minocycline or saline (n = 12 rats, pull of four animals for each PCR array, three repetitions, a total of six arrays, three for minocycline and three for saline). Total RNA was extracted from retinas dissected 8 days following the induction of glaucoma using the Qiagen RNeasy mini kit (Qiagen, Valencia, CA, USA).
RNA quantity and purity was determined using the Nanodrop ND-2000 (Nanodrop Technologies, Wilmington, DE, USA). RNA was reversed transcribed (RT) using the RT2 First Strand Kit (SABiosciences, Frederick, MD, USA), Real-time PCR was performed using the RT2 SYBR Green qPCR Master Mix (SABiosciences). Next, each sample was aliquotted on the rat apoptosis PCR array. All steps were done according to the manufacturer’s protocol for the ABI Prism 7000 Sequence Detection System.
Each 96-well RT2 ProfilerTM PCR Array contains 84 wells with a realtime PCR assay for different genes related to apoptosis cascade, five wells with assays for different housekeeping genes, a genomic DNA control, three replicate reverse transcription controls, and three replicate positive PCR controls.
Data were analyzed with the Excel-based PCR Array Data Analysis Template provided by the manufacturer.
Real-time reverse transcription–PCR
The mRNA levels of selected genes were examined by qPCR to verify array results. Several genes that are not on the microarray but were of particular interest to us were also examined. Total RNA was extracted from retinal samples at multiple time points following IOP elevation. Real time was performed using the Quanti Tect SybrGreen system (Qiagen, Valencia, CA, USA) in the ABI/ Prism 7700 Sequence Detector System (Applied Biosystems, PerkinElmer) and β-Actin mRNA was used as an endogenous control. Primers were purchased from Sigma (Sigma– Aldrich, Rehovot, Israel, Table 1). A standard curve for each gene was created using three 10-fold dilutions of a cDNA sample produced from a retina of an untreated animal. Each sample was analyzed in triplicate by at least three separate PCR reactions. For each sample, we calculated the normalized ratio (i.e., the number of copies of a given gene divided by the number of copies of β-actin in each sample).
Results
A total of 159 Wistar rats were included in this study. All experimental eyes had significantly elevated IOP (an increase in IOP >10 mmHg) compared to their control fellow eyes as judged by both mean and peak IOP (Fig. 1a and b). IOP usually returned to baseline by 2–3 weeks.
Quantitative PCR array for apoptosis
The PCR array used in this study indicates changes in gene expression caused by elevated IOP (saline group) and, more importantly, reveals the changes in gene expression that have been modified by treatment with minocycline. Table 2 summarizes the 22 genes that were either upregulated or downregulated with a more than 2-fold change in the minocycline group, the saline group, or both, compared to controls. In the saline-treated group, there were four genes that had been upregulated by more than 5-fold compared to their control fellow eyes: they were Hrk (BH3 interacting with the Bcl-2 family domain, an apoptosis agonist), PYCARD (PYD and CARD domain containing), Tnfrsf11b (tumor necrosis factor receptor super family, member 11b) and Casp4 (caspase 4, apoptosis-related cysteine peptidase). Lta (lymphotoxin A) was the only gene that was downregulated (by more than 5-fold). In the minocycline group, Tnfrsf11b was the only gene that was upregulated (by more than 5-fold).
There were 11 genes in which the difference in gene expression between the minocycline and saline groups was more than double (Table 2). For example, all investigated caspase members were over-expressed in the glaucomatous eyes compared to their control eyes. However, two members of the caspase family, caspase 1 and 4, were upregulated in the saline group by a more than 2-fold difference compared to the minocycline group.
Verification of gene expression changes at multiple time points
The expression of different genes was evaluated in eyes with elevated IOP and injected with saline or minocycline at four time points. The mRNA levels of selected genes were examined to verify array results, and several genes that were of particular interest to us were investigated as well.
Cytokines
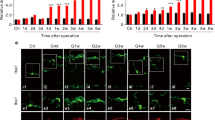
All investigated cytokines were significantly upregulated due to the elevated IOP (Fig. 2). TNF-α was significantly upregulated from day 8 to day 30 (p < 0.05 for all 3 time points) and its 2 receptors, TNF-R1 and TNF-R2, were also upregulated from day 8 to day 14 (p < 0.05 for both time points). The Fas ligand was significantly upregulated in glaucomatous eyes from day 4 to day 30 (p = 0.02, p = 0.05, p = 0.02 and p < 0.001 respectively, for the four time points). In addition, IL-18 was significantly upregulated from day 8 to day 30 (p < 0.05 for all three time points).
a The expression of the TNFα gene increased in glaucomatous eyes compared to their fellow control eyes from day 8 to day 30 in both the minocycline and saline groups (p < 0.01 for all three time points, n = 7 in each group). Treatment with minocycline had no effect on TNFα expression. b The IL-18 gene expression increased significantly in glaucomatous eyes compared to their fellow eyes from day 8 to day 30 in the minocycline and saline groups (p < 0.05 for all 3 time points, n = 7–11 in each group). The expression of IL-18 decreased significantly in minocycline-treated glaucomatous eyes compared to saline-treated eyes from day 14 (n = 7, p = 0.04) to day 30 (n = 7, p = 0.006). c The TNFR1 gene expression increased in glaucomatous eyes compared to their fellow control eyes from day 8 to day 14 in the minocycline and saline groups (p < 0.05 for all time points, n = 7 in each group). Treatment with minocycline had no effect on TNFR1 expression. d TNFR2 gene expression increased in glaucomatous eyes compared to their fellow control eyes from day 8 to day 14 in the minocycline and saline groups (p < 0.05 for the 2 time points, n = 7 in each group). Treatment with minocycline had no effect on TNFR2 expression. e FAS ligand gene expression increased significantly in glaucomatous eyes compared to their fellow control eyes from day 4 to day 30 in the minocycline and saline groups (p < 0.05 for all 4 time points, n = 7 in each group). Treatment with minocycline had no effect on FAS ligan d expression *P < 0.05
Minocycline treatment significantly decreased the expression of IL-18 on day 14 and day 30 (p = 0.04 and p < 0.001 respectively) but it did not affect the expression of any other cytokines.
Bcl-2 family
All investigated members of the Bcl-2 family (the anti-apoptotic Bcl-2 and Bcl-xl, and the pro-apoptotic genes Bax and Bad) were changed in the glaucomatous eyes (Fig. 3). The expression of Bcl-2 was significantly decreased in the glaucomatous eyes compared to their fellow eyes on day 8 and day 14 (p = 0.002 and 0.004 respectively). Bcl-xl expression was also decreased on day 8 (p = 0.04). The expression of Bax was significantly increased in glaucomatous eyes compared to their fellow eyes on day 8 and day 14 (p = 0.046 and p < 0.001 respectively), while Bad expression was increased in glaucomatous eyes on day 8 (p = 0.04). Interestingly, all changes in the Bcl-2 family gene expression were apparent only when the IOP was elevated, and they vanished 2 weeks later when the IOP returned to normal. Minocycline significantly increased the expression of the antiapoptotic gene Bcl-2 on day 8 and day 14 (p = 0.04 and p = 0.03 respectively), but it did not affect any other members of the Bcl-2 family.
a Bcl-2 gene expression decreased in glaucomatous eyes compared to their fellow control eyes at day 8 and day 14 in the minocycline and saline groups (p < 0.05 for the two time points, n = 6–7 in each group). The antiapoptotic gene Bcl-2 expression increased significantly in minocycline-treated glaucomatous eyes compared to saline-treated eyes from day 8 (n = 7 in each group, p = 0.04) to day 14 (n = 7 in each group, p = 0.03). b The proapoptotic gene Bax expression increased significantly in glaucomatous eyes compared to their fellow eyes from day 8 to day 14 in the minocycline and saline groups (p < 0.05 for the two time points, n = 7 in each group).Treatment with minocycline had no effect on Bax expression. c Bad expression increased in glaucomatous eyes compared to their fellow control eyes at day 8 in the minocycline and saline groups (p < 0.05, n = 6–7 in each group). Treatment with minocycline had no effect on Bad expression. d Bcl-xl expression increased in glaucomatous eyes compared to their fellow control eyes at day 8 in the minocycline and saline groups (p < 0.05, n = 6–7 in each group). Treatment with minocycline had no effect on Bcl-xl expression
Anti-apoptotic and pro-apoptotic genes
The expression of the anti-apoptotic gene IAP-1 was significantly increased in glaucomatous eyes compared to their fellow eyes from day 8 to day 30 (p ≤ 0.05 for all three time points, Fig. 4). The expression of the apoptotic gene Gadd45α was significantly increased in glaucomatous eyes compared to their fellow eyes from day 4 to day 30 (p ≤ 0.05 for all four time points). The apoptotic gene iNOS was significantly increased in glaucomatous eyes compared to their fellow eyes from day 4 to day 8 (p <0.05 for all four time points), however, none of these genes were affected by minocycline treatment.
a The anti-apoptotic IAP-1 expression increased in glaucomatous eyes compared to their fellow control eyes from day 8 to day 30 in the minocycline and saline groups (p < 0.05 for all three time points, n = 6–7 in each group). Treatment with minocycline had no effect on IAP-1 expression. b The apoptotic gene Gadd45α expression increased significantly in glaucomatous eyes compared to their fellow control eyes from day 4 to day 30 in the minocycline and saline groups (p < 0.05 for all four time points, n = 6–7 in each group). Treatment with minocycline had no effect on Gadd45α expression. c iNOS gene expression increased significantly in glaucomatous eyes compared to their fellow control eyes from day 8 to day 14 in the minocycline and saline groups (p < 0.05 for the two time points, n = 6–7 eyes in each group). Treatment with minocycline had no effect on iNOS expression. *P < 0.05
Immunohistochemical analysis
We evaluated the effect of minocycline on microglial activation by immunohistochemistry. There was increase in microglial activation among the glaucomatous eyes compared to their control fellow eyes on day 30 but not earlier (Fig. 5, Fig. 6a and b). Elevation of IOP increased the number of activated microglia on day 30 (Fig. 6b). Treatment with minocycline did not affect the total number of microglia, but decreased microglial activation at that time point (Fig. 6d).
Staining for non-activated and activated microglia with Iba-1 (red) and CD68 (green) in retinal cross-sections of glaucomatous eyes on day 14. a Immunohistochemistry for Iba-1, CD68, and DAPI in the retina of control eye. b Immunohistochemistry for Iba-1, CD68, and DAPI in the retina of glaucomatous eye at day 14. Magnification ×40, Scale bars: all images 50 μm
Staining for non-activated and activated microglia with Iba-1 (red) and CD68 (green) in retinal cross-sections of glaucomatous eyes on day 30. a Immunohistochemistry for Iba-1, CD68, and DAPI in the retina of control eye. b Immunohistochemistry for Iba-1, CD68, and DAPI in the retina of a glaucomatous eye at day 30. c Immunohistochemistry for Iba-1, CD68, and DAPI in the retina of a fellow control eye treated with minocycline. d- Immunohistochemistry for Iba-1, CD68, and DAPI in the retina of a glaucomatous eye at day 30. Magnification ×40, Scale bars: all images 50 μm
Immunohistochemistry localized changes in Bcl-2 protein to the RGC layer. There was intense labeling for Bcl-2 and Thy-1, specifically in the RGC layer, in the control fellow eyes of both the saline and minocycline groups (Fig. 7a and c). When elevated IOP was induced, staining for Bcl-2 was decreased in the saline-injected eyes on day 14 compared to their control fellow eyes. However, staining for Bcl-2 was similar between the minocycline-injected eyes and their control fellow eyes (Fig. 7a and b), in support of our RT-PCR results (Fig. 3a).
Immunohistochemistry for Bcl-2, the retinal ganglion cell marker Thy 1 and DAPI in retinal cryosections of control and glaucomatous eyes treated with minocycline or saline. The merged image shows colocalization of Bcl-2 with Thy 1, suggesting that Bcl-2 changes occur mostly in the retinal ganglion cell (RGC) layer. a Colocalization of Bcl-2 and Thy 1 in the control eyes itreated with saline. Bcl-2 staining is localized to the cytosol of the RGCs. b The level of Bcl-2 staining in the cytosol of the RGC is reduced in glaucomatous eyes treated with saline. c Bcl-2 staining is localized to the cytosol of the RGCs in controls eyes treated with minocycline. d The level of Bcl-2 staining in the cytosol of the RGC is slightly reduced in glaucomatous eyes treated with minocycline compared to their fellow eyes. The arrows are pointing to examples of Bcl-2 colocalization. Magnification ×40. Scale bars: all images 50 μm
Discussion
This study investigates the molecular mechanisms underlying the neuroprotective effect of minocycline in experimental glaucoma. The results of this study suggest that minocycline upregulates pro-survival genes and downregulates apoptotic genes, thus shifting the balance toward the anti-apoptotic side. We found that minocycline significantly increased the expression of the pro-survival gene Bcl-2, and decreased the expression of IL-18 in the retina. In addition, PCR arrays suggested that additional genes were affected by minocycline, among them Tp53bp2, TRAF4, osteoprotegerin, caspase 1 and 4, and members of the TNF superfamily. Furthermore, we found that minocycline decreased the number of activated microglia, as suggested by others in the literature [31–34].
Members of the Bcl-2 family are pivotal regulators of the apoptotic process, and include both proteins that promote cell survival (Bcl-2, Bcl-xL, and Bcl-w) and others that antagonize it (e.g., Bax, Bad, Bak, Bik, Bid, BNIP3, and Bim) [35]. In the current study, Bcl-2 expression was significantly decreased in glaucomatous eyes, and this effect was attenuated with minocycline treatment, thus supporting minocycline as a potential neuroprotective drug for glaucoma.
The effect of minocycline on the Bcl-2 family had been observed in other models of ocular injury but not in glaucoma. In one rat model of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis (EAE) with optic neuritis, minocycline acted on two crucial members of the Bcl-2 family by inducing a shift towards the anti-apoptotic side: the expression of Bax was decreased under minocycline treatment, whereas the Bcl-2 expression in RGCs was upregulated [36]. Interestingly, we had recently found that minocycline acted similarly to EAE in an ONT model by inducing a shift towards being anti-apoptotic (upregulating bcl-2 and downregulating Bax), while the expression of IL-18 was not affected (submitted for publication). In the present study, Bcl-2 expression and IL-18 were affected by minocycline, while Bax expression stayed unchanged. These results indicate that the mechanism of action of minocycline may be different among various models of ON injuries.
Our PCR array data suggested that other genes were also affected by minocycline. Tp53bp2, which codes the apoptosis-stimulating protein of p53, a pro-apoptotic member, was downregulated following minocycline treatment. P53 is involved in the apoptosis of RGCs by acting as a transcription factor that upregulates the expression of BAX and downregulates the expression of bcl2 [37]. In support of that, we had previously shown that the proapoptotic genes Ei24 and Gadd45a, members of the P53 pathway, were upregulated in experimental glaucoma [38]. In addition, variants in TNF and TP53 were found to be risk factors for POAG [39]. Taken together, it appears that the downregulation of Tp53bp2 by minocycline contributes to its overall antiapoptotic effect.
Recent evidence has demonstrated that the degeneration of RGCs in glaucoma is associated with subclinical inflammation that plays an important role in the development and progression of the disease. It has also been suggested that the protective effect of minocycline is associated with the reduction of specific cytokines (IL-1β, inducible nitric-oxide synthase [iNOS], and TNF-α) that are mainly expressed by microglia, and that the proliferation of microglia is also inhibited by minocycline [31–34]. This assumption was further supported by studies using models for ocular injuries, such as ONT [16, 40], glaucoma [20] and photoreceptor degeneration [13, 41]. Zhang et al. showed that the microglial cells in the outer nuclear layer and subretinal space were significantly decreased in the minocycline-treated group compared with those in the light-exposed control group 3 days after exposure to light [13]. However, Hughes et al. suggested that minocycline delayed photoreceptor death in a microglia-independent manner [12, 42]. In the present study, we found that minocycline decreased the number of activated microglia in our model for glaucoma, with no effect on the total number of microglia. Furthermore, the only cytokine that was affected by minocycline in our study was IL-18.
In our model, elevation of IOP induced upregulation of IL-18 even at 1 month post-induction. In line with that finding, Zhou et al. demonstrated that the expression of IL-18 and TNF-α increased with age in the retina and ON of DBA/2 J mice in correlation with increased loss of RGCs by apoptosis [43]. Previous studies on models of CNS injuries failed to demonstrate that minocycline affected IL-18 expression [44]. Recently, however, repeated intrathecal injections of minocycline were reported to significantly inhibit the increased expression of IL-18 and IL-18Rs in microglia induced by tetanic sciatic stimulation [45], supporting our findings in this glaucoma model.
TNF-α-mediated cell death is involved in glaucomatous neurodegeneration [46]. TNF-α is a potent immunomediator and proinflammatory cytokine that is rapidly upregulated in the brain after injury. It is an inducer of apoptotic cell death through TNF-α receptor-1 (p55) occupancy in a caspase-mediated pathway. Activation of caspase-8 is known to be a hallmark of the TNF receptor family cell death pathway. In the current study, TNF-α expression levels were significantly higher in the glaucomatous eyes compared to their control fellow eyes, but minocycline had no effect on the expression of TNF-α or its receptors TNFR1 and TNFR2. Our PCR array results revealed that caspase 8 was upregulated in both the saline- and minocycline-injected groups.
Our PCR array and rt-PCR data demonstrated involvement of Fas and the Fas ligand (FasL) in glaucoma. The FasL plays a major role in retinal neurotoxicity, with FasL deficiency having protected RGCs from cell death [47]. However, minocycline did not change the expression of either Fas or the FasL.
TNF receptor-associated factor 4 (TRAF4) was overexpressed in our minocycline group more than in our saline group (PCR array). The physiological and molecular functions of TRAF4 are poorly understood, but there is increasing evidence that links the loss of TRAF4 function to demyelinating or neurodegenerative diseases [48]. The effect of minocycline on TRAF4 observed in our study further supports its potential neuroprotective effect.
Several genes whose expression was affected by minocycline more than 2-fold compared to saline were not directly related to neuroprotection. For example, Cd40lg was downregulated by 3.46-fold in glaucomatous eyes compared to controls, and minocycline abolished this effect. The CD40 ligand (CD154), a member of the TNF superfamily, is a transmembrane protein expressed primarily on activated CD4 T cells [49]. The human CD40 ligand gene (CD40LG) has been mapped to the X chromosome. It is unclear, however, what the relationship is between this finding and minocycline’s neuroprotective effect. Another example is the effect of minocycline on lymphotoxin A. Lymphotoxin A was downregulated by 8.5-fold in glaucomatous eyes that were treated with saline compared to non-treated controls, and minocycline attenuated this effect. Lymphotoxin A is a cytokine that signals through TNFR I and TNFR II, and it has important functions in the development and homeostasis of the immune system. However, the importance of the effect of minocycline on lymphotoxin A is also undetermined.
We have previously shown a simultaneous upregulation of pro-survival and pro-apoptotic genes in glaucoma, indicating that RGCs exhibit an intrinsic neuroprotective mechanism for counteracting apoptosis and for potentially improving RGC survival [38]. The present study demonstrates that minocycline acts in a manner similar to that of the endogenous neuroprotection mechanism. This increase in pro-survival signaling and decrease in pro-apoptotic signaling following the administration of minocycline may partly account for the beneficial effect of minocycline in the setting of glaucoma. We believe that it is time to move forward with clinical studies to investigate the effect of minocycline in patients with progressive glaucoma.
References
Aronson AL (1980) Pharmacotherapeutics of the newer tetracyclines. J Am Vet Med Assoc 176:1061–1068
Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J (1998) Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A 95:15769–15774
Zhang Y, Metz LM, Yong VW, Bell RB, Yeung M, Patry DG, Mitchell JR (2008) Pilot study of minocycline in relapsing-remitting multiple sclerosis. Can J Neurol Sci 35:185–191
Pattison LR, Kotter MR, Fraga D, Bonelli RM (2006) Apoptotic cascades as possible targets for inhibiting cell death in Huntington's disease. J Neurol 253:1137–1142
Kriz J, Nguyen MD, Julien JP (2002) Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 10:268–278
Kim HS, Suh YH (2009) Minocycline and neurodegenerative diseases. Behav Brain Res 196:168–179
Levkovitz Y, Mendlovich S, Riwkes S, Braw Y, Levkovitch-Verbin H, Gal G, Fennig S, Treves I, Kron S (2009) A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 71(2):138–149
Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M (2007) Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 69:1404–1410
Levkovitch-Verbin H, Kalev-Landoy M, Habot-Wilner Z, Melamed S (2006) Minocycline delays death of retinal ganglion cells in experimental glaucoma and after optic nerve transection. Arch Ophthalmol 124:520–526
Levkovitch-Verbin H, Spierer O, Vander S, Dardik R (2011) Similarities and differences between primary and secondary degeneration of the optic nerve and the effect of minocycline. Graefes Arch Clin Exp Ophthalmol 249(6):849–857. doi:10.1007/s00417-010-1608-2
Baptiste DC, Hartwick AT, Jollimore CA, Baldridge WH, Seigel GM, Kelly ME (2004) An investigation of the neuroprotective effects of tetracycline derivatives in experimental models of retinal cell death. Mol Pharmacol 66(5):1113–1122
Hughes EH, Schlichtenbrede FC, Murphy CC, Broderick C, van Rooijen N, Ali RR, Dick AD (2004) Minocycline delays photoreceptor death in the rds mouse through a microglia-independent mechanism. Exp Eye Res 78:1077–1084
Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MO (2004) Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci 45:2753–2759
Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM (2004) Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A 101:3071–3076
Yang LP, Zhu XA, Tso MO (2007) Minocycline and sulforaphane inhibited lipopolysaccharide-mediated retinal microglial activation. Mol Vis 13:1083–1093
Baptiste DC, Powell KJ, Jollimore CA, Hamilton C, LeVatte TL, Archibald ML, Chauhan BC, Robertson GS, Kelly ME (2005) Effects of minocycline and tetracycline on retinal ganglion cell survival after axotomy. Neuroscience 134:575–582
Yuan L, Neufeld AH (2001) Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res 64:523–532
Naskar R, Wissing M, Thanos S (2002) Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci 43:2962–2968
Chauhan BC, LeVatte TL, Jollimore CA, Yu PK, Reitsamer HA, Kelly ME, Yu DY, Tremblay F, Archibald ML (2004) Model of endothelin-1-induced chronic optic neuropathy in rat. Invest Ophthalmol Vis Sci 45:144–152
Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML (2008) Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2 J mouse model of glaucoma. Invest Ophthalmol Vis Sci 49:1437–1446
Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM (2001) Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A 98:14669–14674
Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu du C, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM (2002) Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417:74–78
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat Med 6:797–801
Sanchez Mejia RO, Ona VO, Li M, Friedlander RM (2001) Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery 48:1393–1399, discussion 1399-1401
Wang J, Wei Q, Wang CY, Hill WD, Hess DC, Dong Z (2004) Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J Biol Chem 279:19948–19954
Wang X, Zhu S, Drozda M, Zhang W, Stavrovskaya IG, Cattaneo E, Ferrante RJ, Kristal BS, Friedlander RM (2003) Minocycline inhibits caspase-independent and -dependent mitochondrial cell death pathways in models of Huntington's disease. Proc Natl Acad Sci U S A 100:10483–10487
Levkovitch-Verbin H, Dardik R, Vander S, Melamed S (2009) Mechanism of retinal ganglion cells death in secondary degeneration of the optic nerve. Exp Eye Res 2:127–134
Kozlowski C, Weimer RM (2012) An automated method to quantify microglia morphology and application to monitor activation state longitudinally in vivo. PLoS One 7(2):e31814
Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res 57(1):1–9
Graeber MB, López-Redondo F, Ikoma E, Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW, Kohsaka S (1998) The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res 813(2):241–253
Tikka TM, Koistinaho JE (2001) Minocycline provides neuroprotection against N-methyl-D-aspartate neurotoxicity by inhibiting microglia. J Immunol 166:7527–7533
Suzuki H, Sugimura Y, Iwama S, Nobuaki O, Nagasaki H, Arima H, Sawada M, Oiso Y (2010) Minocycline prevents osmotic demyelination syndrome by inhibiting the activation of microglia. J Am Soc Nephrol 21: 2090–2098
Cho KO, La HO, Cho YJ, Sung KW, Kim SY (2006) Minocycline attenuates white matter damage in a rat model of chronic cerebral hypoperfusion. J Neurosci Res 83:285–291
Wang AL, Yu AC, Lau LT, Lee C, le Wu M, Zhu X, Tso MO (2005) Minocycline inhibits LPS-induced retinal microglia activation. Neurochem Int 47:152–158
Antonsson B, Martinou JC (2000) The Bcl-2 protein family. Exp Cell Res 256:50–57
Maier K, Merkler D, Gerber J, Taheri N, Kuhnert AV, Williams SK, Neusch C, Bahr M, Diem R (2007) Multiple neuroprotective mechanisms of minocycline in autoimmune CNS inflammation. Neurobiol Dis 25:514–525
Nickells RW (1999) Apoptosis of retinal ganglion cells in glaucoma: an update of the molecular pathways involved in cell death. Surv Ophthalmol 43(Suppl 1):S151–S161
Levkovitch-Verbin H, Dardik R, Vander S, Nisgav Y, Kalev-Landoy M, Melamed S (2006) Experimental glaucoma and optic nerve transection induce simultaneous upregulation of proapoptotic and prosurvival genes. Invest Ophthalmol Vis Sci 47:2491–2497
Fan BJ, Liu K, Wang DY, Tham CC, Tam PO, Lam DS, Pang CP (2010) Association of polymorphisms of tumor necrosis factor and tumor protein p53 with primary open-angle glaucoma. Invest Ophthalmol Vis Sci 51: 4110–4116
Baptiste DC, Hartwick AT, Jollimore CA, Baldridge WH, Seigel GM, Kelly ME (2004) An investigation of the neuroprotective effects of tetracycline derivatives in experimental models of retinal cell death. Mol Pharmacol 66:1113–1122
Zhao L, Ma W, Fariss RN, Wong WT (2011) Minocycline attenuates photoreceptor degeneration in a mouse model of subretinal hemorrhage microglial: inhibition as a potential therapeutic strategy. Am J Pathol 179:1265–1277
Hughes E, Schlichtebrede F, Murphy C, Ali R, Dick A (2003) Minocycline suppresses photoreceptor apoptosis in the rds mouse through a mechanism unrelated to microglial inhibition. ARVO abstract #2843
Zhou X, Li F, Kong L, Chodosh J, Cao W (2009) Anti-inflammatory effect of pigment epithelium-derived factor in DBA/2 J mice. Mol Vis 15:438–450
Fox C, Dingman A, Derugin N, Wendland MF, Manabat C, Ji S, Ferriero DM, Vexler ZS (2005) Minocycline confers early but transient protection in the immature brain following focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab 25:1138–1149
Chu YX, Zhang YQ, Zhao ZQ (2012) Involvement of microglia and interleukin-18 in the induction of long-term potentiation of spinal nociceptive responses induced by tetanic sciatic stimulation. Neurosci Bull 28: 49–60
Tezel G, Li LY, Patil RV, Wax MB (2001) TNF-alpha and TNF-alpha receptor-1 in the retina of normal and glaucomatous eyes. Invest Ophthalmol Vis Sci 42:1787–1794
Gregory MS, Hackett CG, Abernathy EF, Lee KS, Saff RR, Hohlbaum AM, Moody KS, Hobson MW, Jones A, Kolovou P, Karray S, Giani A, John SW, Chen DF, Marshak-Rothstein A, Ksander BR (2011)Opposing roles for membrane bound and soluble Fas ligand in glaucoma-associated retinal ganglion cell death. PLoS One 6:e17659
Blaise S, Kneib M, Rousseau A, Gambino F, Chenard MP, Messadeq N, Muckenstrum M, Alpy F, Tomasetto C, Humeau Y, Rio MC (2012) In vivo evidence that TRAF4 is required for central nervous system myelin homeostasis. PLoS One 7:e30917
Armitage RJ, Maliszewski CR, Alderson MR, Grabstein KH, Spriggs MK, Fanslow WC (1993) CD40L: a multi-functional ligand. Semin Immunol 5:401–412
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by the Claire & Amadee Maratier Institute for the study of blindness and visual disorders, Sackler School of Medicine, Tel-Aviv University.
Rights and permissions
About this article
Cite this article
Levkovitch-Verbin, H., Waserzoog, Y., Vander, S. et al. Minocycline upregulates pro-survival genes and downregulates pro-apoptotic genes in experimental glaucoma. Graefes Arch Clin Exp Ophthalmol 252, 761–772 (2014). https://doi.org/10.1007/s00417-014-2588-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-014-2588-4