Abstract
Background
One of the early signs of diabetic retinopathy is the alteration of the blood–retinal barrier (BRB), which may involve the breakdown of endothelial cell tight junctions. Methylglyoxal (MGO) is a cytotoxic metabolite that is produced from glycolysis in vivo. Elevated levels of MGO are observed in a number of pathological conditions, including neurodegenerative disorders and diabetic complications. Herein, we hypothesize that increased levels of MGO disrupt the tight junction protein known as occludin protein by matrix metalloproteinases (MMPs), leading to breakage of the BRB.
Methods
MGO was intravitreally injected into eyes of rats. BRB leakage, MMPs activity, and occludin were investigated in intravitreally MGO-injected eyes.
Results
When normoglycemic rats were intravitreally injected with 400 μM MGO, there was widespread leakage of fluorescein isothiocyanate–bovine serum albumin (FITC-BSA) from the retinal vasculature when compared to control retinas. In addition, MGO-injected retinas demonstrated increases of both activity and expression of MMP-2 and MMP-9, and the degradation of occludin was found in the MGO-injected retinas.
Conclusions
The results suggest that the activation of MMPs by elevated levels of MGO in the retina may facilitate an increase in vascular permeability by a mechanism involving proteolytic degradation of occludin. These findings may have implications for the role of MGO in the pathogenesis of diabetic retinopathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy is associated with progressive damage of the capillary basement membrane, loss of microvascular intramural pericytes, and leaky dilation [1, 2]. A major clinicopathological hallmark of diabetic retinopathy is increased capillary permeability culminating in an overt breakdown of the inner blood–retinal barrier (BRB) [3, 4]. The pathogenic mechanism of the compromise of the BRB during diabetic retinopathy remains unclear.
One group of extracellular proteinases that have been shown to play a role in the retinal neovascularization seen in the later stages of diabetic retinopathy is the matrix metalloproteinases (MMPs). MMPs are a class of approximately 25 known proteinases that serve to degrade at least one component of the extracellular matrix in addition to other substrates. Recently, it was reported that an elevated expression of MMPs in the retina facilitated an increase in vascular permeability by a mechanism involving proteolytic degradation of the tight junction proteins [5].
Endogenous reactive carbonyl species (RCS), such as 3-deoxyglucosone (3-DG), glyoxal (GO), and methylglyoxal (MGO), play an important role in mediating carbonyl stress in various cells [6, 7]. RCS are highly produced where there is a high level of glucose in the physiological system. RCS in the cells can react with proteins, especially lysine and arginine residues, and with DNA to form a group of compounds called advanced glycation end products (AGEs). Chemical modifications of the proteins and DNA cause structural damage to cells and eventually lead to tissue injury. An accumulation of MGO in diabetes may contribute to diabetic retinopathy [8]. Our recent study showed that the production of MGO in retinal tissue was closely correlated with the loss of retinal pericytes, an early sign of diabetic retinopathy. Interestingly, MGO increases the expression of MMP-2 in the basement membrane of peritoneal microvessels [9]. However, the underlying mechanisms of MGO cytotoxicity in BRB breakage have remained uncertain. Thus, in this study, we investigate whether MGO can induce BRB dysfunction in rat retinas and, if so, whether MGO-induced retinal vasopermeability occurs via the enhanced expression of MMPs in the retina.
Materials and methods
Animals and induction of diabetes
Diabetes was induced by a single injection of streptozotocin (STZ, 60 mg/kg, i.p.) in 7-week-old male Sprague-Dawley (SD) rats. Age-matched control rats received an equal volume of vehicle (0.01 M citrate buffer, pH 4.5). One week after the STZ injection, the blood glucose level was measured from the tail vein. Rats with a blood glucose level over 300 mg/dl were considered to be diabetes-induced rats. The animals were then divided into two groups: (1) normal SD rats (n = 8) and (2) STZ-induced diabetic rats (n = 8). Five months after the induction of diabetes, the animals were fasted for at least 15 h and immediately anesthetized and killed. Blood was obtained for fasting blood glucose determination using a Beckman glucose analyzer II (Beckman, CA, USA). Levels of MGO in the retinal tissues were assessed by HPLC. Animals were deeply anesthetized using ketamine/xylazine, and eyes were enucleated. Retinas were isolated and homogenized in 300 μl of lysis buffer (50 mM Tris-HCl pH 7.4, 250 mM NaCl and 1x protease inhibitor cocktail), incubated for 1 h on ice and briefly sonicated. Protein concentration was determined and normalized. Subsequently, 100 μl of sample at a concentration of 1 μg/μl was mixed with 100 μl HCl (0.2 M). The concentration of MGO was determined according to a modification of a previously reported method [10, 11]. All experiments were approved by the Korea Institute of Oriental Medicine Institutional Animal Care and Use Committee.
Intravitreal injection of MGO
Thirty-two male SD rats (7 weeks old) were used in this study. Each rat was anesthetized with a 1:1 mixture of xylazine hydrochloride (4 mg/kg) and ketamine hydrochloride (10 mg/kg). A single dose of 24 mM MGO in a volume of 3μl was injected into the vitreous of the right eye with a microinjector (Hamilton Co., NV, USA) under a dissecting microscope. Assuming the vitreous volume of an adult rat eye to be approximately 56 μl [12], the final intravitreal concentration of MGO was approximately 400 μM. For normal control, 3 μl of physiological saline was injected into the left eye. The needle was left in position for 30–60 s and then slowly withdrawn to minimize fluid loss from the eye. The rats were monitored regularly for infection associated with the injection site. Eyes with injection-damaged lenses or retinas were excluded from the study. At 1 day after the intravitreal injection, rats were anesthetized and killed. The 32 rats were subjected to measurement of BRB permeability, trypsin-digested vessel preparation, gelatin zymography, and extraction of protein, respectively. All experiments were approved by the Korea Institute of Oriental Medicine Institutional Animal Care and Use Committee.
Measurement of BRB permeability
Eight rats previously injected intravitreally with MGO or saline were deeply anesthetized using ketamine/xylazine. One hundred mg/kg each of fluorescein isothiocyanate–bovine serum albumin (FITC-BSA, Sigma, St. Louis, MO) in sterile PBS was injected into the left ventricle. The tracer was allowed to circulate for 5 min and one eye was then enucleated and immediately fixed in 4% paraformaldehyde for 2 h. The retinas were dissected, flat-mounted onto a glass slide, and viewed by fluorescence microscopy (BX51, Olympus, Tokyo, Japan). For quantification of retinal vascular leakage, the eyes were enucleated, embedded in an OCT compound (Sakura. Finetechnical, Tokyo, Japan), and immediately frozen in liquid nitrogen. The plasma was collected and assayed for fluorescence with a spectrofluorophotometer (Synergy™ HT, Bio-Tek, VT, USA) based on standard curves of FITC-BSA in normal plasma. Frozen retinal sections (4 to 8 μm thick) were collected every 30 μm and viewed with a fluorescence microscope (BX51, Olympus). Six images from nonvascular retinas (200 μm2) in each section were collected. Quantification of the FITC-BSA fluorescence intensity was calculated by ImageJ software (NIH) and normalized to the plasma fluorescence intensity for each animal.
Trypsin-digested vessel preparation
The eyes were enucleated from eight rats previously injected intravitreally with MGO or saline, and the retinas were isolated. The retinal samples were then placed in 10% formalin for 2 days. After fixation, the retinas were incubated in trypsin (3% in sodium phosphate buffer) for approximately 60 min. The vessel structures were isolated from the retinal cells by gentle rinsing in distilled water. The vascular specimens were then mounted on a slide.
Immunofluorescence staining
Immunofluorescence staining was performed on the trypsin-digested retinal vessels. Antibody was rabbit anti-occludin (1:200, Invitrogen Life Technologies, Carlsbad, CA, USA). To detect occludin, the vessels were incubated with Texas red-conjugated goat anti-rabbit antibody (1:500, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The intensity of the fluorescence was analyzed in five randomly selected 200-mm2 areas using ImageJ software (NIH).
Gelatin zymography
The activities of MMP-2 and MMP-9 were measured by gelatin zymography using human recombinant pro- and active MMP-2/9 (Calbiochem, Gibbstown, NJ, USA) as standards. Retinal tissues from eight rats previously injected intravitreally with MGO or saline were homogenized in a lysis buffer (50 mmol/l Tris-HCl, pH 7.6, 150 mmol/l NaCl, 5 mmol/l CaCl2, 0.05% BRIJ-35, 0.02% NaN3, 1% Triton X-100), and centrifuged. Aliquots containing equal amounts of protein from each sample were incubated for 1 h with gelatin-Sepharose 4B (Amersham Biosciences, NJ, USA) with constant shaking. The pellets were washed with a working buffer (lysis buffer without Triton X-100) and resuspended in 100 μl of elution buffer (working buffer with 10% dimethyl sulfoxide) for 30 min and then centrifuged. The samples were loaded on 10% zymogram gelatin gels (Invitrogen, CA, USA). After electrophoresis, the gels were incubated in renaturing buffer (Invitrogen) for 1 h at room temperature followed by incubation in developing buffer (Invitrogen) overnight at 37°C. The gels were stained for 1 h in 1% Coomassie blue (Invitrogen) and then washed with water to obtain the clearest background for photography.
Western-blot analysis
Retinal proteins were extracted from eight rats previously injected intravitreally with MGO or saline and then separated by SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membrane was probed with rabbit anti-occludin antibody (1:1000, Invitrogen) and mouse anti-MMP-2 (1:1000, Santa Cruz) and anti-MMP-9 antibodies (1:1000, Santa Cruz), and then the immune complexes were visualized with an enhanced chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ, USA).
Statistical analysis
The results obtained in the studies were analyzed with nonparametric statistics because the data were not normally distributed. Comparisons between two groups were performed with the Mann–Whitney rank test. Statistical analysis was performed by GraphPad Prism 4.0 (GraphPad, La Jolla, CA, USA).
Results
Increased MGO in the retinas of diabetic rat with hyperpermeable retinal vessels
Streptozotocin-induced diabetic rat with hyperglycemia (Table 1) were examined for increases in retinal vascular permeability using a FITC-BSA leakage assay. We observed a modest increase in the dye leakage from the retinal vessels of diabetic rats when compared to the control normoglycemic rats (Fig. 1a and b). In addition, we examined the accumulation of MGO in the retinas of the control and diabetic rats. The retinas of diabetic rats have increased levels of MGO as compared to control retinas (Fig. 1c).
Blood–retinal barrier breakdown (a and b) and MGO intracellular accumulation (c) in normal rats (NOR) and STZ-induced diabetic rats (DM). Retinal permeability as determined by the FITC-BSA technique is increased in rats following 5 months of diabetes. The intracellular accumulation of MGO in the retinal vasculature is nearly twofold higher in diabetic rats compared to nondiabetic rats. Values in the bar graphs represent the means ± SE, n = 8. *p < 0.01 vs. normal rats
An increase in retinal MGO causes increased retinal vascular permeability in normoglycemic rats
To determine if the increase of MGO seen in diabetic rat could contribute to increased retinal vascular permeability, we examined whether an increased concentration of MGO in the retina could induce retinal vascular leakage in normal rats. To evaluate increased vascular permeability in MGO-injected retinas, fluorescein angiography was performed using FTIC-BSA. Figure 2a shows representative fluorescence micrographs of FITC-BSA in control and MGO-injected eyes. The fluorescence intensity is limited to the vasculature in the control retinas and diffusely increased throughout the retinal parenchyma in the MGO-injected retinas. Figure 2b shows the change in the fluorescence intensity in the control and MGO-injected retinas after normalizing to plasma fluorescence. MGO increased retinal fluorescence by 71% (p < 0.01).
MGO-induced BRB breakdown. Normoglycemic rats were intravitreally injected for 1 day with 3 mM of MGO. Control retinas showed no leakage of the tracer into the retina as evidenced by the clear delineation of retinal capillaries. MGO-injected retinas demonstrated a widespread breakdown of their BRB with tracer leakage into the neural retina and a loss of delineation of the retinal capillaries. Values in the bar graphs represent the means ± SE, n = 8. *p < 0.01 vs. control group
Tight junction protein loss and association with MGO accumulation
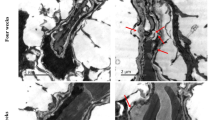
Given the correlation between increased vascular permeability and loosening of the tight junctions in MGO-injected retinas, we investigated the expression of the tight junction protein known as occludin. A marked decrease in occludin was detected in the MGO-injected retinas (Fig. 3a). To confirm whether expression of occludin decreases due to an increased concentration of MGO, we also performed a Western-blot analysis. With an injection of MGO, occludin was significantly reduced (Fig. 3b). These results indicate that increased vascular permeability of MGO-injected retinas is accompanied by a decrease of tight junction proteins in retinal vessels.
MGO-induced occludin loss. a Representative retinal vessels from control and MGO-injected retinas stained with anti-occludin antibody. Occludin expression was evident at the interfaces between adjacent endothelial cells in the control retinas, while it was mostly eliminated from the microvessels in the MGO-injected retinas (arrow). b Total protein was isolated, and a Western blot was performed. The MGO-injected retinas demonstrated less occludin than the control retinas. Values in the bar graphs represent the means ± SE, n = 8. *p < 0.01 vs. control group
Activation of retinal MMP-2 and MMP-9 in MGO-injected eyes
Because MMPs are increased in the retinas of diabetic animals and because the breakdown of occludin may be involved in the activation of MMP-2 and MMP-9 in retinal endothelial cells [5], we wished to determine whether the decrease in occludin content observed in MGO-injected eyes could result from the activation of these proteolytic enzymes. MGO-injected retinas were analyzed by zymography and demonstrated a marked increase in both the pro and active forms of MMP-2 and MMP-9 (Fig. 4a). Figure 4b also shows the retinal protein levels for MMP-2 and MMP-9 in the retinas that were evaluated with a Western-blot analysis. The MMP-2 and MMP-9 levels increased approximately 1.5 fold and 1.8 fold, respectively, in the MGO-injected eyes compared to the controls.
Activation of MMP-2 and MMP-9 in MGO-injected retinas. a Representative gelatin zymography of retinal samples. MGO increases MMP-2 and MMP-9 activation in retinas. Following an MGO treatment, an increase in production of both the pro and activated forms of the enzymes was seen. b Protein expression of MMP-2 and MMP-9. Western-blot analysis shows the increased expression of MMP-2 and MMP-9 in the MGO-injected retinas. Values in the bar graphs represent the means ± SE, n = 8. *p < 0.01 vs. control group
Discussion
In this study, the direct involvement of MGO in the development of diabetic retinopathy was examined. The current study has demonstrated that MGO can induce BRB dysfunction in normoglycemic animals, an important hallmark and sight-threatening lesion of diabetic retinopathy. Diabetic retinopathy is a common complication of diabetes [13]. In diabetic retinopathy, the earliest visible sign is capillary hyperpermeability. Increased capillary permeability results in the leaking of fluid into the surrounding retinal tissue, which pools around the macula, thus causing macular edema and visual loss. Our results suggest that MGO contributes to increased retinal vascular leakage in diabetes.
One of the major consequences of hyperglycemia is the formation of MGO. MGO is a major precursor of advanced glycation end products (AGEs) and is increased in diabetic tissues [14]. MGO is associated with the formation of acellular capillaries and a loss of pericytes in diabetic rats [15, 16]. Cytotoxicity induced by MGO has already been reported in various cells (rat Schwann cells, human vascular endothelial cells, rat mesangial cell and bovine retinal pericytes) [17–20]. In this study, we show that the exposure of retinas to MGO leads to an alteration of the BRB. The data obtained in our intraocular injection systems are consistent with observations in the retinas of a diabetic animal model [5]. Altogether, data suggests that the MGO-induced MMP activation increases the breakdown of endothelial cell tight junctions, possibly contributing to BRB leakage in situations of an increased availability of MGO, such as diabetes.
The plasma methylglyoxal levels have been estimated to be about 0.5 μM in healthy individuals and can increase twofold in cases of diabetes [21], and others have demonstrated that the plasma methylglyoxal concentration in poorly controlled human diabetic patients is about 400 μM [22]. Cells produce large amounts of methylglyoxal [23]. Intracellular levels are probably much higher than plasma levels [14]. Moreover, incubation of retinal epithelial cells with 3 mM MGO resulted in an intracellular accumulation of MGO. This intracellular level of MGO is consistent with the levels found in retinal tissues of diabetic animals [11]. In our previous study, the intravitreal injection of 400 μM MGO into normoglycemic rat eyes induced the injury of retinal pericytes [24]. Based on these previous reports, we chose a dose of 400 μM MGO to mimic the exposure to elevated levels of MGO under diabetic conditions.
Previous work demonstrated that the activation of MMP-2, MMP-9, and MMP-14 by both high glucose in bovine retinal endothelial cells and hyperglycemia in diabetic rats induced alterations of tight junction function [5]. MGO increased the expression of MMP-2 in the basement membrane of peritoneal microvessels [9]. We also showed that MGO induced the increases in both activity and expression of MMP-2 and MMP-9 as well as the loss of occludin in retinas. These results indicate that the harmful effect of MGO on the BRB could be linked to the activation of MMPs.
MMPs are a family of proteolytic enzymes that degrade extracelluar matrix (ECM) proteins such as collagen and elastin and are essential for cellular migration and tissue remodeling under physiological and pathological conditions [25]. The major MMP species expressed in the vasculature include MMP-1, MMP-2, and MMP-9, and both endothelial and smooth muscle cells can synthesize these enzymes. The major sources of MMPs in the vessel are endothelial and vascular smooth muscle cells [26]. The expression of MMP species is upregulated by a number of factors, including growth factors, cytokines, and physical stress [27]. A recent study demonstrated increased MMP-9 expression in aortic tissue homogenates obtained from diabetic animals [28]. High glucose stimulated the synthesis of MMP-9 in endothelial cells [27]. Conversely, MMP-2 gene expression is downregulated in the glomeruli and tubulointerstitial tissue obtained from patients with diabetes [29]. In experimental diabetes, significant decreases in MMP-2 and MMP-9 in the renal tissue have been reported [30]. Increased endothelial permeability may involve the activation of MMPs [31, 32]. Giebel et al. previously reported on the upregulation of MMPs in the diabetic retina and their possible role in the proteolytic breakdown of the important components of the BRB [5]. On the basis of these observations, it has been proposed that augmented MMP activity might contribute to the alteration of the BRB in diabetes. The increase of MMPs in diabetic animals may be partly due to the direct effects of hyperglycemia [33], increased VEGF expression [34], or the production of other diabetes-related products including reactive oxygen species [28] and AGEs [35]. In the present study, we confirmed that high levels of MGO resulted in an increased production of MMP-2 and MMP-9 in retinas.
Next, to further investigate the role of MMPs in BRB breakdown, we examined the integrity of occludin. In this study, a loss of occludin was noted in MGO-injected retinas. This suggests that the degradation of occludin within the tight junction complex may result in an overall breakdown of the barrier. Occludin is a 65-kDa protein specific to cells that contain tight junctions, and it is thought to span the plasma membrane, conferring the cell-to-cell interaction of tight junctions [36]. The expression of occludin correlates with an increased function in the barrier. Tight junction proteins are expressed in the endothelial cells of the blood–brain barrier and the BRB [37]. Occludin expression has been shown to be specific for vascular endothelial cells with strong barrier properties [38]. Although occludin by itself cannot form a functionally tight barrier, it likely plays an important role in the organization and stabilization of the tight junction [39, 40]. Occludin cleavage by MMPs is another possible mechanism underlying vascular barrier impairment [41].
In conclusion, the major findings of this study are (1) that MGO is intracellularly accumulated in the retina in an animal model of early diabetic retinopathy at a time when there is an increase in BRB permeability, (2) that high levels of MGO cause an increase of the activity and production of MMP-2 and MMP-9 in MGO-injected retinas, and (3) that MGO affects the function of the vascular permeability barrier in MGO-injected retinas via the degradation of the tight junction protein known as occludin. Together, these findings suggest that elevated levels of MGO in the retina of diabetic animals may facilitate an increase in vascular permeability by a mechanism involving proteolytic degradation of occludin.
References
Cunha-Vaz J, Bernardes R (2005) Nonproliferative retinopathy in diabetes type 2. Initial stages and characterization of phenotypes. Prog Retin Eye Res 24:355–377
Roy S, Sato T, Paryani G, Kao R (2003) Downregulation of fibronectin overexpression reduces basement membrane thickening and vascular lesions in retinas of galactose-fed rats. Diabetes 52:1229–1234
Sander B, Larsen M, Engler C, Lund-Andersen H, Parving HH (1994) Early changes in diabetic retinopathy: capillary loss and blood–retina barrier permeability in relation to metabolic control. Acta Ophthalmol 72:553–559
Patz A (1980) Studies on retinal neovascularization. Friedenwald Lecture. Invest Ophthalmol Vis Sci 19:1133–1138
Giebel SJ, Menicucci G, McGuire PG, Das A (2005) Matrix metalloproteinases in early diabetic retinopathy and their role in alteration of the blood–retinal barrier. Lab Invest 85:597–607
Sander CS, Hamm F, Elsner P, Thiele JJ (2003) Oxidative stress in malignant melanoma and non-melanoma skin cancer. Br J Dermatol 148:913–922
Kuniyasu H, Oue N, Wakikawa A, Shigeishi H, Matsutani N, Kuraoka K, Ito R, Yokozaki H, Yasui W (2002) Expression of receptors for advanced glycation end-products (RAGE) is closely associated with the invasive and metastatic activity of gastric cancer. J Pathol 196:163–170
Stitt AW, McGoldrick C, Rice-McCaldin A, McCance DR, Glenn JV, Hsu DK, Liu FT, Thorpe SR, Gardiner TA (2005) Impaired retinal angiogenesis in diabetes: role of advanced glycation end products and galectin-3. Diabetes 54:785–794
Hirahara I, Kusano E, Yanagiba S, Miyata Y, Ando Y, Muto S, Asano Y (2006) Peritoneal injury by methylglyoxal in peritoneal dialysis. Perit Dial Int 26:380–392
Schalkwijk CG, Posthuma N, ten Brink HJ, ter Wee PM, Teerlink T (1999) Induction of 1,2-dicarbonyl compounds, intermediates in the formation of advanced glycation end-products, during heat-sterilization of glucose-based peritoneal dialysis fluids. Perit Dial Int 19:325–333
Bento CF, Fernandes R, Matafome P, Sena C, Seica R, Pereira P (2010) Methylglyoxal-induced imbalance in the ratio of vascular endothelial growth factor to angiopoietin 2 secreted by retinal pigment epithelial cells leads to endothelial dysfunction. Exp Physiol 95:955–970
Berkowitz BA, Lukaszew RA, Mullins CM, Penn JS (1998) Impaired hyaloidal circulation function and uncoordinated ocular growth patterns in experimental retinopathy of prematurity. Invest Ophthalmol Vis Sci 39:391–396
Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL 3rd, Klein R (1998) Diabetic retinopathy. Diabetes Care 21:143–156
Randell EW, Vasdev S, Gill V (2005) Measurement of methylglyoxal in rat tissues by electrospray ionization mass spectrometry and liquid chromatography. J Pharmacol Toxicol Methods 51:153–157
Hammes HP (2003) Pathophysiological mechanisms of diabetic angiopathy. J Diabetes Complicat 17:16–19
Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M (2003) Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 9:294–299
Akhand AA, Hossain K, Mitsui H, Kato M, Miyata T, Inagi R, Du J, Takeda K, Kawamoto Y, Suzuki H, Kurokawa K, Nakashima I (2001) Glyoxal and methylglyoxal trigger distinct signals for map family kinases and caspase activation in human endothelial cells. Free Radic Biol Med 31:20–30
Fukunaga M, Miyata S, Liu BF, Miyazaki H, Hirota Y, Higo S, Hamada Y, Ueyama S, Kasuga M (2004) Methylglyoxal induces apoptosis through activation of p38 MAPK in rat Schwann cells. Biochem Biophys Res Commun 320:689–695
Huang WJ, Tung CW, Ho C, Yang JT, Chen ML, Chang PJ, Lee PH, Lin CL, Wang JY (2007) Ras activation modulates methylglyoxal-induced mesangial cell apoptosis through superoxide production. Ren Fail 29:911–921
Kim J, Son JW, Lee JA, Oh YS, Shinn SH (2004) Methylglyoxal induces apoptosis mediated by reactive oxygen species in bovine retinal pericytes. J Korean Med Sci 19:95–100
Lapolla A, Flamini R, Dalla Vedova A, Senesi A, Reitano R, Fedele D, Basso E, Seraglia R, Traldi P (2003) Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med 41:1166–1173
Lapolla A, Reitano R, Seraglia R, Sartore G, Ragazzi E, Traldi P (2005) Evaluation of advanced glycation end products and carbonyl compounds in patients with different conditions of oxidative stress. Mol Nutr Food Res 49:685–690
Chaplen FW, Fahl WE, Cameron DC (1998) Evidence of high levels of methylglyoxal in cultured Chinese hamster ovary cells. Proc Natl Acad Sci USA 95:5533–5538
Kim J, Kim OS, Kim CS, Kim NH, Kim JS (2010) Cytotoxic role of methylglyoxal in rat retinal pericytes: Involvement of a nuclear factor-kappaB and inducible nitric oxide synthase pathway. Chem Biol Interact 188:86–93
Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS (1996) Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest 98:2572–2579
Vincenti MP (2001) The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. Transcriptional and posttranscriptional regulation, signal transduction and cell-type-specific expression. Methods Mol Biol 151:121–148
Portik-Dobos V, Anstadt MP, Hutchinson J, Bannan M, Ergul A (2002) Evidence for a matrix metalloproteinase induction/activation system in arterial vasculature and decreased synthesis and activity in diabetes. Diabetes 51:3063–3068
Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, Harrison DG, Tsao PS (2001) Diabetes mellitus enhances vascular matrix metalloproteinase activity: role of oxidative stress. Circ Res 88:1291–1298
Del Prete D, Anglani F, Forino M, Ceol M, Fioretto P, Nosadini R, Baggio B, Gambaro G (1997) Down-regulation of glomerular matrix metalloproteinase-2 gene in human NIDDM. Diabetologia 40:1449–1454
Song RH, Singh AK, Leehey DJ (1999) Decreased glomerular proteinase activity in the streptozotocin diabetic rat. Am J Nephrol 19:441–446
Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J Neurosci 21:7724–7732
Alexander JS, Elrod JW (2002) Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J Anat 200:561–574
Grant MB, Caballero S, Tarnuzzer RW, Bass KE, Ljubimov AV, Spoerri PE, Galardy RE (1998) Matrix metalloproteinase expression in human retinal microvascular cells. Diabetes 47:1311–1317
Behzadian MA, Windsor LJ, Ghaly N, Liou G, Tsai NT, Caldwell RB (2003) VEGF-induced paracellular permeability in cultured endothelial cells involves urokinase and its receptor. FASEB J 17:752–754
Moore TC, Moore JE, Kaji Y, Frizzell N, Usui T, Poulaki V, Campbell IL, Stitt AW, Gardiner TA, Archer DB, Adamis AP (2003) The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthalmol Vis Sci 44:4457–4464
Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S (1993) Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788
Watson PM, Anderson JM, Vanltallie CM, Doctrow SR (1991) The tight-junction-specific protein ZO-1 is a component of the human and rat blood–brain barriers. Neurosci Lett 129:6–10
Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL (1997) Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci 110(Pt 14):1603–1613
Chen Y, Merzdorf C, Paul DL, Goodenough DA (1997) COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos. J Cell Biol 138:891–899
Wong V, Gumbiner BM (1997) A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol 136:399–409
Liu W, Hendren J, Qin XJ, Shen J, Liu KJ (2009) Normobaric hyperoxia attenuates early blood–brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem 108:811–820
Acknowledgments
This research was supported by a grant [K11040] from the Korea Institute of Oriental Medicine (KIOM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J., Kim, CS., Lee, Y.M. et al. Methylglyoxal induces hyperpermeability of the blood–retinal barrier via the loss of tight junction proteins and the activation of matrix metalloproteinases. Graefes Arch Clin Exp Ophthalmol 250, 691–697 (2012). https://doi.org/10.1007/s00417-011-1912-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-011-1912-5