Abstract
Purpose
The aim of this study is to image and describe the in vivo choroidal changes in various retinal dystrophies using the technique of enhanced depth imaging (EDI) optical coherence tomography (OCT) and to correlate these findings with the clinical appearance. Associations between choroidal change and genotype, visual acuity and results of retinal electrophysiology are also explored.
Design
Retrospective observational case series.
Methods
Twenty patients attending the medical retina clinics at Moorfields Eye Hospital underwent EDI OCT choroidal scans as part of the scanning protocol when they underwent OCT imaging with the Spectralis HRA and OCT. The choroidal images were obtained by moving the Spectralis camera close enough to obtain an inverted image of the retina. The scans were read by two experienced OCT readers assessing the choroidal thickness as well as the choroidal contour for focal areas of choroidal thinning corresponding to the areas of RPE/outer retinal atrophy. The spectrum of patients included those with Stargardt macular dystrophy, macular dystrophies secondary to known mutations such as peripherin/RDS, uncharacterised macular dystrophies, Best disease, bifocal chorioretinal atrophy, Bietti crystalline retinal dystrophy and choroideraemia.
Results
The choroidal appearance was symmetrical in all patients who had both eyes scanned. Ten patients showed no choroidal thinning, five had focal mild to moderate choroidal thinning, three had focal severe choroidal thinning, and two patients had diffuse severe choroidal thinning. There was no association between choroidal thinning and visual acuity [Fisher’s exact test, p = 0.350 (right eye), p = 1.000 (left eye)], or extent of retinal dysfunction on electrophysiology (Fisher’s exact test, p = 1.000).
Conclusion
Enhanced depth imaging using spectral domain OCT can be used to identify choroidal changes in inherited retinal disease. The pattern of choroidal change correlates well with the clinical appearance. It appears that the extent and pattern of choroidal thinning is dependent on the stage of the disease in some cases, and in others the causative gene defect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inherited retinal disorders primarily affect the outer retina and retinal pigment epithelium (RPE), resulting in eventual photoreceptor and RPE cell death. In the late stage of disease, RPE and photoreceptor cell death may be associated with variable loss of the underlying choriocapillaris, visible clinically as a well-defined area of chorioretinal atrophy [1]. Several histopathological studies have confirmed loss of photoreceptors, RPE and choriocapillaris localised to this area of clinically apparent atrophy [2–4]. In other inherited retinal dystrophies, however, a more diffuse process of choroidal atrophy occurs [5, 6].
The mechanism of choriocapillaris atrophy is unclear, but appears to be related to RPE cell death. In animal models, loss of the RPE results in subsequent atrophy of the choriocapillaris [7, 8]. RPE-derived vascular endothelial growth factor (VEGF) is essential for the maintenance of the choriocapillaris [9], and it is plausible that reduced levels of VEGF following RPE cell death lead to choriocapillaris atrophy.
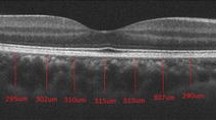
Despite extensive clinicopathological descriptions of the RPE and neuroretinal changes in inherited retinal disease [10–12], there is virtually no similar analysis of the corresponding in vivo choroidal changes. Reliable techniques for in vivo choroidal imaging are now available [13–15]. Enhanced depth imaging (EDI) is a technique employed to image the choroid by moving the Spectralis spectral domain optical coherence tomography (OCT) device closer to the eye such that an inverted image is obtained [13, 14]. This places the deeper structures closer to zero delay from the Fourier transformation, hence the acquired images have an improved ability to visualise the choroid, allowing the thickness and contour of the choroid to be assessed. The appearance of the choroid on EDI OCT in normal eyes is that of a smooth outer contour convex in shape, and thickest in the subfoveal or parafoveal region [13]. An example of this is shown in Fig. 1.
a EDI OCT of the choroid in a normal eye. The outer choroidal contour demarcated by the white arrows has a smooth convex contour. The choroid is thickest in the subfoveal region. b The graph depicts a plot of choroidal thickness across the full length of the OCT section, plotted using manual segmentation lines of the proprietary software. The vertical black line in the graph shows the subfoveal choroidal thickness in the location of the white arrowhead in a to be 317 microns
The aim of this study is to image and describe the in vivo choroidal changes in various retinal dystrophies using the technique of EDI OCT, and to correlate these findings with the clinical appearance. The symmetry between eyes and possible associations with genotype, visual acuity, and electrophysiological findings are also examined.
Methods
This study is a retrospective observational case series approved by the research governance committee at Moorfields Eye Hospital. The scans of 20 patients attending the medical retina clinics at the hospital who underwent EDI OCT choroidal scans with the Spectralis HRA and OCT (Heidelberg Engineering, Heidelberg, Germany) as part of the scanning protocol were retrospectively reviewed. These patients attended clinics between March and November 2009. Three groups of patients were selected based on the clinical appearance:
-
1)
patients with mottled outer retinal and retinal pigment epithelial atrophy
-
2)
patients with discrete outer retinal, RPE and choriocapillaris atrophy
-
3)
patients with diffuse chorioretinal atrophy.
Eighteen patients had both eyes scanned; two patients who had previously had complex retinal surgery involving the use of silicone oil in one eye had scanning data only for the fellow eye.
The scans were read by two experienced OCT readers and the outer choroidal contour identified. Steps in the choroidal contour representing focal areas of thinning were recorded, and in cases of focal choroidal thinning, two measurements of choroidal thickness were made with the manual calipers provided by the planimetric software of the proprietary device. The first measurement was of the choroidal thickness in the area of focal thinning, and the second measurement was of the choroidal thickness in the adjacent area of thicker (normal) choroid. In eyes where there was no focal area of thinning in the choroidal contour, measurements of the subfoveal choroidal thickness were made.
The absence or presence of choroidal thinning was correlated with the clinical appearance of the infrared fundus photographs captured simultaneously with the choroidal tomograms. The symmetry of the choroidal changes between the two eyes was assessed. Genotype, visual acuity, and results of retinal electrophysiology were also reviewed.
Statistical methods
The difference in choroidal thickness measurements between the two eyes was compared using the paired t-test and the relationship between visual acuity, extent of electrophysiological changes and presence of choroidal thinning was analysed using the Fisher’s exact test.
Results
A summary of the choroidal changes, clinical features and diagnoses of the patients scanned is provided in Table 1.
In ten patients, there was no evidence on EDI OCT of any choroidal thinning under the area where the maculopathy was evident, relative to the adjacent choroid in non-affected areas. Nine of these patients had the phenotype of mottled outer retinal and RPE atrophy with no clinically evident choroidal atrophy. Of these, three had Stargardt macular dystrophy, four had uncharacterised macular dystrophies and two had rod cone dystrophy, one of which was due to an MERTK mutation. The three patients with Stargardt macular dystrophy had early stage disease, with the clinical picture of mottled outer retinal and RPE atrophy with flecks (Fig. 2, patient 2), whilst the four patients with uncharacterised macular dystrophy had the clinical appearance of mottled outer retinal and RPE atrophy without flecks. One patient with an uncharacterised macular dystrophy had markedly thickened choroid measuring 545 and 523 microns in the right and left eye respectively on EDI (patient 7.). The remaining patient had Best disease in the vitelliruptive stage.
a (patient 2) Autofluorescence imaging of the right macula in a patient with Stargardt macular dystrophy showing mottled outer retinal and RPE atrophy with flecks. There is speckled hyperfluorescence centrally with a surrounding ring of hyperfluorescence. b Corresponding EDI OCT choroidal scan of the region demarcated by the line in a shows no areas of focal choroidal thinning. The white arrows delineate the external choroidal margins. The black arrows demarcate the zone of outer retinal atrophy centrally. Similar changes were observed in the other eye. c The graph depicts the plot of choroidal thickness across the full length of the OCT section, plotted using manual segmentation lines of the proprietary software. The vertical black line in the graph shows the subfoveal choroidal thickness in the location of the white arrowhead in b to be 380 microns
Five patients had focal areas of mild to moderate choroidal thinning on EDI OCT, which corresponded with the clinically visible areas of discrete outer retinal, RPE and choriocapillaris atrophy. Of these five patients, two had Stargardt macular dystrophy in the later stage of disease, one had a macular dystrophy secondary to the peripherin/RDS (R172W) mutation, and two had uncharacterised macular dystrophies (Fig. 3, patient 14). The degree of thinning in three of these cases of focal thinning was estimated to be between 10% and 15% of the adjacent choroidal thickness.
a (patient 14) Uncharacterised macular dystrophy with discrete patches of outer retinal and RPE atrophy at the right macula. Similar changes were observed in the left eye. b The EDI OCT choroidal scan shows focal mild choroidal thinning visible under the area of chorioretinal atrophy. The white arrowheads delineate the zone of outer retinal atrophy. c The graph depicts a plot of choroidal thickness across the full length of the OCT section, plotted using manual segmentation lines of the proprietary device. The choroidal thickness in the region of focal choroidal thinning measured 220 microns compared with 247 microns in the region of the adjacent thicker choroid, as indicated by the vertical black lines in Fig. 3c
Three patients had focal areas of severe choroidal thinning under the area of dystrophy relative to the adjacent choroid. The clinical appearance was that of focal chorioretinal atrophy involving the medium-sized choroidal vessels. Of these three patients, one had macular atrophy associated with Stargardt disease (Fig. 4, patient 17), one had progressive bifocal chorioretinal atrophy, and one patient had an uncharacterised macular dystrophy with an excavated chorioretinal pigmented scar.
a (patient 17) Infrared photograph of the left macula in a patient with late-stage Stargardt disease showing a discrete area of marked chorioretinal atrophy involving the medium-sized choroidal vessels. Symmetrical changes were observed in the right eye. b The vertical EDI OCT section through the subfoveal choroid shows focal severe choroidal thinning. The yellow arrowed line shows that the large choroidal vessel visible on the infrared photo is visible on EDI OCT as a large vacuole. c Magnified view of the choroidal section with arrows delineating the choroidal margins. The choroidal thickness between the black arrows was 70 microns and 209 microns between the white arrows. The white arrowhead points to the start of the zone of outer retinal atrophy. d The graph depicts a plot of choroidal thickness across the full length of the OCT section, plotted using manual segmentation lines of the proprietary device. The vertical black lines in the graph show the choroidal thicknesses measured at the locations of the black and white arrows in c
The patients with Bietti crystalline retinal dystrophy (Fig. 5, patient 19) and choroideraemia both had diffuse severe choroidal thinning with the subfoveal choroidal thickness measuring less than 100 microns in each eye.
a (patient 19) Color fundus photograph of the left eye in a patient with Bietti crystalline retinal dystrophy showing widespread RPE atrophy and hyper-reflective crystals. b The corresponding EDI OCT choroidal scan shows diffuse severe choroidal thinning (choroidal margins are delineated by arrows). The subfoveal choroidal thickness was measured to be 60 microns. c The graph depicts a plot of choroidal thickness across the full length of the OCT section, plotted using manual segmentation lines of the proprietary device. The vertical black line in the graph shows the subfoveal choroidal thickness in Fig. 5b to be 60 microns
The mean (standard deviation) subfoveal choroidal thickness in patients with no choroidal thinning was 317 (89) microns and 312 (86) microns in the right and left eyes respectively (Table 2). The difference in the subfoveal choroidal thicknesses between the two eyes was not statistically significant (paired t-test, p = 0.07). In eyes where there was focal mild–moderate or severe choroidal thinning, the change in choroidal thickness between the region of focal thinning and the adjacent thicker (normal) choroid was symmetrical between the two eyes (Table 2). In these eyes, the difference between eyes was not statistically significant when comparing the regions of focal choroidal thinning (paired t-test, p = 0.39) as well as the regions of adjacent thicker (normal) choroid (paired t-test, p = 0.40).
There was no association between choroidal thinning and visual acuity [Fisher’s exact test, p = 0.350 (right eye), p = 1.000 (left eye)]or extent of retinal dysfunction on electrophysiology (Fisher’s exact test, p = 1.000) (Tables 3 & 4 in Appendix)
Discussion
EDI OCT of the choroid in the various retinal dystrophies imaged in this study demonstrates that the choroid can be variably affected. We observed four main patterns of choroidal involvement:
-
1)
No choroidal involvement apparent whereby the outer choroidal contour is smooth without any steps representing areas of focal thinning (Fig. 2).
-
2)
Focal mild to moderate choroidal thinning under the area of clinically apparent discrete outer retinal, RPE and choriocapillaris atrophy (Fig 3). We defined focal mild to moderate choroidal thinning as choroidal thinning of up to 50% when compared to adjacent thicker normal choroid.
-
3)
Focal severe choroidal thinning under the area of clinically apparent focal chorioretinal atrophy involving the medium-sized choroidal vessels (Fig. 4) defined as choroidal thinning of greater than 50% when compared to adjacent thicker normal choroid.
-
4)
Diffuse choroidal thinning (Fig. 5) defined as a smooth outer choroidal contour with the subfoveal choroidal thickness measuring less than 135 microns, 135 microns being 2 standard deviations from the mean choroidal thickness in normal subjects as described by Margolis and Spaide [13].
We observed that eyes with the clinical appearance of mottled RPE and outer retinal atrophy showed no choroidal thinning on the choroidal scans. The mean (SD) subfoveal choroidal thicknesses in these eyes were 317(89) and 312 (86) microns in the right and left eyes respectively. The subfoveal choroidal thickness in these eyes could be considered within the normal range, given two recent studies which found the mean (SD) subfoveal choroidal thickness in normal eyes to be 287 (76)[13] and 354 (111) microns[16].
Of the ten cases which showed no evidence of choroidal thinning on EDI OCT, three patients had Stargardt macular dystrophy where there were flecks at the macula and mottled outer retinal and RPE atrophy (Fig. 2), and four other patients had uncharacterised macular dystrophies with a similar appearance but without the flecks.
Conversely, in eyes with the clinical appearance of discrete, well-circumscribed areas of chorioretinal atrophy, there was a corresponding focal area of choroidal thinning on EDI OCT. The extent of focal choroidal thinning varied from mild–moderate to severe. Two patients in the late stages of Stargardt macular dystrophy and three patients with non-Stargardt macular dystrophy, had clinically apparent areas of discrete outer retinal, RPE and choriocapillaris atrophy at the macula all of whom had focal mild to moderate thinning of the choroid on EDI OCT under the area of outer retinal and RPE atrophy (Fig. 3).
In keeping with this observation, focal severe choroidal thinning was detected on EDI OCT in three patients under the area of discrete chorioretinal atrophy which clinically involved the medium-sized choroidal vessels (Fig. 4). Choroidal imaging showed marked thinning of the choroid, with only a residual layer of large choroidal vessels remaining under the area of atrophy suggesting that there is loss of both choriocapillaris and medium-sized choroidal vessels.
The pattern of choroidal change was found to be symmetrical between the two eyes in all patients. This is consistent with the fact that retinal dystrophies are known to affect both eyes in symmetrical fashion.
It is unclear what the observed area of focal choroidal thinning on EDI OCT corresponds to anatomically, given that the resolution afforded by EDI OCT of the choroid is insufficient to resolve the choriocapillaris. One histological study has estimated choriocapillaris thickness to be between 6.5 to 9.8 microns and choroidal thickness to be between 84 and 193 microns [17]. The choriocapillaris should therefore range between 5 and 10 % of total choroidal thickness. High-resolution OCT imaging of the choroid by Povazay et al. has shown the choriocapillaris to represent approximately 10% of choroidal thickness [15]. In Fig. 3, the choroid appears thinner in the area of chorioretinal atrophy by up to 10% when compared with the adjacent choroid, with preservation of the larger choroidal vessels.
It is therefore possible, that the mild focal choroidal thinning under the area of chorioretinal atrophy in some cases represents choriocapillaris atrophy whilst moderate focal choroidal thinning represents an extension of this atrophic process to involve the medium sized choroidal vessels. Severe focal thinning on the other hand, may represent loss, or failure of development of the choroidal vasculature as is the case of patient 18 who had progressive bifocal chorioretinal atrophy. Bifocal chorioretinal atrophy is an autosomal dominant macular dystrophy linked to chromosome 6q where marked chorioretinal atrophy resembling staphylomas develop at an early age [18].
Factors related to the primary genetic mutation are likely to play a role regarding the extent and pattern of choroidal atrophy present in certain dystrophies such as Bietti crystalline retinal dystrophy and choroideraemia which are progressive, clinically involve the choroid, and ultimately result in severe visual loss [5, 6, 19]. These patients in our study had severely thinned choroids, measuring less than 100 microns in each eye (Fig. 5). Although there is relatively little normative data available for normal in vivo choroidal thickness, Margolis and Spaide reported the average subfoveal choroidal thickness in 30 normal subjects to be 287 (standard deviation ± 76) microns [13]. A subfoveal choroidal thickness measurement of 100 microns would therefore lie more than 2 standard deviations outside this range, and therefore might be regarded as being pathologically thinned. In contrast, the two patients with generalised rod/cone dystrophy did not show any evidence of choroidal thinning.
Although genetic factors play a role in determining the degree of choroidal atrophy present in some dystrophies, our study shows that the stage of disease is also likely to be an important variable. Our patients with Stargardt macular dystrophy showed choroidal involvement, varying from no involvement to focal severe thinning. The pathogenesis of Stargardt disease involves the abnormal deposition of lipofuscin in the RPE followed by RPE degeneration and finally photoreceptor cell death [20]. It is likely that choriocapillaris atrophy occurs at a late stage of this disease process when there is significant RPE cell loss [1]. Significantly, RPE cell death in animal models of disease leads to secondary choriocapillaris atrophy [7–9]. Further longitudinal studies of choroidal imaging in patients with known genetic mutations are needed to clarify the relative roles of genetic factors and non-specific RPE cell death in the pathogenesis of choroidal thinning.
In our study, there was no relationship between choroidal thinning and visual acuity, or extent of retinal dysfunction on electrophysiology. To demonstrate an association between choroidal change and these parameters, a larger population of one disease entity may be required. Nevertheless, this study provides a novel classification of the in vivo choroidal changes in inherited retinal disease, setting a framework for future investigation.
Conclusion
Enhanced depth imaging using spectral domain OCT can be used to identify choroidal changes in inherited retinal disease. The pattern of choroidal change correlates well with the clinical appearance and is also symmetrical between eyes. It appears that the extent and pattern of choroidal thinning is dependent on the stage of the disease in some cases, and in others the causative gene defect. Focal mild to moderate choroidal thinning may represent choriocapillaris atrophy secondary to RPE cell death, whereas more severe thinning could be due to genetic factors causing atrophy of the larger-sized choroidal vessels.
References
Oh KT, Weleber R, Stone E, Oh D, Rosenow J, Billingslea A (2004) Electroretinographic findings in patients with Stargardt disease and fundus flavimaculatus. Retina 24(6):920–928
Ashton N (1953) Central areolar choroidal sclerosis; a histopathological study. Br J Ophthalmol 37:140–147
Ferry AP, Llovera I, Shafer DM (1972) Central areolar choroidal dystrophy. Arch Ophthalmol 88:39–43
Voo I, Glasgow BJ, Flannery J, Udar N, Small KW (2001) North Carolina Macular Dystrophy: clinicopathological correlation. Am J Ophthalmol 132(6):933–935
Mura M, Sereda C, Jablonski MM, MacDonald IM, Iannaccone A (2007) Clinical and functional findings in choroideraemia due to complete deletion of the CHM gene. Arch Ophthalmol 125(8):1107–1113
Ayata A, Tatlipinar S, Unal M, et Ersanli D, Bilge AH (2008) Autofluorescence and OCT features of Bietti’s crystalline dystrophy. Br J Ophthalmol 92:718–720
Korte GE, Reppucci V, Henkind P (1984) RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci 25:1135–1145
Neuhardt TH, May CA, Wilsch C, Eichorn M, Lutjen-Drecoll E (1999) Morphological changes of retinal pigment epithelium and choroid in mice. Exp Eye Res 68:75–83
Saint-Geniez N, Kurihara T, Sekiyama E, Maldonado A, D’Amore P (2009) An essential role for RPE-derived soluble VEGF in the maintainance of the choriocapillaris. Proc Natl Acad Sci U S A 106(44):18751–18756, Epub
Wirtitsch MG, Ergun E, Hermann B, Unterhuber A, Stur M, Schloda C, Sattman HH, Ko TH, Fujimoto JG, Drexler W (2005) Ultrahigh resolution optical coherence tomography in macular dystrophy. Am J Ophthalmol 140(6):976–983
Ergun E, Hermann B, Wirtitsch M, Unterhuber A, Ko TH, Sattmann H, Scholda C, Fujimoto JG, Stur M, Drexler W (2005) Assessment of central visual function in Stargardt’s disease/fundus flavimaculatus with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci 46:310–316
Lim JL, Tan O, Fawzi AA, Hopkins JJ, Gil-Flamer JH, Huang D (2008) A pilot study of Fourier-domain optical coherence tomography of retinal dystrophy patients. Am J Ophthalmol 146(3):417–426
Margolis R, Spaide RF (2009) A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol 147(5):811–815
Spaide RF, Koizumi H, Pozzoni MC (2008) Enhanced depth imaging spectral domain optical coherence tomography. Am J Ophthalmol 146(4):496–500
Povazay B, Hermann B, Hofer B, Kajic V, Simpson E, Bridgford T, Drexler W (2009) Wide-field optical coherence tomography of the choroid in vivo. Invest Ophthalmol Vis Sci 50:1856–1863
Ikuno Y, Kawaguchi K, Nouchi T, Yasuno Y (2010) Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci 51:2173–2176
Ramrattan R, Van Der Schaft T, Mooy C, Bruijin W, Mulder P, de Jong P (1994) Morphometric analysis of Bruch’s membrane, the choriocapillaris and the choroid in aging. Invest Ophthalmol Vis Sci 35:2857–2864
Godley BF, Tiffin PA, Evans K, Kelsell RE, Hunt DM, Bird AC (1996) Cinical features of progressive bifocal chorioretinal atrophy: a retinal dystrophy linked to chromosome 6q. Ophthalmology 103(6):893–898
MacDonald I, Russell L, Chi-Chao C (2009) Choroideraemia: new findings from ocular pathology and review of recent literature. Surv Ophthalmol 54:401–407
Cideciyan AV, Aleman TS, Swinder M et al (2004) Mutations in ABCA4 result in accumulation of lipofuschin before slowing of the retinoid cycle. A reappraisal of the human disease sequence. Hum Mol Genet 13:525–534
Acknowledgments
We would like to acknowledge Ms Genevieve Wright (genetics co-ordinator) and Ms Sophie Devery (genetics counsellor), Moorfields Eye Hospital for their help in obtaining molecular diagnoses of the patients from the database.
Funding
NIHR Biomedical Research Centre, Moorfields Eye Hospital and UCL Institute of Ophthalmology
Financial Disclosures
The Authors report no financial interests in the Spectralis HRA and OCT device used to obtain choroidal scans in the study
Study Approval
The study was approved by the Moorfields Eye Hospital Clinical Governance Committee (Approval Number: YEOHJ1002)
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Yeoh, J., Rahman, W., Chen, F. et al. Choroidal imaging in inherited retinal disease using the technique of enhanced depth imaging optical coherence tomography. Graefes Arch Clin Exp Ophthalmol 248, 1719–1728 (2010). https://doi.org/10.1007/s00417-010-1437-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1437-3