Abstract
Background
Automatically measurements of retinal thickness by optical coherence tomography (OCT) facilitate the assessment of various retinal diseases.The aim of this retrospective study was to report macular thickness measurements in eyes with vascular pigment epithelial detachment (PED) due to age-related macular degeneration (AMD) by using two different commercially available spectral domain (SD) OCT instruments and to consequently point out differences in their algorithm software.systems.
Methods
OCT images of patients with vascular PED due to AMD, obtained with Cirrus and Spectralis OCT, were retrospectively analyzed. Main objectives were to observe differences in central retinal thickness (CRT) values and failures in automated threshold delineation, as well as central point thickness values obtained after manual correction of threshold lines. Scanning with the Cirrus HD OCT was performed with the 512 × 128 scan pattern; scans performed with the Spectralis OCT were 20 × 15 degree raster scans consisting of 19 high-speed line scans.
Results
OCT images of 34 eyes of 28 patients with a mean age of 71 years and a mean distance visual acuity (VA) of 0.70 ETDRS were analyzed. Mean central retinal thickness (CRT) was 262.38 μm ± 133.18 (176–507 μm) in Cirrus and 337.82 μm ± 137.75 (277–790 μm) in Spectralis scans,mainly caused by different software approaches in positioning the posterior threshold line, following the PED in Cirrus OCT whereas remaining unelevated in Spectralis OCT. There were failures in positioning the outer retinal boundary line in 50% of Cirrus scans and in 73.52% of Spectralis scans. We obtained the mean value of central point neurosensory retinal thickness of each central single scan after manual delineation, and found a significant correlation (r = 0.819, p < 0.001).
Conclusions
Our study indicates that there are significant differences in CRT values in patients with vascular PED, due to different segmentation algorithms and a high error rate in automatically set threshold lines. When planning and conducting multicenter studies, one has to be especially aware of the differences in delineating threshold algorithm lines by different SD OCT devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Optical coherence tomography (OCT) was first introduced in 1991 as an imaging technique, providing cross-sectional images of the retina [1]; due to its easy handling, this non-invasive and non-contact technique got established into clinical ophthalmology in the mid-nineties [2]. Nowadays, evaluating retinal morphology by OCT is commonly used for detection and monitoring of diseases such as epiretinal membranes, macular holes and age-related macular degeneration (AMD), enabling objective disease progression and response to therapy. [3].
Contrary to fluorescein angiography (FA), where the exact amount of fluid accumulation can only be estimated, which makes the entire procedure subjective, changes in retinal morphology and values of retinal thickness (RT) obtained by OCT are easily comparable to values of previous measurement data [4].
The reliability of such change analysis depends on the accuracy of segmentation software.
For years, there has been only one time domain (TD) OCT software system available for clinical use (Stratus OCT, Carl Zeiss Meditec).
Improvements in OCT technology have been introduced in the recent past, and OCT software systems from different manufacturers using SD technology have now entered the market. In comparison to conventional TD technology, SD-OCT uses a stationary reference arm to obtain an interference spectrum, which then undergoes Fourier transformation to allow for simultaneous measurement of all the echo time delays of light, leading to significant improvements in system speed and sensitivity [5].
SD-OCT devices feature a greater resolution, which translates into enhanced capability to observe fine ocular pathologic features [6].
Although much progress has been made in improving the accuracy of segmentation, segmentation errors still occur in both TD- and SD-OCT instruments [7–10].
Various studies compare the performances of RT analysis between TD-OCT systems and SD-OCT systems with each other [11, 12], whereas studies evaluating the RT measurements obtained by SD-OCT devices from different manufacturers are still rare [13–15].
The present study was performed to investigate the comparability of retinal thickness measurements, and to assess the segmentation performance of current SD-OCT devices from two different manufacturers in patients with fibrovascular PED.
We decided to put the study’s focus on fibrovascular PED, as this is a very common clinical condition in exudative AMD with a rather uniform phenotype.
The results of this study will provide information on how OCT macular measurements by two different software systems can be compared, and will further our understanding of how different systems of data capture and analysis in OCT systems can influence the measurements.
Material and methods
The two spectral-domain OCT scanners evaluated in this retrospective study were the Cirrus HD-OCT (Carl Zeiss Meditec, Dublin, CA, USA) and the Spectralis OCT (Heidelberg Engineering, Inc., Heidelberg, Germany).
OCT images of eyes of patients examined between September 2008 and December 2008 with a single Cirrus OCT machine and a single Spectralis OCT machine presenting with fibrovascular PED verified by FA were included, and retrospectively evaluated.
All patients had imaging performed on both scans on the same day in random order, after visual acuity testing with ETDRS (early treatment diabetic retinopathy) charts at a distance of 4 m and pupil dilation with one drop of Mydriaticum (Agepha Pharmaceuticals, agent:Tropicamid); a minimum diameter of pupil was not defined.
All patients had given consent to OCT imaging as part of their routinely clinical care; examinations with both devices were performed to get experience with the newly acquired Spectralis OCT.
Scanning with the Cirrus HD-OCT was performed with the 512 × 128 scan pattern, where a 6 × 6 mm area on the retina is scanned with 128 horizontal lines, each consisting of 512 A scans per line; scans performed with the Spectralis OCT were 20 × 15 degree raster scans consisting of 19 high-speed line scans.
Scans performed by Cirrus OCT had to reach at least 5 out of 10 points at the quality score, Spectralis OCT scans at least 20 out of 40 possible points to become included in this retrospective study.
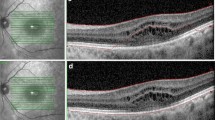
Both OCT systems provide an internal software algorithm for retinal thickness measurements, and both SD-OCT devices declare the inner limiting membrane (ILM) as inner retinal boundary. Cirrus OCT segmentation software sets the outer retinal threshold line above the third hyperreflective band of the RPE–choriocapillaris complex, whereas Spectralis sets it beyond the third hyperreflective band, referring to it as Bruch’s membrane ( see Fig. 1a,b and 2a,b)
Each single scan was separately analyzed in respect of the correct position of the inner and outer threshold line, by one independent examiner (ES), who was not involved in the scanning process.The results were confirmed 100% by a second opinion (IK).
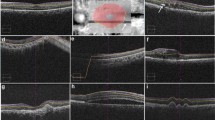
All single B scans, used for calculating the CRT (28 single scans in Cirrus OCT; three single scans in Spectralis OCT) were individually analyzed and classified into one of three consistent categories in increasing error severity. Errors in length scan were graded as negligible (≤50 μm), moderate (50-200 μm) and severe (≥200 μm) errors, and errors in width scan as negligible (≤0.5 mm), moderate (0.5-2 mm) and severe (≥2 mm) [15]. The values for length and width were selected based on our clinical opinion (ES and IK) without statistical meaning. The greatest error present in a single B-scan determined the classification of the error type in this scan.
Furthermore, at each central single scan (scan number 64 in Cirrus OCT and scan number 10 in Spectralis OCT), manual delineating of the threshold lines was performed to obtain the mean central point thickness of the neurosensory retina of both devices.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) (Chicago, IL, USA).
The CRT values of Cirrus OCT and Spectralis OCT were compared by using SPSS t-test. To investigate a linear correlation between the neurosensory retinal thickness values of Cirrus and Spectralis OCT, the Pearson correlation coefficient was used.
Results
OCT scans of 34 eyes, showing fibrovascular PED due to exudative AMD of 28 patients were included.
Patients´ characteristics are shown in Table 1.
The surface of the retina, represented by ILM, was correctly detected in 30 eyes (88.23%) by Cirrus and in 34 (100%) eyes by Spectralis.
An average of 2.8 ± 1.1 (range 0 - 49) single scan lines were affected by failures in correctly delineating the inner threshold line in Cirrus OCT; the average number of misdetected single scans in the four affected eyes is 23.8.
The evaluation of Cirrus scan cubes showed failures in finding the correct position of the lines at the outer threshold border in 17 (50%) of all examined eyes. An average of 12.04 scan lines of 128 scans per eye (9.41%) was affected by failures in finding the correct position.
The surface of the outer retinal boundary was not correctly detected and positioned by the threshold algorithm in 25 out of 34 eyes (73.52%) in scans done by Spectralis OCT.
Inaccurate setting affected 6.03 (31.76%) single scans out of a total of 19 scans for each examination.
The average CRT was 262.38 μm ± 133.18 (176–507 μm) in Cirrus and 337.82 μm ± 137.75 (277–790 μm) in Spectralis scans (p < 0.001).
The quality of positioning of the lines was significantly correlated to distance visual acuity neither in Cirrus (p = 0.084) nor in Spectralis (p = 0.077).
The reasons for algorithm failures were analyzed by two independent examiners (ES and IK) in 100% concordance:
-
1.
associated with the morphology of the RPE due to the AMD in 88.06% of Cirrus scans and 81.49% of Spectralis scans.
-
2.
based on impeded scanning conditions, due to cataract, vitreal or corneal opacities in 11.94% of Cirrus and 19.51% of Spectralis scans.
All central single B scans of both SD-OCT devices were analysed for correct segmentation, respectively identifying the threshold algorithm lines, finding 8.8% severe and 8.8% moderate errors in Cirrus and 8.8% severe and 21.23% moderate segmentation errors in Spectralis (see Fig. 3a, b).
We obtained the mean value of central point neurosensory retinal thickness of each central single scan by manually delineation, respectively setting the inner threshold line on the ILM and the outer threshold line right above the third hyperreflective band of the RPE–choriocapillary complex.
A significant correlation could be shown, with a mean central point neurosensory retinal thickness of 271.22 μm ± 82.63 (176–507 μm) in Cirrus images and 278.41 ± 92.31 (247–524 μm) in Spectralis images. (r = 0.819, p < 0.001) (Fig. 4).
Discussion
This study was performed to investigate the comparability of retinal thickness measurements, and to assess the segmentation performance of current SD-OCT devices from two different manufacturers in patients with fibrovascular PED in exudative AMD.
Retinal thickness measurement analysis is playing an increasingly important clinical role for tracking treatment outcomes in patients with neovascular AMD.
There is a multitude of SD-OCT devices on the market, but still very little literature about the comparability of the different threshold algorithm softwares, especially in patients with severe morphological changes in the retina and RPE.
Wolf-Schnurrbusch et al. [16] compared measurements of the central retinal thickness with six different OCT devices in healthy subjects, and found similar mean CRT values in Spectralis (289 μm) and Cirrus OCT (277 μm). These values were significantly higher than in all other evaluated instruments (p < 0.01).
Mylonas et al. [15] compared retinal thickness measurements of four different SD and TD OCT devices in patients with neovascular AMD, finding highest values of central millimeter thickness (CMMT) in Spectralis SD OCT and significant correlation between CMMT measurements of Cirrus (327.25 μm) and Spectralis (383.54 μm) (r = 0.87).
In contrast to the results of the studies mentioned earlier, we found that the tested OCT systems provide statistically significant different values for central retinal thickness in patients with vascular PED, with a mean CRT of 262.38 μm in Cirrus and 337.82 μm in Spectralis believed to be mainly caused by different retinal segmentation algorithms used by these evaluated OCT softwares: Although both systems perform automated delineations of retinal boundaries, each system uses different anatomic landmarks in the specification of the outer retinal boundary, which makes the posterior line follow the PED in Cirrus OCT whereas it remains unelevated in Spectralis OCT, which leads to predictably different CRT values. Clinicians should be aware of the differences in retinal thickness measurements, especially in routine clinical care of patients and in conducting multicenter studies.
These different performances in segmentation line position mentioned above have resulted in particular attention being paid to controversial opinions as to whether PED is a sign of activity in exudative AMD or not [3, 4]. Both segmentation softwares have reasonable approaches: Cirrus OCT software measures the neurosensory retina only when calculating the CRT and does not detect the PED itself, whereas Spectralis OCT software accounts for both PED and neurosensory retina, which leads to significant differences of the CRT even when both programs set the threshold lines correctly. In this regard, if one decides to include the PED in the measurement of CRT during follow up, a manual measurement should be preferred.
Reasons for failures were merely disease-related, as the segmentation software systems of the OCT instruments seemed to be compromised by the hemorrhagic and highly reflective nature of the PED, which makes the underlying RPE line less detectable. In our present study, we found lack of precision in positioning the outer retinal boundary line in 50% (n = 17) of all Cirrus scans and in 73.52% (n = 25) of all Spectralis scans. The average number of single lines affected by misdetection of the outer retinal boundary was 12.04 (9.41%) in Cirrus and 6.03 (31.76%) in Spectralis.
Krebs et al. [11] performed a study including 104 patients with exudative AMD, and found an overall failure rate of 25% in Cirrus OCT.
Of these algorithm line failures, 37.5% were associated with misidentification of the posterior border.
Ahlers et al. [8] reported on 22 eyes with fibrovascular RPE detachment, and found significant errors in 27.35% and limited quality in 9.89% of all OCT scans performed with a prototype of Cirrus OCT. Mylonas et al. [15] found 2% of severe and 4% of moderate segmentation errors in Cirrus OCT, and 2% of severe and 25% of moderate segmentation errors in Spectralis OCT, in eyes affected by neovascular AMD.
In our study, we found no correlation between VA and the rate of algorithm failures, neither in Cirrus nor in Spectralis OCT images. Nevertheless, although we found no correlation, fixation problems arising due to reduced VA may lead to a lower scan quality based on a more difficult examination [16].
Another focus of interest was the manual adjustment of the inner and outer boundary lines of the central scan. By doing so, we obtained CRT central point thickness values of the neurosensory retina that were comparable and highly correlating, with a mean central point neurosensory retinal thickness of 271.22 μm in Cirrus images and 278.41 in Spectralis images.We are aware that manual correction of threshold lines adds some subjectivity, but again, in the presence of PED, it should be given preference to automatically acquired values. [10].
This study highlights the problems of segmentation errors in Cirrus and Spectralis OCT scans of eyes with vascular PED in patients with AMD.
A limiting factor of this study was its retrospective nature and the small number of evaluated OCT scans.
In our study, in contrast to the cube scan with 128 single scans performed by the Cirrus OCT, only 19 scans were performed in Spectralis OCT.
Spectralis OCT has the option to increase B scan density further for even better retinal coverage; however, higher scan density leads to longer examination time.
Therefore, 19 B scans were used as a compromise between sufficient retinal coverage and acceptable examination duration, since these examinations were part of a control check-up of AMD patients.
When carrying out the OCT examination, the main focus was put on setting the center of the scanned area to the fovea, but using different OCT systems the scan lines could not be superimposed, which made a point-to-point registration impossible.
In future prospective studies dealing with comparing SD-OCT devices, one would increase the number of B scans to obtain more detailed information with less need of data interpolation, since segmentation errors can influence the final result negatively because most of the area is interpolated between the single scans.
Results obtained from this study can be generalized only to this subset of patients.
This study concentrated on two different SD-OCT devices, and found significant differences in segmentation algorithm. Further investigations are necessary to see if the retinal thickness values of other SD-OCT devices lead to similar results in eyes with fibrovascular PED due to exudative AMD.
A general awareness of different algorithm software systems should be raised amongst those using OCT for patient assessment and follow-up. Measurements obtained from different SD OCT systems may not be used interchangeably with each other.
References
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA et al (1991) Optical coherence tomography. Science 254(5035):1178–1181
Puliafito CA, Hee MR, Lin CP, Reichel E, Schuman JS, Duker JS, Izatt JA, Swanson EA, Fujimoto JG (1995) Imaging of macular diseases with optical coherence tomography. Ophthalmology 102(2):217–229
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, Puliafito CA, Davis JL, Flynn HW Jr, Esquiabro M (2007) An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 143(4):566–583
Krebs I, Ansari-Shahrezaei S, Goll A, Binder S (2008) Activity of neovascular lesions treated with bevacizumab: comparison between optical coherence tomography and fluorescein angiography. Graefes Arch Clin Exp Ophthalmol 246(6):811–815, Epub 2008 Jan 15
Choma M, Sarunic M, Yang C, Izatt J (2003) Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express 11(18):2183–2189
Wojtkowski M, Leitgeb R, Kowalczyk A, Bajraszewski T, Fercher AF (2002) In vivo human retinal imaging by Fourier domain optical coherence tomography. J Biomed Opt 7(3):457–463
Krebs I, Haas P, Zeiler F, Binder S (2008) Optical coherence tomography: limits of the retinal-mapping program in age-related macular degeneration. Br J Ophthalmol 92:933–935
Ahlers C, Simader C, Geitzenauer W, Stock G, Stetson P, Dastmalchi S, Schmidt-Erfurth U (2008) Automatic segmentation in three-dimensional analysis of fibrovascular pigment epithelial detachment using high-definition optical coherence tomography. Br J Ophthalmol 92(2):197–203, Epub 2007 Oct 26
Patel PJ, Chen FK, da Cruz L, Tufail A (2008) Segmentation error in Stratus optical coherence tomography for neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 50(1):399–404, Epub Aug 1
Sadda SR, Joeres S, Wu Z, Updike P, Romano P, Collins AT, Walsh AC (2007) Error correction and quantitative subanalysis of optical coherence tomography data using computer-assisted grading. Invest Ophthalmol Vis Sci 48(2):839–848
Krebs I, Falkner-Radler C, Hagen S, Haas P, Brannath W, Lie S, Ansari-Shahrezaei S, Binder S (2009) Quality of the threshold algorithm in age-related macular degeneration: Stratus versus Cirrus OCT. Invest Ophthalmol Vis Sci 50(3):995–1000
Leung CK, Cheung CY, Weinreb RN, Lee G, Lin D, Pang CP, Lam DS (2008) Comparison of macular thickness measurements between time domain and spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 49(11):4893–4897
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT (2008) Evaluation of time domain and spectral domain optical coherence tomography in the measurement of diabetic macular edema. Invest Ophthalmol Vis Sci 49(10):4290–4296
Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, Iliev ME, Frey M, Rothenbuehler SP, Enzmann V, Wolf S (2009) Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci 50(7):3432–3437
Mylonas G, Ahlers C, Malamos P, Golbaz I, Deak G, Schütze C, Sacu S, Schmidt-Erfurth U (2009) Comparison of retinal thickness measurements and segmentation performance of four different spectral and time domain OCT devices in neovascular age-related macular degeneration. Br J Ophthalmol 93(11):1453–1460, Epub 2009 Jun 10
Hee MR (2005) Automated measurements of retinal thickness with optical coherence tomography. Am J Ophthalmol 139(1):18–29
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support
No financial support was provided for this study.
Conflicts of interest
The authors have no conflict of interest to declare.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00417-010-1446-2
Rights and permissions
About this article
Cite this article
Smretschnig, E., Krebs, I., Moussa, S. et al. Cirrus OCT versus Spectralis OCT: differences in segmentation in fibrovascular pigment epithelial detachment. Graefes Arch Clin Exp Ophthalmol 248, 1693–1698 (2010). https://doi.org/10.1007/s00417-010-1415-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-010-1415-9