Abstract
Background
Angiotensin II type 1 (AT1) receptor-antagonists are widely used for treatment of hypertension. Recent studies have demonstrated a protective effect of renin angiotensin system (RAS) antagonism against immune-mediated inflammatory diseases such as myocarditis, chronic allograft rejection, antiglomerular basement membrane nephritis, colitis, and arthritis. However, only a few reports have demonstrated the effect of RAS in ocular inflammatory conditions. The purpose of this study was to investigate the anti-inflammatory effect of a selective AT1 receptor antagonist, losartan, on endotoxin-induced uveitis (EIU) and compare the effect on experimental autoimmune uveoretinitis (EAU).
Methods
To induce EIU, 7-week-old Lewis rats were injected subcutaneously with 200 μg lipopolysaccharide (LPS). Losartan was administered intravenously at the same time. The aqueous humor was collected from eyes 24 h after LPS injection. The number of infiltrating cells, protein concentration, and levels of tumor necrosis factor (TNF)-α and monocyte chemoattractant protein-1 (MCP-1) in the aqueous humor were determined. The collected eyes were immunohistochemically stained with monoclonal antibody for activated nuclear factor (NF)-κB. To induce EAU, C57BL/6 mice (6–8 weeks old) were immunized with human interphotoreceptor retinoid binding protein (hIRBP)-derived peptide emulsified in complete Freund’s adjuvant (CFA) and concomitantly injected with purified Bordetella pertussis toxin (PTX). Clinical severity of EAU and T cell proliferative response were analyzed.
Results
Losartan significantly suppressed the development of EIU. Numbers of aqueous cells of control EIU rats, those from EIU rats treated with 1 or 10 mg/kg of losratan were 75.3 ± 45.6 × 105, 27.9 ± 8.1 × 105, or 41.3 ± 30.9 × 105 cells/ml respectively (p < 0.01 vs control). Aqueous protein, TNF-α, and MCP-1 levels were also significantly decreased in a manner dependent on the amount of losartan administered (p < 0.01). Treatment of EIU rats with losartan suppressed activation of NF-κB at the iris ciliary body. Thus, the suppressive effect of losartan on ocular inflammation in EIU appeared to result from down-regulation of NF-κB activation and reduction of inflammatory cytokine production. On the other hand, in the EAU model, neither the clinical score nor the antigen-specific T cell proliferative response was significantly influenced by the treatment with losartan.
Conclusions
The present findings indicate that RAS may be involved in the acute inflammation of the eye, but not in T cell-dependent ocular autoimmunity. Antagonism of the RAS may be a potential prophylactic strategy for treatment of the human acute ocular inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human endogeneous uveitis is an intraocular inflammatory disease causing severe visual loss, and even blindness. There are various animal models of uveitis including EIU and EAU. EIU is an animal model for acute ocular inflammation induced by injection of the LPS, a component of gram-negative bacterial cell wall [36]. EIU is characterized by protein leakage in the anterior chamber and by infiltration of macrophages and neutrophils into the eye, with a peak at 24 h after LPS injection [9, 28]. Acute inflammation develops mainly in the anterior chamber (iridocyclitis) and the inflammatory cells infiltrate also in the vitreous as well as the retina [47]. It has been reported that inflammatory cytokines play an essential role in development of the EIU. Elevated expression of cytokines and chemokines such as TNF-α and MCP-1 has been observed concomitant with the peak of EIU [13, 34]. Local angiotensin II (AII) expression has also been found to be elevated during retinal inflammation in the EIU model [26].
EAU is another animal model for relatively chronic endogeneous uveitis such as Behçet disease, birdshot retinochoroidopathy, sympathetic ophthalmia and Vogt–Koyanagi–Harada disease [8]. EAU represents a Th1 cell-mediated and organ-specific autoimmune disease that is induced by immunization with retinal proteins, such as soluble antigen (SA) or hIRBP-derived peptide, or by the adoptive transfer of specific T cells for these antigens into naïve syngeneic recipients [7, 18, 30].
RAS is well known to play an important role in regulating blood pressure and body fluid regulation. It has been considered that the function of RAS is to control vascular tonus and maintain the fluid homeostasis by regulating water and electrolyte absorption and/or excretion in the kidney and gastrointestinal tract [27]. Recent studies, however, have demonstrated that AII is also involved in broad biological actions such as apoptosis, remodeling, and inflammation of vascular wall [37, 51, 52]. In addition, peripheral AII may be involved in an attack of fever and the peripheral interleukin (IL)-1β production following systemic injection of LPS in rats [29, 42]. When angiotensin-converting enzyme (ACE) inhibitor was administered to an experimental myocarditis model to block RAS, an anti-inflammatory effect was observed [17]. Similarly, RAS inhibitor showed a significant attenuation of hepatic IL-1β production in rats treated with LPS [29]. We previously reported that captopril, an ACE inhibitor, suppressed the inflammation in a rat EIU model [21].
AT1 receptor antagonists have been developed as drugs that selectively and markedly blocked the RAS at the receptor level, differently from ACE inhibitors [46]. Indeed, it has been reported that the AT1 receptor antagonist effectively attenuates various inflammatory processes [11, 16, 33, 35]. One of the AT1 receptor blockers, telmisartan, showed a neuroprotective effect via modulating AT1 receptor and AT2 receptor signaling in retinal inflammation in a mouse EIU model [26]. It was also reported in a retinal inflammation model that telmisartan reduced the number of infiltrating cells in aqueous humor [32]. Another AT1 receptor antagonist, losartan, is now widely used to treat hypertension. Notably, it was reported that losartan acted not only as an AT1 receptor antagonist but as an anti-inflammatory agent via pathways perhaps other than modulation of AT1 and AT2 receptor signaling [24].
Recent studies have collectively demonstrated the protective effect of RAS antagonism against immune-mediated inflammatory diseases such as myocarditis, chronic allograft rejection, antiglomerular basement membrane nephritis, colitis, and arthritis [1, 2, 6, 14, 15, 20, 22, 35, 38, 43]. However, no report has clearly demonstrated beneficial contribution of the RAS antagonism in ocular inflammatory conditions. The purpose of the present study was to investigate the anti-inflammatory effect of losartan on an animal model of acute uveitis (EIU). We analyzed the effects of losartan on cellular infiltration, extravasation of protein, and levels of TNF-α and MCP-1 in the aqueous humor of EIU rats. To further clarify the mechanism of the anti-inflammatory effect, we also examined the expression of NF-κB in the EIU lesion. Finally, to examine and compare the effect of losartan on a relatively chronic inflammatory model (EAU) with that on EIU, we analyzed the clinical score and generation of antigen-specific T cell proliferative response in the EAU mice, either in the presence or absence of losartan.
Materials and methods
Experimental animals and reagents
Seven-week-old male Lewis rats (220–250 g) were obtained from Clear Japan (Tokyo, Japan). Six- to 8-week-old C57BL/6 female mice were obtained from Japan SLC (Hamamatsu, Japan). All rats and mice were bred and maintained in a specific pathogen-free condition. All experimental animals were treated in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research. All experiments were conducted with the approval and supervision of Hokkaido University Animal Care and Use Committee.
Losartan was provided by Merck and Co Inc (Rahway, NJ, USA). LPS from Salmonella typhimurium was purchased from Sigma-Aldrich (St. Louis, MO, USA). hIRBP peptide sequence 1–20 (GPTHLFQPSLVLDMAKVLLD) was synthesized by Sigma-Genosys (Ishikari, Japan). Purified PTX was purchased from Sigma-Aldrich (St. Louis). CFA and Mycobacterium tuberculosis strain H37Ra were purchased from Difco (Detroit, MI, USA).
Induction of EIU and collection of the aqueous humor
EIU was induced by subcutaneous injection of 200 μg of LPS from Salmonella typhimurium in 0.1 ml of phosphate buffered saline (pH 7.4, PBS) [21]. At the same time, these rats were injected intravenously with 1 mg/kg or 10 mg/kg of losartan (losartan potassium) diluted in 0.1 ml of PBS. Control EIU rats or naïve rats (negative control) were intravenously administered PBS alone (no losartan). Twenty-four hours after LPS injection, the rats were sacrificed and the aqueous humor (15–20 μl/rat) was collected as described below.
Histopathologic evaluation
Rats were euthanized 24 h after LPS injection. The eyes were enucleated immediately, stored in 10 % buffered formalin for 24–48 h, and embedded in paraffin. Sagittal sections (5 μm) were cut near the optic nerve head and stained with hematoxylin and eosin (H&E). Anterior chamber, iris-ciliary body (ICB), vitreous and retina were observed under light microscopy.
Number of infiltrating cells and protein concentration in aqueous humor
At 24 h after LPS injection, the rats were euthanized, and the aqueous humor was collected immediately from both eyes by an anterior chamber puncture (15–20 μl/rat), using a 30-gauge needle under a surgical microscope. The aqueous humor was then accurately diluted 10-fold with PBS. For cell counting, the aqueous humor sample was suspended in an equal amount of Türk stain solution, and the cell number was counted with a hemocytometer under a light microscope. The number of cells per field (an equivalent of 0.1 μl) was manually counted by two independent researchers.
Measurement of cytokines
The total protein concentration in the aqueous humor samples was measured with a bicinchoninic acid protein assay kit (Pierce, Rockford, IL, USA). The aqueous humor samples were stored on ice until using. The levels of TNF-α and MCP-1 concentrations in aqueous humor were measured with enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instruction.
Immunohistochemical studies for NF-κB
At 3 h after LPS injection, rats were anesthetized and the eyes were fixed by an intracardiac perfusion of 4 % paraformaldehyde in 0.1 M PBS. The eyes were enucleated and immersed in the same fixative for 12 h. After dehydration and paraffin embedment of the eyes, 5-μm sagittal sections near the optic nerve head were obtained. The sections were rinsed in PBS twice and incubated with normal goat serum before staining with anti-p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Binding of the primary antibody was visualized using Cy-3 conjugated goat anti-rabbit IgG (Jackson Immuno-Research Laboratories, West Grove, PA, USA). Nuclei were then stained with PBS containing YO-PRO-1 (Molecular Probes, Eugene, OR, USA) for 5 min. The sections were examined by laser scanning confocal microscopy (MRC-1024: Bio-Rad, Richmond, CA, USA; and LSM 510: Carl Zeiss, Oberkochen, Germany). Two areas of ICB were randomly photographed and the number of activated NF-κB positive cells was counted. The results were averaged for each sample in each group. This analysis was performed in the six eyes from three rats in each group.
Induction and assessment of EAU
Mice were immunized subcutaneously in the back with hIRBP peptide as described elsewhere [23]. IRBP 1–20 (200 μg) in 0.2 ml was emulsified in CFA (1:1 v/v) that had been supplemented with M.tuberculosis to 5 mg/ml. These mice were given 0.1 μg of PTX in 100 μl of PBS intraperitoneally as an additional adjuvant. Losartan was dissolved in PBS solution for in vivo injections. Mice were administered orally with losartan (20 mg/kg) or vehicle alone for 14 days. Two groups of mice were given oral administration of losartan or PBS on days 0–14, and other two groups of mice received the oral administration on days 14–28 after immunization. A disposable feeding needle was used for the oral administration.
Fundus examination was performed every 3 or 4 days from day 7 post-immunization by two ophthalmologists [23], and the severity of EAU in each eye was evaluated according to EAU clinical score (grade 0–5) as described previously [44].
Antigen specific T cell proliferative assay
Mice were immunized with 100 μg of hIRBP1-20 that was emulsified in CFA (1:1 v/v). Immunized mice orally received either losartan (20 mg/kg) or vehicle alone every day. Ten days after immunization, cells were collected from draining lymph nodes, suspended at 5 × 105 /well and cultured in vitro in the presence of graded concentrations of hIRBP peptide for 48 h [23]. Then, the cells were pulsed-labeled with 3H-thymidine and cultured for additional 16 h. Quantitation of these responses was accomplished by measuring incorporation of 3H-thymidine.
Statistical analysis
Data in EIU are presented as means±SD. Data were analyzed by analysis of variance (ANOVA), and statistical significances were analyzed using a Student’s t-test with Bonferroni’s collection. EAU scores were compared by Mann-Whitney U-test. A p-value was considered statistically significant when lower than 0.05.
Results
Histopathological findings in eyes of EIU rats
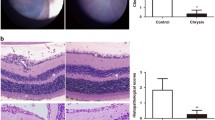
The eyes of control group rats that had been administered PBS alone showed no inflammation (Fig. 1a,e). Representative histological changes in eyes of LPS-treated EIU group but untreated with losartan were shown as a positive control (Fig. 1b,f). A large number of inflammatory cells were found in the anterior and posterior segment at 24 h after LPS administration. In contrast, only a few inflammatory cells were infiltrating in the eyes of rats treated with losartan (1 or 10 mg/kg) (Fig. 1c,g or d,h). These findings demonstrated that inflammation of eyes was ameliorated in the losartan-treated rats compared with the losartan-untreated rats.
Histologic changes of ICB, vitreous and retina 24 h after LPS injection. Eyes were enucleated 24 h after LPS injection, and fixed. Then they were sectioned, and stained with H & E. Photographs on the left side show ICB legion and those on the right side show vitreous and retina in rat. Rats of control group (a, e) were not injected with LPS. No inflammation is observed. Severe inflammatory cell infiltration is observed in EIU rats (b, f). In the group of EIU rats treated with losartan (1 mg/kg ; c, g , 10 mg/kg ; d, h), reductions of cell infiltration are observed compared to EIU rats. Inflammatory cells around the ICB and in the anterior chamber and vitreous cavity are present (arrows in b, c, f, g and h). Original magnification, ×200
Effect of losartan on cellular infiltration and protein concentration in the aqueous humor in eyes of EIU rats
In the EIU group, the mean number of inflammatory cells in aqueous humor 24 h after LPS administration was 75.3 ± 45.6 × 105 cells/ml. Aqueous cell numbers of rats injected with 1 mg/kg or 10 mg/kg losartan were 27.9 ± 8.1 or 41.3 ± 30.9 × 105 cells/ml respectively. Thus, treatment of EIU rats with losartan significantly reduced the number of inflammatory cells in the anterior segment (p < 0.01, Fig. 2a).
Effect of losartan administration on cellular infiltration and protein concentration in the aqueous humor. Rats were injected with LPS and the aqueous humor was collected 24 h later. a Mean cell number in the aqueous humor. b Mean protein concentration. All data show the mean±SD from 8–13 rats of each group. ** Significantly different from the LPS group (p < 0.01)
Next, we examined aqueous protein levels. The protein concentrations in aqueous humor collected from rats treated with 0, 1, or 10 mg/kg of losartan were 72.3 ± 3.4, 53.0 ± 5.5, or 42.9 ± 6.0 mg/ml respectively. The aqueous protein levels were significantly lower in either dose of losartan-treated rats than those in losartan-non-treated rats (p < 0.01). Further, the decrease in the protein level was losartan dose-dependent (Fig. 2b). In naïve rats administered PBS alone, the protein concentration in the aqueous humor was negligible (1.3 ± 0.13 mg/ml).
Effect of losartan administration on aqueous TNF-α and MCP-1 levels in EIU rats
Then, TNF-α level in aqueous humor of EIU rats was measured. No detectable TNF-α and MCP-1 was observed in naïve rats (data not shown). The concentration of TNF-α collected from LPS-treated EIU rats reached 2.91 ± 0.36 ng/ml at 24 h after LPS administration, while EIU rats injected with low-dose (1 mg/kg) or high-dose (10 mg/kg) losartan showed 2.50 ± 0.26 or 1.91 ± 0.78 ng/ml TNF-α respectively (Fig. 3a). Thus, aqueous TNF-α levels were reduced by losartan treatment in a dose-dependent manner, and the reduction by losartan (10 mg/kg) was regarded as significant compared to TNF-α levels in control EIU rats (p < 0.01).
Effect of losartan administration on TNF-α and MCP-1 concentration in the aqueous humor. Rats were injected with LPS and the aqueous humor was collected 24 h after LPS injection. a TNF-α concentration. b MCP-1 concentration. Data are the mean±SD of results from 8–13 rats. ** Significantly different from the LPS group (p < 0.01)
Next, we quantified aqueous levels of a chemokine, MCP-1. Aqueous humor of losartan-untreated EIU rats contained 1164.3 ± 276.5 pg/ml of MCP-1 (Fig. 3b). MCP-1 levels in the aqueous humor collected from rats injected with 1 or 10 mg/kg of losartan were 847.6 ± 265.9 or 595.6 ± 256.7 pg/ml respectively. Thus, aqueous MCP-1 concentrations were also significantly decreased (p < 0.01) in rats treated with losartan in a dose-dependent fashion compared to those in control EIU rats.
Immunohistochemistry of NF-κB p65 in the ICB after LPS injection in EIU
NF-κB activation is involved in various inflammatory responses. We then analyzed immunohistochemically activated NF-κB expression in the lesion. No activated NF-κB-positive nuclei were found in the ICB before EIU induction (Fig. 4a[A]). Three hours after LPS injection, a considerable expression of activated NF-κB p65 was observed in the ICB of EIU rats (Fig. 4a[B]). By contrast, only a few NF-κB p65 positive nuclei were detected in losartan (10 mg/kg)-injected EIU rats (Fig. 4a[C]). The mean proportion of activated NF-κB-positive cells in EIU rats was 27.6 ± 14.1%, whereas those in rats treated with 1 or 10 mg/kg of losartan were 12.5 ± 2.7% or 10.6 ± 4.5%, respectively (Fig. 4b). The proportion of activated NF-κB positive cells was significantly lower in EIU rats treated with either dose of losartan (1 or 10 mg/kg) than that in untreated EIU rats (p < 0.01).
Effect of losartan administration on NF-κB p65 activation in the ICB 3 h after LPS injection. a Immunohistochemistry of NF-κB p65 (red) in the ICB of the rats 3 h after LPS injection. Dual-immunofluorescence labeling shows the colocalization of p65 (yellow) in nuclei (green). [A], control group; rats were not injected with LPS. [B], LPS group; rats were injected with LPS and 0.1 ml PBS. [C], losartan-treated group; rats were injected with LPS and 10 mg/kg of losartan diluted in 0.1 ml PBS. Magnification: ×400. Arrows: activated NF-κB positive cells. b Quantitative analysis of NF-κB-positive cells in the ICB. Control rats, LPS-injected EIU rats, and EIU rats treated with 1 mg/kg or 10 mg/kg losartan are shown. Data are the mean±SD (n = 6). ** Significantly different from LPS group (p < 0.01)
Effect of losartan on EAU mice
To determine the effect of losartan on Th1-induced inflammation, EAU, mice were immunized with hIRBP peptide emulsified in CFA as described in “Materials & methods”. Two groups of experimental mice were prepared. In the first group (“early”), for 14 days after immunization with hIRBP B6 mice were orally administered with losartan (20 mg/kg) or PBS alone, every day (Fig. 5a). Another group of mice was treated with losartan or PBS alone from day 14 to 28 after immunization (“late”) (Fig. 5b). The funduscopic examination was performed from day 7 after immunization every 3–4 days.
Clinical score of EAU in mice treated with losartan. EAU was induced in B6 mice by injection of hIRBP peptide in CFA. Mice were treated with losartan (○) or PBS alone (●). Daily administration of losartan or PBS was performed for 2 weeks after immunization, days 0–14 (“early” group, a) or days 14–28 (“late” group, b). Results are presented as the mean score for all eyes of each group of mice (8 mice per group) ±SEM. Closed triangles (▲) indicate daily administration of losartan or PBS. * Significance was determined using Mann-Whitney U-test (p < 0.05)
As shown in Fig. 5a,b, similar levels of EAU were observed in both groups of losartan-treated and vehicle-treated mice (n = 8 in each experimental group of “early” and “late” groups). In the “early” group, however, mean EAU severity of the losartan-treated mice was significantly milder (0.25 ± 0.11, mean±SE) than that in the controls (0.75 ± 0.11, p < 0.05) on day 10 after immunization (Fig. 5a). However, significant differences in the severity were no longer detected between losartan- and vehicle-treated mice on day 14 or later stages. In mice of the “late” group, no difference in EAU severity was seen between losartan- and vehicle-treated mice for the entire period of the experiment (Fig. 5b). These findings suggest that losartan is effective only in an early phase.
Next, we examined whether losartan showed a suppressive effect on the antigen-specific proliferative response of hIRBP-specific T cells. Th1 cells play an essential role in development of EAU. Draining lymph nodes were collected from hIRBP plus CFA-immunized mice treated with either losartan or PBS alone (control) for 10 days after immunization. Lymph node cells were cultured in vitro in the presence of graded concentrations of hIRBP peptide for 48 h. Comparable levels of antigen-specific proliferation were seen in a dose-dependent manner in both losartan or PBS-treated groups (Fig. 6). No difference was observed in the T cell proliferation between these two groups of EAU mice.
Antigen-specific T cell proliferative response. 3H-thymidine incorporations of lymphocytes collected from losartan-treated (○) or PBS alone (●) mice were demonstrated. Cells were obtained from draining lymph nodes of B6 mice 10 days after immunization. The results are presented as the mean±SEM (n = 3)
Discussion
In the present study, we examined the therapeutic effect of a blocker of AT1 receptor, losartan, on LPS-induced acute ocular inflammation (EIU). We found that a single administration of losartan significantly ameliorated EIU in rats. Aqueous cell infiltration and levels of protein, inflammatory cytokines and chemokines in the aqueous humor were significantly decreased in EIU rats treated with losartan as compared to those in non-treated EIU rats. Activation of NF-κB in the legion was also significantly suppressed by the treatment with losartan. These results suggest that the AT1 receptor antagonist, losartan, exerts an anti-inflammatory influence in ocular tissue via directly inhibiting activation of NF-κB pathway, which leads to subsequent reduction of inflammatory cytokine production.
NF-κB is a key transcription factor that regulates various inflammatory processes [4]. The activated form of NF-κB is a heterodimer, usually consisting of two proteins, p65 (relA) and p50 subunit. In unstimulated cells, NF-κB is found in cytoplasm and bound to IκBα and IκBβ. When cells are stimulated, NF-κB is released from IκB by specific kinases phosphorilate IκB, and moves into the nucleus, where it binds to specific sequences in promoter regions of the target genes [3]. With various stimuli including LPS, proinflammatory cytokines and reactive oxygen species activate NF-κB [4]. It has been reported that AII also rapidly activates NF-κB [25] through AT1 and AT2 receptors [12, 31].
The present study showed that LPS-induced NF-κB p65 nuclear translocation in the ICB was significantly suppressed by an administration of losartan. The activation of NF-κB leads to an increase in the expression of numbers of genes, including TNF-α and IL-1β, that mediate inflammation and immune responses. These cytokines sometimes activate and, on another occasion, are activated by NF-κB [5]. It has been reported that losartan inhibited the LPS-induced production of IL-1β [42]. In the present study, we demonstrated that increased levels of aqueous TNF-α in EIU rats was significantly down-regulated by injection of a high dose of losartan. Thus, it seems that losartan inhibits not only IL-1β production but also the positive cycle of NF-κB and TNF-α, which results in the anti-inflammatory effect in EIU rats.
It has been reported that various chemokines are involved in ocular inflammation. In the present study, we focused on MCP-1 expression as a representative inflammatory chemokine. MCP-1 is an important mediator of monocyte infiltration [19] and shown to be over-expressed in human eyes during acute anterior uveitis [49] and during EIU in rats [13]. NF-κB up-regulates transcription of MCP-1 gene [48, 50]. Indeed, we found augmented production of MCP-1 in the aqueous humor of EIU rats. The augmented MCP-1 level was significantly decreased when treated with losartan in a dose-dependent manner. We consider that the suppression of MCP-1 production by an administration of losartan leads to reduced monocyte recruitment in the inflamed ocular tissue.
It has recently been reported that the AT1 receptor antagonist shows an anti-inflammatory effect by inhibiting retinal ICAM-1 upregulation, leukocyte adhesion and infiltration [26, 32]. It seems that the AT1 receptor antagonist has several pathways to suppress ocular inflammation. Our present results demonstrated a new pathway by which an AT1 blocker directly inhibited NF-κB activation and ameliorated acute ocular inflammation in the EIU system.
On the other hand, losartan administration suppressed a clinical score of EAU only at an early phase. As mentioned previously, losartan has an anti-inflammatory effect by inhibiting the expression of adhesion molecules. Thus, it seems possible that this very limited suppression of EAU is attributable to the inhibitory effect of losartan on infiltration of the inflammatory cells into the eyes that express reduced adhesion molecules. However, we found that oral administration of losartan showed no suppressive effect on generation of hIRBP-specific T cell in the lymph nodes. These results suggest that losartan did not affect activation of hIRBP-specific T cells by the antigen presenting cells in lymph nodes. There are various differences between EIU and EAU models examined in the present study. EIU was induced rapidly by LPS in rats, whereas EAU was induced by immunization of mice with hIRBP, an analogue protein antigen for self murine IRBP, and it took a relatively long period to induce inflammatory manifestation in the latter model. Furthermore, a single intravenous administration was given to EIU rats, whereas multiple oral administrations were performed in EAU mice. We considered that the oral administration of losartan did not affect NF-κB activation of antigen presenting cells in the regional lymph nodes, and stimulation of hIRBP-specific Th1 cells normally occurred in the EAU mice. These points, especially the status of NF-κB activation of antigen-presenting cells in the regional lymph nodes in the EAU model in the presence of losartan should be pursued in further studies.
We have reported that a high-potency NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC), ameliorated EAU when intraperitonealy administered for a long period [23]. It is possible that PDTC is more potent inhibitor of NF-κB than losartan. Indeed, PDTC inhibited the translocation of NF-κB into the nucleus of EAU retina [23]. However, PDTC has never been used yet in clinical practice, since there are concerns about its safety. Meanwhile, losartan is used widely in safety, and a clinical dose of losartan showed the anti-inflammatory effect on EIU in the present study. Thus, it should be examined whether the losartan (clinical dose) really suppresses the NF-κB activation in the antigen-presenting cells in the regional lymph nodes and retina in the EAU model as well, when administered intraperitoneally.
Another point between EIU and EAU may be species. We induced EIU in rats and EAU in mice in this study. Human, rat, and mouse AT1 receptor isoforms are pharmacologically indistingishable [10]. Rat and mouse ANGII receptors have coevolved, and cloned mammalian ANGII receptors share their AT1 subtypes, which can recognize losartan with high affinity [40, 41]. It means that species may not be really acknowledged as a problem in this study.
There is growing evidence suggesting that among various AT1 receptor antagonists only losartan has AT1 receptor-independent actions primarily related to anti-inflammatory and antiaggregatory mechanisms. These properties are not shared by other angiotensin receptor blockers, such as candesartan, valsartan, and ACE inhibitors [39]. Losartan is a prodrug that is converted to active form in vivo. Thus, losartan undergoes the firstpass metabolism in the liver and is converted into EXP3174, whose affinity to the AT1 receptor is ten times higher than that of losartan. Another metabolite of losartan, EXP3179, seems to possess anti-inflammatory and anti-aggregatory properties, though it does not interfere with ligand binding to the AT1 receptor [24]. Since EXP3179 is structurally similar to indomethacin, a conventional nonsteroidal anti-inflammatory drug (NSAID), it seems possible that these metabolites function like as NSAID [45] in suppression of EIU in the present study. Though the exact mechanism of anti-inflammatory effect of losartan still remains unclear, the anti-inflammatory and anti-aggregatory activities of losartan metabolite may be responsible for the suppression of EIU.
In the present study, rats were injected with a usual dose (1 mg/kg) and a 10-times dose (10 mg/kg) of losartan. Though aqueous cytokines and chemokines were suppressed in a dose-dependent manner, the numbers of aqueous humor cells were not significantly different between 1 mg/kg and 10 mg/kg. Since rats have a very small volume of aqueous humor (5 μl/eye), some errors may be observed in case of counting the aqueous cells. Another possible cause may be that a common dose of losartan is good enough to decrease the cell infiltration in the aqueous chamber. In any case, losartan administration ameliorated EIU and suppressed the inflammatory parameters in aqueous humor definitely. When viewed from the point of view of cytokines and chemokines, a higher dose (10 mg/kg) of losartan might be better to suppress the acute ocular inflammation than the common dose of the drug. Since the treatment with losartan induced no marked changes in rat behavior or food and water consumption in the present study, a higher dose of losartan administration may be one option of future clinical application.
In conclusion, we demonstrated that RAS was involved in acute ocular inflammation, and inhibition of AT1 receptor ameliorated EIU in rats. Further, we showed that losartan exerted a suppressive effect on acute inflammation by inhibiting the NF-κB pathway. Although the AT1 receptor blocker did not show an impressive regulatory effect on the antigen-specific T cell proliferative response in EAU model mice, modification of the method of losartan administration, including the dose, route and timing to the mice, should be pursued to eventually determine whether the AT1 receptor blocker is effective in relatively chronic ocular inflammation like EAU. At the present time, we consider that RAS/ AT1 receptor antagonism is one of the prophylactic strategies for ocular acute inflammatory disorders.
References
Amuchastegui SC, Azzollini N, Mister M, Pezzotta A, Perico N, Remuzzi G (1998) Chronic allograft nephropathy in the rat is improved by angiotensin II receptor blockade but not by calcium channel antagonism. J Am Soc Nephrol 9:1948–1955
Andersson P, Cederholm T, Johansson AS, Palmblad J (2002) Captopril-impaired production of tumor necrosis factor-α-induced interleukin-1β in human monocytes is associated with altered intracellular distribution of nuclear factor-κB. J Lab Clin Med 140:103–109
Baldwin AS Jr (1996) The NF-κB and I κB proteins: new discoveries and insights. Annu Rev Immunol 14:649–683
Barnes PJ (1997) Nuclear factor-κB. Int J Biochem Cell Biol 29:867–870
Barnes PJ, Karin M (1997) Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336:1066–1071
Benediktsson H, Chea R, Davidoff A, Paul LC (1996) Antihypertensive drug treatment in chronic renal allograft rejection in the rat. Effect on structure and function. Transplantation 62:1634–1642
Caspi RR, Roberge FG, McAllister CG, el-Saied M, Kuwabara T, Gery I, Hanna E, Nussenblatt RB (1986) T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol 136:928–933
Caspi RR, Roberge FG, Chan CC, Wiggert B, Chader GJ, Rozenszajn LA, Lando Z, Nussenblatt RB (1988) A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol 140:1490–1495
Chan CC, Tuaillon N, Li Q, Shen DF (2000) Therapeutic applications of antiflammin peptides in experimental ocular inflammation. Ann N Y Acad Sci 923:141–146
Chiu AT, Dunscomb J, Kosierowski J, Burton CR, Santomenna LD, Corjay MH, Benfield P (1993) The ligand binding signatures of the rat AT1A, AT1B and the human AT1 receptors are essentially identical. Biochem Biophys Res Commun 197:440–449
Chua CC, Hamdy RC, Chua BH (1998) Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta 1401:187–194
de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52:415–472
de Vos AF, van Haren MA, Verhagen C, Hoekzema R, Kijlstra A (1994) Kinetics of intraocular tumor necrosis factor and interleukin-6 in endotoxin-induced uveitis in the rat. Investig Ophthalmol Vis Sci 35:1100–1106
Fukuzawa M, Satoh J, Sagara M, Muto G, Muto Y, Nishimura S, Miyaguchi S, Qiang XL, Sakata Y, Nakazawa T, Ikehata F, Ohta S, Toyota T (1997) Angiotensin converting enzyme inhibitors suppress production of tumor necrosis factor-α in vitro and in vivo. Immunopharmacology 36:49–55
Furukawa Y, Matsumori A, Hirozane T, Sasayama S (1996) Angiotensin II receptor antagonist TCV-116 reduces graft coronary artery disease and preserves graft status in a murine model. A comparative study with captopril. Circulation 93:333–339
Godsel LM, Leon JS, Engman DM (2003a) Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists in experimental myocarditis. Curr Pharm Des 9:723–735
Godsel LM, Leon JS, Wang K, Fornek JL, Molteni A, Engman DM (2003b) Captopril prevents experimental autoimmune myocarditis. J Immunol 171:346–352
Gregerson DS, Obritsch WF, Fling SP, Cameron JD (1986) S-antigen-specific rat T cell lines recognize peptide fragments of S-antigen and mediate experimental autoimmune uveoretinitis and pinealitis. J Immunol 136:2875–2882
Gu L, Tseng SC, Rollins BJ (1999) Monocyte chemoattractant protein-1. Chem Immunol 72:7–29
Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K (1999) Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest 103:627–635
Ilieva I, Ohgami K, Jin XH, Suzuki Y, Shiratori K, Yoshida K, Kase S, Ohno S (2006) Captopril suppresses inflammation in endotoxin-induced uveitis in rats. Exp Eye Res 83:651–657
Inokuchi Y, Morohashi T, Kawana I, Nagashima Y, Kihara M, Umemura S (2005) Amelioration of 2,4,6-trinitrobenzene sulphonic acid induced colitis in angiotensinogen gene knockout mice. Gut 54:349–356
Kitamei H, Iwabuchi K, Namba K, Yoshida K, Yanagawa Y, Kitaichi N, Kitamura M, Ohno S, Onoé K (2006) Amelioration of experimental autoimmune uveoretinitis (EAU) with an inhibitor of nuclear factor-κB (NF-κB), pyrrolidine dithiocarbamate. J Leukoc Biol 79:1193–1201
Kramer C, Sunkomat J, Witte J, Luchtefeld M, Walden M, Schmidt B, Tsikas D, Boger RH, Forssmann WG, Drexler H, Schieffer B (2002) Angiotensin II receptor-independent antiinflammatory and antiaggregatory properties of losartan: role of the active metabolite EXP3179. Circ Res 90:770–776
Kranzhofer R, Browatzki M, Schmidt J, Kubler W (1999) Angiotensin II activates the proinflammatory transcription factor nuclear factor-κB in human monocytes. Biochem Biophys Res Commun 257:826–828
Kurihara T, Ozawa Y, Shinoda K, Nagai N, Inoue M, Oike Y, Tsubota K, Ishida S, Okano H (2006) Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Investig Ophthalmol Vis Sci 47:5545–5552
Levens NR (1984) Modulation of jejunal ion and water absorption by endogenous angiotensin after dehydration. Am J Physiol 246:G700–G709
Li Q, Peng B, Whitcup SM, Jang SU, Chan CC (1995) Endotoxin induced uveitis in the mouse: susceptibility and genetic control. Exp Eye Res 61:629–632
Miyoshi M, Nagata K, Imoto T, Goto O, Ishida A, Watanabe T (2003) ANG II is involved in the LPS-induced production of proinflammatory cytokines in dehydrated rats. Am J Physiol Regul Integr Comp Physiol 284:R1092–R1097
Mochizuki M, Kuwabara T, McAllister C, Nussenblatt RB, Gery I (1985) Adoptive transfer of experimental autoimmune uveoretinitis in rats. Immunopathogenic mechanisms and histologic features. Investig Ophthalmol Vis Sci 26:1–9
Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE (1991) Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351:233–236
Nagai N, Oike Y, Noda K, Urano T, Kubota Y, Ozawa Y, Shinoda H, Koto T, Shinoda K, Inoue M, Tsubota K, Yamashiro K, Suda T, Ishida S (2005) Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Investig Ophthalmol Vis Sci 46:2925–2931
Peeters AC, Netea MG, Kullberg BJ, Thien T, van der Meer JW (1998) The effect of renin-angiotensin system inhibitors on pro- and anti-inflammatory cytokine production. Immunology 94:376–379
Planck SR, Huang XN, Robertson JE, Rosenbaum JT (1994) Cytokine mRNA levels in rat ocular tissues after systemic endotoxin treatment. Investig Ophthalmol Vis Sci 35:924–930
Price A, Lockhart JC, Ferrell WR, Gsell W, McLean S, Sturrock RD (2007) Angiotensin II type 1 receptor as a novel therapeutic target in rheumatoid arthritis: in vivo analyses in rodent models of arthritis and ex vivo analyses in human inflammatory synovitis. Arthritis Rheum 56:441–447
Rosenbaum JT, McDevitt HO, Guss RB, Egbert PR (1980) Endotoxin-induced uveitis in rats as a model for human disease. Nature 286:611–613
Rossig L, Dimmeler S, Zeiher AM (2001) Apoptosis in the vascular wall and atherosclerosis. Basic Res Cardiol 96:11–22
Ruiz-Ortega M, Bustos C, Hernandez-Presa MA, Lorenzo O, Plaza JJ, Egido J (1998) Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-κB activation and monocyte chemoattractant protein-1 synthesis. J Immunol 161:430–439
Sadoshima J (2002) Novel AT(1) receptor-independent functions of losartan. Circ Res 90:754–756
Sandberg K (1994) Structural analysis and regulation of angiotensin II receptors. TEM 5:28–35
Sasamura H, Hein L, Krieger JE, Pratt RE, Kobilka BK, Dzau VJ (1992) Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem Biophys Res Commun 185:253–259
Shimizu H, Miyoshi M, Matsumoto K, Goto O, Imoto T, Watanabe T (2004) The effect of central injection of angiotensin-converting enzyme inhibitor and the angiotensin type 1 receptor antagonist on the induction by lipopolysaccharide of fever and brain interleukin-1β response in rats. J Pharmacol Exp Ther 308:865–873
Tanaka A, Matsumori A, Wang W, Sasayama S (1994) An angiotensin II receptor antagonist reduces myocardial damage in an animal model of myocarditis. Circulation 90:2051–2055
Taylor AW, Yee DG, Nishida T, Namba K (2000) Neuropeptide regulation of immunity. The immunosuppressive activity of α-melanocyte-stimulating hormone (α-MSH). Ann NY Acad Sci 917:239–247
Tegeder I, Pfeilschifter J, Geisslinger G (2001) Cyclooxygenase-independent actions of cyclooxygenase inhibitors. Faseb J 15:2057–2072
Timmermans PB, Smith RD (1994) Angiotensin II receptor subtypes: selective antagonists and functional correlates. Eur Heart J 15(Suppl D):79–87
Tuaillon N, Shen DF, Berger RB, Lu B, Rollins BJ, Chan CC (2002) MCP-1 expression in endotoxin-induced uveitis. Investig Ophthalmol Vis Sci 43:1493–1498
Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, Fukushima J, Kawamoto S, Ishigatsubo Y, Okubo T (1994) NF-κB and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol 153:2052–2063
Verma MJ, Lloyd A, Rager H, Strieter R, Kunkel S, Taub D, Wakefield D (1997) Chemokines in acute anterior uveitis. Curr Eye Res 16:1202–1208
Wang Y, Rangan GK, Goodwin B, Tay YC, Harris DC (2000) Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-κB dependent. Kidney Int 57:2011–2022
Wesselman JP, De Mey JG (2002) Angiotensin and cytoskeletal proteins: role in vascular remodeling. Curr Hypertens Rep 4:63–70
Wolf G, Wenzel U, Burns KD, Harris RC, Stahl RA, Thaiss F (2002) Angiotensin II activates nuclear transcription factor-κB through AT1 and AT2 receptors. Kidney Int 61:1986–1995
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research (S), (B) and (C) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, by Grant-in-Aid for Scientific Research on Priority Areas from MEXT Japan, and by a Grant for Researches on Sensory and Communicative Disorders, Ministry of Health, Labour and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyazaki, A., Kitaichi, N., Ohgami, K. et al. Anti-inflammatory effect of angiotensin type 1 receptor antagonist on endotoxin-induced uveitis in rats. Graefes Arch Clin Exp Ophthalmol 246, 747–757 (2008). https://doi.org/10.1007/s00417-007-0730-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0730-2