Abstract
Background
Ruptured retinal arterial macroaneurysms (RAM) can bleed into the various spaces of the eye. The hemorrhage of the inner layer conceals hemorrhage of the outer layer, making it difficult to diagnose the distribution of hemorrhage accurately and to predict the prognosis in clinical examinations. The objective of this study was to examine the clinical features and prognosis of ruptured RAM on the basis of surgical observations.
Methods
Retrospective review of 33 eyes of 31 patients with impairment of visual acuity due to hemorrhage from a ruptured RAM, who had undergone pars plana vitrectomy. A study of the location of hemorrhage was made from the preoperative fundus photographs and video of the surgical procedure.
Results
Hemorrhage from the RAM was present in two or more locations, consisting of the vitreous cavity, beneath the internal limiting membrane (sub-ILM), or the subretinal space in all but one eye. Sub-ILM hemorrhage was presented in the macular region in 22 of the 27 eyes presenting with sub-ILM hemorrhage (81%). Submacular hemorrhage was only detected when sub-ILM hemorrhage removed during surgery in 12 of the 22 eyes (55%) with sub-ILM hemorrhage. The preoperative VA ranged from hand motion to 0.1, while postoperative VA improved 0.01 to 1.0 (average: 0.2, paired t-test, P < 0.01). The VA was poor in eyes with dense submacular hemorrhage, while it was good in eyes with other hemorrhage.
Conclusions
The effects of vitrectomy were influenced by the location of hemorrhage from the RAM. The VA was poor in eyes exhibiting dense submacular hemorrhage. However, since hemorrhage from a RAM was present at various levels within the eye, it was difficult to evaluate the amount of submacular hemorrhage prior to surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ruptured retinal arterial macroaneurysms (RAM) can bleed into the subretinal space, into the retina, into the area beneath the internal limiting membrane (sub-internal limiting membrane [sub-ILM]), or into the vitreous, and the hemorrhage is frequently observed at more than one of these sites [1]. In the case of bleeding at several sites, hemorrhage of the inner layer conceals hemorrhage of the outer layer (if such hemorrhage is present), making it difficult to diagnose the distribution of hemorrhage accurately in clinical examinations. Consequently, many previous reports of the natural history of ruptured RAM have described results without an accurate diagnosis of the distribution of hemorrhage, thereby leaving behind a significant problem in terms of selecting an appropriate treatment [1–4]. In addition, although there have been several reports related to the surgical results of submacular hemorrhage associated with the rupture of RAM [5–7], we are unaware of reports describing surgical results on large numbers of cases and targeted to all cases of hemorrhage associated with ruptured RAM (including vitreous hemorrhage and premacular hemorrhage).
We have hypothesized that assessing the level of hemorrhage that has the greatest effect on impairment of a patient’s visual acuity is important in terms of predicting prognosis following treatment. In addition, this level is believed to be determined most reliably from surgical findings.
The objective of this study was to examine the clinical features and prognosis of ruptured RAM on the basis of surgical observations.
Patients and methods
Subjects had impairment of visual acuity due to hemorrhage from a ruptured RAM, had undergone pars plana vitrectomy from November 2001 through Jun 2006, and were monitored for at least 6 months after surgery. All patients were informed of the risks, benefits, and alternatives to surgery, and final informed consent was obtained from each patient before surgery.
A comprehensive ophthalmologic examination, including best corrected visual acuity, slit-lamp examination, intraocular pressure, and fundus examination with biomicroscopy and indirect ophthalmoscopy, was performed preoperatively as well as postoperatively. In those cases in which the fundus was visible, fundus photographs were taken. In cases in which the presence of RAM was questionable, surgical observations and fundus photographs taken immediately after surgery were examined. In all cases, the surgical procedure was recorded with a video recorder, and was subsequently examined by viewing the video. Cases with other ocular diseases, such as age-related macular degeneration (ARMD) and diabetic retinopathy, were excluded from the study. The data of the cases used in this study are shown in Table 1.
The surgical procedure consisted of a standard three-port pars plana vitrectomy with retrobulbar anesthesia. Cataract surgery was performed on phakic eyes. If the posterior hyaloid cortex had adhered to the retina following core vitrectomy, the cortex was aspirated with a cutter and separated from the retinal surface. In cases in which macular hemorrhage was in the form of a sub-ILM hemorrhage, an incision was made in the ILM using a micro-hooked needle, and blood was aspirated passively with a soft-tipped extrusion cannula. The ILM was resected using a forceps where a sub-ILM hemorrhage was present. The surgical procedure was terminated at this point in cases with no or only a small amount of submacular hemorrhage.
In cases of dense submacular hemorrhage, following the above-mentioned procedure and according to the method described by Lewis with minor modifications [8], a partial retinotomy was made with a 30-gauge subretinal spatula, followed by lavage of the hemorrhage from the subretinal space using a balanced salt solution (BSS) or tissue plasminogen activator (tPA). After adjusting the concentration of tPA (Monteplase; Eisai Pharmaceutical Inc., Tokyo; 10 μg/0.1 ml in physiological saline), 0.1 ml was injected beneath the retina. After 30 minutes, a small amount of perfluorodecalin was injected into the vitreous cavity to force out the submacular hemorrhage. Subsequently, lavage was performed once on the subretinal space by injecting BSS. Fluid-air exchange was performed in nine, eyes followed by injection of 1.5 ml of 100% sulfur hexafluoride into the vitreous cavity. Patients were instructed to maintain a prone position postoperatively. Photocoagulation was not performed for RAM and retinotomy during surgery.
A study of those sites in which hemorrhage was present due to a ruptured RAM was made from the preoperative fundus photographs and video of the surgical procedure. Moreover, hemorrhage that was thought to have caused mainly a decrease in central visual acuity in each case was classified into three groups (Fig. 1). The three groups consisted of: 1) a vitreous hemorrhage group, in which the fundus was not visible due to vitreous hemorrhage prior to surgery in which no macular hemorrhage was observed during surgery, 2) a sub-ILM hemorrhage group, in which premacular hemorrhage was present and either no submacular hemorrhage was present, or hemorrhage, if present, was small enough to allow visualization of the choroidal markings, and 3) a submacular hemorrhage group, in which the submacular hemorrhage was large enough to prevent visualization of the choroidal markings beneath the macula.
Best-corrected visual acuity was measured using a Landolt ring, expressed using decimal notation, and examined by a technician who was masked to the surgical procedure. Decimal visual acuity was converted to the logarithm of the minimal angle of resolution (logMAR) visual acuity, and visual acuity was assumed to have improved or worsened if there was a change of two levels or more. For statistical analyses, decimal visual acuity was used after it was converted to the logMAR visual acuity. The paired t-test was used to compare visual acuity before and after surgery. Values of P < 0.05 were considered to be significant.
Results
Clinical characteristics
Thirty-three consecutive eyes (14 right, 19 left) of 31 patients with hemorrhage from ruptured RAM were enrolled in this clinical study. Nine patients (nine eyes) were male, and 22 (24 eyes) were female, and their average age was 78 years (range: 48–92 years). Twenty-two patients had systemic hypertension, three had diabetes, four had cerebral infarction, three had cardiovascular disease, three had asthma or bronchitis, three were taking small amounts of aspirin, and three were taking ticlopidine hydrochloride. The mean amount of time that elapsed between the onset of symptoms and surgery was 28 days (range: 5–90 days).
The average aneurysm size was a disc diameter of 0.32 (DD, range: 0.08–0.79 DD). The aneurysms were in a supratemporal artery in 13 eyes, in an inferotemporal artery in 18 eyes, superior to the optic disc in one eye, and inferior to the optic disc in one eye.
Location of hemorrhage from RAM
Hemorrhage from the RAM was present in two or more locations, consisting of the vitreous cavity, beneath the ILM, or the subretinal space in all but one eye (and this one eye exhibited vitreous hemorrhage and exudative changes around the aneurysm). Hemorrhage was observed beneath the ILM and subretinal in 21 eyes; beneath the ILM, subretinal, and in the vitreous cavity in six eyes; subretinal and in the vitreous cavity in five eyes; and in the vitreous cavity in one eye. Sub-ILM hemorrhage was present in the macular region in 22 of the 27 eyes presenting with sub-ILM hemorrhage (81%). In these 22 eyes, ten eyes had no hemorrhage beneath the macula, seven eyes had a small hemorrhage beneath the macula enough so as to enable visualization of choroidal markings, and five eyes had dense hemorrhage beneath the macula. That is, submacular hemorrhage was present in 12 of the 22 eyes (55%) with premacular (sub-ILM) hemorrhage concealing the presence of submacular hemorrhage. There were seven eyes in which preretinal hemorrhage was suspected based on the formation of a liquid surface (niveau) by preoperative assessment, and none of these eyes permitted aspiration of the sub-ILM hemorrhage without an incision in the ILM. Classification of the type of hemorrhage revealed that four of 33 eyes had a vitreous hemorrhage (12%); 17 eyes had a sub-ILM hemorrhage (52%); and 12 eyes had a submacular hemorrhage (36%). In cases 19 and 22, the sites where sub-ILM hemorrhage was present coincided following the removal of sub-ILM hemorrhage, exhibiting mild subretinal hemorrhage and prominent subretinal hemorrhage surrounding the periphery. Hemorrhage from the aneurysms reached the macula in all types of hemorrhage except for those resulting from vitreous hemorrhage. Macular edema was observed in five of ten eyes free of submacular hemorrhage.
Surgical procedure
Phacoemulsification, aspiration, and intraocular lens implantation were performed on 26 eyes. There were four eyes in which only vitrectomy was performed, 29 eyes in which ILM resection was performed in addition to vitrectomy, and nine eyes in which lavage was performed for subretinal hemorrhage. There were three eyes in which lavage was unable to be performed due to organized subretinal hemorrhage.
Visual acuity results
The average observation period after surgery was 11 months (range: 6–46 months). Preoperative visual acuity was 0.1 or lower using hand motion at a distance of 20 cm, while postoperative visual acuity was 0.01 to 1.0 (average: 0.2), thus indicating a significant improvement (paired t-test, P < 0.01). Visual acuity improved in 31 of the 33 eyes, remained unchanged in two eyes, and did not worsen in any of the eyes. Thirteen eyes (39%) demonstrated a final visual acuity of 0.5 or better; eight eyes (24%) demonstrated a final visual acuity of 0.15 to less than 0.5; and 12 (36%) eyes demonstrated a final visual acuity of 0.1 or worse (Table 2). In terms of the type of hemorrhage, among cases of vitreous hemorrhage, no eye had a visual acuity of 0.1 or worse (Table 3). Among cases of sub-ILM hemorrhage, ten eyes (59%) demonstrated a final visual acuity of 0.5 or better, and three eyes (18%) had a visual acuity of 0.1 or worse. Among cases of submacular hemorrhage, nine eyes (75%) had a visual acuity of 0.1 or worse.
The fundus photographs of selected cases treated with this method are shown in Figs. 2, 3, and 4.
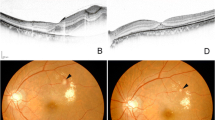
Patient 11. a In this fundus photograph taken at the time of the initial visit, a dense premacular sub-ILM hemorrhage is present within a vascular arcade, making it unclear as to the presence of subretinal hemorrhage. b Postoperatively, an organized aneurysm is present on the superotemporal side of the macula, and indicates the absence of subretinal hemorrhage. c The fundus photograph was taken 8 months after surgery, and shows the presence of recurrent bleeding from the aneurysm in the subretinal space. d On the other hand, the fundus photograph taken after photocoagulation shows that the aneurysm has again organized
Patient 31. a This preoperative fundus photograph shows sub-ILM hemorrhage and preretinal hemorrhage anterior to the macula, and as a result thereof, the presence of submacular hemorrhage is unknown. b This postoperative fundus photograph was taken following lavage performed for submacular hemorrhage, and shows that although hemorrhage is no longer present, a macular hole occurred
Complications
Surgical complications consisted of a peripheral retinal tear in six eyes and a macular hole in six eyes. There were three eyes in which the presence of a macular hole was suspected during removal of sub-ILM hemorrhage. A macular hole occurred in two eyes when BSS and tPA were injected into the subretinal space to perform lavage for subretinal hemorrhage. In addition, a hole formed when the retina was aspirated accidentally during aspiration of sub-ILM hemorrhage of the macula in the remaining eye. The hole eventually closed in only one of these eyes.
Postoperative complications consisted of a macular hole in two eyes, recurrent bleeding from the aneurysm in six eyes, posterior capsule opacification in one eye, and retinal detachment in two eyes. In one eye, a macular hole occurred on day 9 after surgery, despite the fact that the patient had not undergone a subretinal procedure, and this hole closed spontaneously in 2 months. In the other eye, a macular hole occurred 1 month after surgery and has remained open. There were seven eyes (21%) with recurrent bleeding from the same aneurysm after surgery; all of these cases were women, and the time of recurrent bleeding ranged from 1 to 10 months after surgery. Treatment performed for recurrent bleeding consisted of direct photocoagulation of the aneurysm in five eyes and repeat surgery for another eye. Treatment using a neodymium-yttrium-aluminum-garnet (Nd-YAG) laser was performed on the eye presenting with posterior capsule opacification. Repeat surgery was performed on the two eyes presenting with retinal detachment to restore the retina.
Discussion
In this study, hemorrhage distributed at several histological levels in eyes with ruptured RAM was eliminated surgically in a stepwise manner, moving from the vitreous towards the subretinal space. As a result, hemorrhage occurring both beneath the ILM and beneath the retina was determined to be the type of hemorrhage most frequently observed in eyes having ruptured RAM. Moreover, hemorrhage causing impaired visual acuity in each eye was classified according to the predominant histological level at which the hemorrhage was present according to observations made during surgery. The histological level of the hemorrhage was determined to have an effect on the visual acuity prognosis in eyes with ruptured RAM. Visual prognosis was better in eyes in which hemorrhage was present in the vitreous or sub-ILM than in eyes in which it was present beneath the macula. The amount of hemorrhage present beneath the macula, in particular, had a considerable effect on the visual acuity prognosis. As far as we know, this study is the first such study ever conducted.
Hemorrhage from a ruptured RAM is frequently distributed beneath the ILM anterior to the macula, thereby concealing the presence of submacular hemorrhage. In this series, the largest number of cases demonstrated hemorrhage from ruptured RAM that was both beneath the ILM and subretinal. Sub-ILM hemorrhage was located anterior to the macula in 22 of the 27 eyes exhibiting with sub-ILM hemorrhage (81%). Why does sub-ILM hemorrhage occur anterior to the macula in this manner? A common finding observed in such cases was the presence of the aneurysm either within a vascular arcade or in the vicinity thereof. We believe that, in addition to this finding, the following two anatomical characteristics are reasons for the frequent occurrence of sub-ILM hemorrhage anterior to the macula. The first is that the thickness of the ILM at the posterior pole, 1887 nm, is greater than that at other sites [9]. When a ruptured aneurysm is located within a vascular arcade, the resulting hemorrhage remains beneath the ILM, because it is unable to puncture the thick and rigid ILM. The second is the strength of the attachment of the vitreous and major retinal vessels that form a vascular arcade [10]. The hemorrhage also probably flows out toward the macular region without flowing to the periphery beyond the vascular arcade, due to its strong adhesion to the vitreous. These presumptions are supported paradoxically by the hemorrhage outside a vascular arcade puncturing the ILM and flowing into the vitreous cavity in cases 3 and 4 (Fig. 2), and by the sub-ILM hemorrhage in the vicinity of a vascular arcade occurring in the macular region, while the subretinal hemorrhage spread to the periphery beyond a vascular arcade in cases 21 and 28. On the other hand, sub-ILM hemorrhage did not extend toward the macular region in three of 27 eyes with sub-ILM hemorrhage. In case 22, a large volume of subretinal hemorrhage was present beneath the macula, and the retina at the macula was distended. As a result, sub-ILM hemorrhage was in a form that was pushed toward the temporal side. Sub-ILM hemorrhage in the right eye of case 15 and in case 29 appeared to have punctured the ILM, resulting in VH. Hemorrhage appeared to remain beneath the ILM following puncture in these two eyes. This was probably due to the force of the hemorrhage being quite strong when the aneurysm ruptured in these two eyes. Although sub-ILM hemorrhage in two of the 27 eyes with sub-ILM hemorrhage can be assumed to have been premacular, preoperative fundus photographs revealed the formation of niveau, and the sub-ILM hemorrhage deviated from the macula (cases 25 and 26). In these eyes, in which sub-ILM hemorrhage occurred anterior to the macula, it was difficult to determine the state of subretinal hemorrhage accurately before surgery. This is because the sub-ILM hemorrhage anterior to the macula was concealing the submacular hemorrhage. This study made it possible to determine the hemorrhage status accurately at locations deeper than sub-ILM. Submacular hemorrhage was observed in 55% of the eyes having premacular, sub-ILM hemorrhage, with the hemorrhage being mild in roughly half of these cases and prominent in the remaining half.
In those eyes exhibiting sub-ILM hemorrhage anterior to the macula in which subretinal hemorrhage is absent or small, visual acuity can be expected to be restored by surgery. We did not perform a subretinal drainage if the amount of submacular hemorrhage was low or of an extent that still allowed visualization of the choroidal markings. Although the report by Zhao et al. did not provide a description of criteria for assessing the amount of subretinal hemorrhage [5], the visual acuity prognosis was better in the eyes on which a subretinal drainage was not performed than in the eyes in which there was dense submacular hemorrhage. Bennett et al. reported that the thickness of the subretinal hemorrhage had an effect on the visual acuity prognosis [3]. Our results suggested that even if a small amount of hemorrhage is present beneath the macula, removal is not necessary, since the effect on the retina is minimal. However, the issue here is assessment of the amount of submacular hemorrhage. As described by McCabe et al. who monitored the natural history in eyes with premacular hemorrhage [2], it is not possible to determine the extent of submacular hemorrhage. Accordingly, when monitoring the natural history without surgical intervention [1–4], the case in question cannot be certain to present with sub-ILM hemorrhage only, and there is still the potential for a prominent, thick hemorrhage beneath the macula. Treatment involving the dispersion of sub-ILM hemorrhage to the vitreous cavity using a YAG laser has been reported [11]. The use of a YAG laser makes it possible to determine the amount of submacular hemorrhage. However, since blood present soon after onset has a high consistency, there are cases in which the blood may not be able to be dispersed into the vitreous cavity with a YAG laser alone [11].
The natural history of the submacular hemorrhage secondary to a ruptured retinal macroaneurysm is viewed differently depending on the researcher. The percentages of best-corrected visual acuity being 20/200 or worse have been reported to be 100% by Bennett et al. [3], 50% by Berrocal et al. [4], and 34% by McCabe et al. [2]. The percentages of best-corrected visual acuity being 20/40 or better were reported to be 0%, 50% and 37% respectively. This is considered to be favorable in comparison with that of ARMD.
In our study, the postoperative visual acuity was poorer in eyes in which there was dense submacular hemorrhage. According to our study, those eyes that presented with a large amount of submacular hemorrhage demonstrated significantly poorer postoperative visual acuity in comparison with other eyes. We considered two possible causes as to why we obtained this finding. The first is that, among the cases in our study that underwent subretinal lavage, there was only one eye in which it was performed within 7 days after onset; in the rest, surgery was performed after an average of 15 days. The second possible cause is that a macular hole occurred at a high frequency during creation of artificial retinal detachment, clearly demonstrating that invasiveness caused by mechanical manipulation has an effect on the visual acuity prognosis.
Experimental studies of submacular hemorrhage in animal eyes have shown considerable damage to the retina within 1 week [12]. In laboratory animals, a mechanism for metabolic impairment of the sensory retina, iron toxicity [12 ] or fibrin-mediated retinal disorders [13] caused by interruption of the pathway with the retinal pigment epithelium due to subretinal hemorrhage has been indicated, and similar phenomena probably occur in humans as well if dense hemorrhage is present beneath the retina. Humayun et al. reported that a visual acuity of 20/40 or better was obtained in five of nine eyes on which subretinal lavage was performed within 7 days after onset [6]. This difference in the amount of time until surgery may have resulted in the difference between our results and theirs.
Recently, there have been some reports describing cases of ruptured RAM complicated with a macular hole [14, 15]. Since RAM occur in the nerve fiber layer comprising the innermost layer of the retina, the fact that hemorrhage resulting from rupture thereof reaches the subretinal space means that the bleeding has penetrated nearly the entire layer of the retinal parenchyma [16]. There is also the possibility of the neural retina being subjected simultaneously to mechanical damage. If retinal detachment is created artificially in such cases presenting with submacular hemorrhage, the anatomically fragile macula will most likely be torn easily. On the other hand, case 7 suffered a macular hole postoperatively, despite the absence of subretinal hemorrhage and not having undergone a submacular procedure. In addition, among those cases in which there was only sub-ILM hemorrhage without submacular hemorrhage, macular edema was found postoperatively in roughly half of those cases. On the basis of these findings, the macula has the possibility of being fragile even in cases free of subretinal hemorrhage.
The morphology of subretinal hemorrhage in two eyes (cases 19 and 22) was particularly interesting. Following removal of sub-ILM hemorrhage in those eyes, there was mild subretinal hemorrhage coinciding with the site of the sub-ILM hemorrhage, while dense hemorrhage was present in the shape of a doughnut around the periphery. It appeared as if the sub-ILM hemorrhage had pushed out the blood beneath the retina. A treatment has been reported involving pneumatic displacement of submacular hemorrhage with or without tPA for a mechanism of treatment resembling this phenomenon [17, 18]. Moreover, a treatment has also been reported by which favorable results were obtained by injecting tPA directly into a subretinal hemorrhage to displace the hematoma downward, in order to more reliably affect displacement of the hematoma [19, 20]. According to these methods, the submacular hematoma can be relocated from the macula with a lower degree of invasiveness.
Recurrent bleeding from the RAM occurred in 21% of patients in this series following rupture. McCabe et al., who observed the natural course, reported that recurrent bleeding was observed in one of 41 eyes [2]. In cases that underwent vitrectomy, although Hotta et al. reported one case of recurrence of postoperative subretinal hemorrhage [21], there are many reports in which there were no cases of recurrent bleeding. In this study, recurrent bleeding was observed in 21% of the cases, and some cases exhibited recurrent bleeding over a period of time as long as 10 months after surgery. The increased likelihood of recurrent bleeding in cases taking anticoagulants and in cases in which the aneurysm is pulsating has yet to be confirmed (data not shown), and the reason for the frequent occurrence of recurrent bleeding in this study is unknown. One possible hypothesis is that, as a result of having removed the vitreous and ILM in contact with the aneurysm, the tamponade effect on the aneurysm was weakened, thereby hindering organization of the aneurysm. Thus, even if the postoperative prognosis is favorable, long-term monitoring is required.
In summary, the effects of vitrectomy for hemorrhage due to ruptured retinal arterial macroaneurysms were clearly determined to be influenced greatly by the type of hemorrhage from the retinal arterial macroaneurysms. The visual acuity prognosis was poor in cases exhibiting dense subretinal hemorrhage of the macula, while it was good in other types of hemorrhage. However, since there were many cases in which hemorrhage from a retinal arterial macroaneurysm was present at various levels within the eye, it was difficult to evaluate the amount of submacular hemorrhage prior to surgery. Surgical complications included a macular hole, while postoperative complications included a high frequency of recurrent bleeding from the aneurysm. We believe this study has provided important suggestions in terms of determining the indication for surgery with respect to retinal arterial macroaneurysms, for which a final decision has yet to be reached. Since this study was conducted retrospectively at a single institution on a relatively small number of cases, it will be necessary in the future to conduct a multi-center, prospective, randomized study.
References
Asdourian GK, Goldberg MF, Jampol L, Rabb M (1997) Retinal macroaneurysms. Arch Ophthalmol 95:624–628
McCabe CM, Flynn HW Jr, Mclean WC, Brod RD, McDonald HR, Johnson MW, Williams GA, Mieler WF (2000) Nonsurgical manegement of macular hemorrhage secondary to retinal artery macroaneurysms. Arch Ophthalmol 118:780–785
Bennett SR, Folk JC, Blodi CF, Klugman M (1990) Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol 109:33–37
Berrocal MH, Lewis ML, Flynn HW Jr (1996) Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol 122:486–493
Zhao P, Hayashi H, Oshima K, Nakagawa N, Ohsato M (2000) Vitrectomy for macular hemorrhage associated with retinal arterial macroaneurysm. Ophthalmology 107:613–617
Humayun M, Lewis H, Flynn HW Jr, Sternberg P Jr, Blumenkranz MS (1998) Management of submacular hemorrhage associated with retinal arterial macroaneurysms. Am J Ophthalmol 126:358–361
Lim JI, Drews-Botsh C, Sternberg P Jr, Capone A Jr, Aaberg TM Sr (1995) Submacular hemorrhage removal. Ophthalmology 102:1393–1399
Lewis H (1994) Intraoperative fibrinolysis of submacular hemorrhage with tissue plasminogen activator and surgical drainage. Am J Ophthalmol 118:559–568
Foos RY (1972) Vitreoretinal juncture; topographical variations. Invest Ophthalmol 11:801–808
Kuwabara T, Cogan DG (1960) Studies of retinal vascular patterns. 1. Normal architecture. Arch Ophthalmol 64:904–911
Iijima H, Satoh S, Tsukahara S (1998) Nd:YAG laser photodisruption for preretinal hemorrhage due to retinal macroaneurysm. Retina 18:430–434
Glatt H, Machemer R (1982) Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol 94:762–773
Toth CA, Morse LS, Hjelmeland LM, Landers MB 3rd (1991) Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol 109:723–729
Colucciello M, Nachbar JG (2000) Macular hole following ruptured retinal arterial macroaneurysms. Retina 20:94–96
Tashimo A, Mitamura Y, Ohtsuka K, Okushiba U, Imaizumi H, Takeda M (2003) Macular hole formation following ruptured retinal arterial macroaneurysm. Am J Ophthalmol 135:487–492
Perry HD, Zimerman LE, Benson WE (1997) Hemorrhage from isolated aneurysm of a retinal artery: Report of two cases simulating malignant melanoma. Arch Ophthalmol 95:281–283
Hassan AS, Johnson MW, Schneiderman TE, Regillo CD, Tornambe PE, Poliner LS, Blodi BA, Elner SG (1999) Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology 106:1900–1906
Ohji M, Saito Y, Hayashi A, Lewis JM, Tano Y (1998) Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch Ophthalmol 116:1326–1332
Oliver S, Chow DR, Packo KH, MacCumber MW, Awh CC (2004) Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in age-related macular degeneration. Ophthalmology 111:1201–1208
Singh Singh RP, Patel C, Sears JE (2006) Management of subretinal macular hemorrhage by direct administration of tissue plasminogen activator. Br J ophthalmol 90:429–431
Hotta K, Hotta J (2006) Case of recurrent macular haemorrhage after removal of a sub-internal limiting membrane haematoma secondary to retinal artery macroaneurysm. Clin Experiment Ophthalmol 34:610–612
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have no proprietary interest in any aspect of this report.
Rights and permissions
About this article
Cite this article
Nakamura, H., Hayakawa, K., Sawaguchi, S. et al. Visual outcome after vitreous, sub-internal limiting membrane, and/or submacular hemorrhage removal associated with ruptured retinal arterial macroaneurysms. Graefes Arch Clin Exp Ophthalmol 246, 661–669 (2008). https://doi.org/10.1007/s00417-007-0724-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0724-0