Abstract
Background
To report on the spontaneous closure of a full thickness juxtafoveolar idiopathic macular hole (IMH) monitored with fundus autofluorescence (AF) as well as optical coherence tomography (OCT) imaging.
Methods
Observational case report. Fundus Autofluorescence with a confocal SLO (HRA, Heidelberg Engineering,Germany) and OCT imaging were used to monitor the spontaneous evolution of a stage II IMH.
Results
A 70 year-old woman with unremarkable ocular history received a diagnosis of idiopathic macular hole in the left eye. Bright autofluorescence corresponding to the IMH was documented with the confocal SLO and OCT imaging could confirm the presence of an hour glass shaped full thickness juxtafoveolar IMH. Biomicroscopy revealed no posterior vitreous detachment (PVD). Few months later clinical examination demonstrated the presence of typical symptoms and signs of PVD (miodesopsias and Weiss ring). The bright autofluorescence corresponding to the IMH disappeared and OCT imaging documented a normal fovea in morphology and thickness.
Conclusions
Spontaneous closure of full thickness juxtafoveolar IMH can occur and may be properly monitored with fundus AF as well as OCT imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The macular hole is a full thickness defect of retinal tissue involving the anatomical fovea. Significant controversy still exists regarding the pathogenesis, prognosis and finally the management of this lesion [1].
In the last years optical coherence tomography (OCT) has been introduced into clinical practice and has improved our knowledge about the onset and progression of idiopathic macular hole (IMH). A new classification of macular hole based upon OCT findings has been proposed [2]. Fundus Autofluorescence (AF) imaged using a confocal scanning laser ophthalmoscope (SLO) derives from the lipofuscin laden retinal pigment epithelium (RPE) [3]. The latter is attenuated by the luteal pigment in the macula. Therefore, an autofluorescent spot in the macula is consistent with a loss of foveal tissue, either partial (lamellar macular hole) or complete (full thickness macular hole) [4].
Although full thickness macular hole rarely close spontaneously, we followed up and monitored with OCT and AF the spontaneous closure of a stage II small eccentric IMH.
Case report
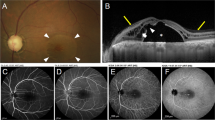
In February 2003, a 70 year-old woman with unremarkable ocular history presented a complaint of decreased vision in her left eye for two months. She did not report any trauma. Her best corrected visual acuity (VA) was 0.3 with metamorphopsia LE and 0.8 RE. Slit lamp examination showed a mild nuclear sclerosis bilaterally with no further abnormalities of the anterior segment. Fundus examination revealed no pathologic findings in the right eye. In the left eye, slightly superior to the fovea, a small stage II (Gass definition) IMH [1] with no posterior vitreous detachment (PVD) was evident. OCT could confirm the presence of an hour glass shaped stage III IMH (Azzolini et al. definition) (Fig. 1) [2]. Bright autofluorescence corresponding to the IMH was documented with a confocal SLO (HRA, Heidelberg Engineering, Germany) (Fig. 2).
Optical coherence tomography tomogram (5 mm vertical scan) of the full-thickness foveal defect at baseline. There is no residual retinal tissue on the bottom of the hole (Stage 3 according to the classification of Azzolini et al.) [2]
Vitrectomy was suggested but the patient denied surgical intervention. She was scheduled for monthly controls in which visual acuity and fundus evaluation were substantially stable. In particular, a Weiss ring remained absent biomicrosopically.
In December 2003, she presented a report of improved VA in the left eye for a few weeks associated with miodesopsias and disappearance of metamorphopsia. Best corrected VA was 0.8 LE and 0.8 RE. Biomicroscopy of the left fundus revealed a Weiss ring associated with PVD. No macular lesions were visible bilaterally. OCT imaging of the left fovea was substantially normal in morphology and thickness, demonstrating the complete closure of the iuxta-foveolar hole (Fig. 3).
Consistently, autofluorescence imaging showed no AF in the macula bilaterally (Fig. 4). Five months later, in May 2004, VA, ophthalmoscopy, OCT and AF remained stable.
Discussion
Macular hole can be traumatic or idiopathic, and although several reports have been published concerning spontaneous closure of macular holes, information is still limited. Various explanations have been proposed for spontaneous resolution of IMH: the release of vitreous traction on the edge of the hole, cell proliferation at the base of the hole, and contractile epiretinal membrane proliferation, resulting in the shrinkage and closure of the hole are likely to be important factors.
In our case, OCT showed a small full thickness microhole ( 50–150 micron) that can be classified as stage II IMH according to Gass [1]. The typical signs of partial or complete detachment of the posterior hyaloid from the macular area were absent either by ophthalmoscopy or OCT. A posterior vitreous detachment with a Weiss ring became evident only a few months after first presentation and corresponded to VA improvement, disappearance of metamorphopsia and sudden occurrence of miodesopsias. Consequently it’s likely that the release of vitreous traction in the macular area facilitated the spontaneous closure of the hole.
Our hour-glass shaped microhole had also a small opening area. That could have allowed glial cell proliferation to bridge the gap between the edges leading to spontaneous closure.
Disappearance of AF from the macular hole, like after successful surgical repair [4, 5], suggests that indeed in our case the RPE was again covered by retinal and/or glial tissue, as demonstrated also by OCT images.
In summary we showed that spontaneous closure of stage II full thickness juxtafoveolar IMH may be properly monitored with fundus AF as well as OCT imaging. Fundus autofluorescence may be considered a useful alternative to OCT in the investigation of macular holes.
References
Gass JDM (1995) Reappraisal of biomicroscopic classification of stages of development of a macular hole. Am J Ophthalmol 119:752–759
Azzolini C, Patelli F, Brancato R (2001) Correlation between optical coherence tomography data and biomicroscopic interpretation of idiopathic macular hole. Am J Ophthalmol 132:348–355
Von Ruckmann A, Fitzke FW, Bird AC (1995) Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Brit J Ophthalmol 79:407–412
Von Ruckmann A, Fitzke FW, Gregor ZJ (1998) Fundus autofluorescence in patients with macular holes imaged with a laser scanning ophthalmoscope. Brit J Ophthalmol 82:346–351
Ciardella AP, Lee GC, Langton K, Sparrow J, Chang S (2004) Autofluorescence as a novel approach to diagnosing macular holes. Am J Ophthalmol 137:956–959
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milani, P., Seidenari, P., Carmassi, L. et al. Spontaneous resolution of a full thickness idiopathic macular hole: fundus autofluorescence and OCT imaging. Graefes Arch Clin Exp Ophthalmol 245, 1229–1231 (2007). https://doi.org/10.1007/s00417-006-0530-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-006-0530-0