Abstract
Background
Fundus autofluorescence (AF) in some patients with retinitis pigmentosa is characterized by a parafoveal ring of increased AF which surrounds the centre as hypofluorescent changes appear at the periphery. The aim of this study was to evaluate the AF patterns in relation to retinal function measured by electroretinography and visual fields.
Methods
Thirty-two patients with RP were included in the study. AF imaging of the macular area was performed with the scanning laser ophthalmoscope. Patients were divided in two groups according to their fundus AF patterns. All patients from group 1 had a ring of increased AF of different size but no atrophic areas inside vascular arcades. Patients with a ring of increased AF and round atrophic changes at different eccentricities from their fovea were selected in group 2. Visual fields were tested with kinetic, automated perimetry and microperimetry; the radius of the hyperfluorescent ring and the smallest distance of hypofluorescent areas from the fovea were compared to visual fields, PERG P50 and N95 and mfERG P1 amplitudes of the inner three rings.
Results
A linear relationship was found in group 1 between the radius of the ring of increased AF and both the automated (r=0.82) and kinetic perimetry (r=0.80). The radius of the AF ring correlated highly with the PERG P50 (r=0.72) and N95 (r=0.74) amplitudes. In all patients, mfERG responses were reduced at all retinal locations, more pronounced at periphery. There was a good correlation between the ring of increased AF and the P1 amplitude of ring 2 of mfERG (r=0.62). Patients from group 2 had significantly reduced or non-recordable PERGs and mfERGs. The eccentricities of hypofluorescent changes did not correlate with any type of perimetry.
Conclusions
Our results show that in stages of retinitis pigmentosa, before atrophic lesions spread inside the vascular arcades, the pattern of fundus autofluorescence correlates well with functional tests such as perimetry and electroretinography. The ring of increased AF appears to represent the border between functional and dysfunctional retina. This shows that autofluorescence, as a quick and non-invasive imaging tool, may be related to retinal function as well.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fundus autofluorescence (AF) imaging with confocal scanning laser ophthalmoscope is a relatively new method that shows distribution of lipofuscin in the RPE in vivo [4–6, 24]. Lipofuscin accumulates in lysosomes as a byproduct of the photoreceptor outer segment renewal [15]. Different patterns of abnormal AF were observed in patients with retinitis pigmentosa (RP), showing a high interocular symmetry in most cases. In certain stages of RP, where there appears to be a higher metabolic demand for the RPE due to photoreceptor loss, accumulation of lipofuscin is frequently shown in the form of a narrow parafoveolar ring of increased autofluorescence [12, 20, 25]. In regions and stages where RPE is no longer viable, AF is reduced or absent. This manifests itself in the form of dark circular areas, usually in the periphery, but they may also be observed in the foveolar region [12, 26]. It has been shown that decrease in retinal sensitivity, measured by the pattern ERG (PERG) and perimetric fine matrix mapping, is associated with the retinal area outside the hyperfluorescent ring [20, 21]. No studies have been published, however, that would show how different patterns of AF in RP correspond to classic perimetric methods in addition to fundus-controlled microperimetry, and relate these to electrophysiology. Only recent data have provided some information on multifocal ERG (mfERG) in relation to AF [22]. The objective of the study is therefore to evaluate features of hyper-and hypo-fluorescent areas as seen on AF imaging in relation to the retinal function measured by perimetry and electroretinography in RP patients with well preserved central visual acuity.
Materials and methods
Thirty-two patients with rod-cone RP of autosomal dominant, autosomal recessive and sporadic form were included in the study. Clinical diagnosis of RP has previously been confirmed with full field ERG [18]. All patients had good visual acuity, 0.67 or better. The study protocol included the complete clinical examination, kinetic and static perimetry, AF imaging, and electrophysiology (PERG and mfERG). Eyes with complications of RP, such as cataract or macular edema or abnormalities other than RP, were excluded from the study.
The tenets of the Declaration of Helsinki were followed and all patients signed informed consent after a full explanation of the procedures had been given. The study was approved by the medical ethics committee at the Ministry of Health of the Republic of Slovenia.
Fundus autofluorescence
AF images were obtained using a confocal scanning laser ophthalmoscope (Heidelberg Retina Angiograph, HRA; Heidelberg Engineering, Heidelberg, Germany). AF images of a rectangular 30°×30° field of view were recorded after pupillary dilatation with tropicamide 1%. The ametropic corrector was used to correct for refractive error and to focus on the central retina. To record AF, the argon blue laser light (488 nm) was used for excitation and a wide band-pass filter with cut-off at 521 nm was inserted in front of the detector. A series of at least 16 images were recorded, digitized, aligned for eye movements and averaged to reduce noise. As a result, a single image measuring 512×512 pixels was obtained.
Electrophysiology
PERG and mfERG were recorded using the RETIscan system (Roland Consult, Wiesbaden, Germany) with HK-loop electrodes [8] according to ISCEV standards and guidelines [2, 17]. PERG was evoked by a checkerboard pattern reversing at 3 revs/sec, subtending the field size of 12° vertically and 16° horizontally. The check size was 1° and the contrast 98%. Recordings were done binocularly with optimal refraction. The stimulus for mfERG consisted of 61 hexagons displayed on a monitor with 75 Hz frame rate and scaled with eccentricity. The recordings were done binocularly with dilated pupils to obtain responses from the 30° on each side of fixation. Two narrow diagonal lines were used for fixation. Five ring averages were calculated by averaging amplitudes for areas of the same eccentricity.
Visual fields
Goldmann kinetic and automated static perimetry (Octopus 101, Interzeag, Switzerland) were used. As in almost all cases the pattern of visual field loss in our subjects was concentric, the preserved visual field in kinetic perimetry was calculated as the average radius of all meridians tested with target II/4e. In static perimetry, the G2 TOP program measuring retinal sensitivity at 59 locations in central 30° of visual field was used. The mean sensitivity index (MS), the average of all measured values of retinal sensitivity in dB, was used for comparisons. Microperimetry was done with microperimeter MP1 (Nidek Technologies, Japan). MP1 is an instrument for functional mapping that superimposes the sensitivity map on the retinal image, taken by incorporated non-mydriatic fundus camera. It measures the retinal sensitivity at selected locations while the examiner simultaneously controls patients’ fixation on fundus image. Eye movements are corrected by the incorporated eye tracking system. Retinal sensitivity at selected location is measured as the threshold sensitivity of different intensities of stimuli and is represented on numerical and colour coded scale. Number 0 designates the absolute scotoma for a chosen stimulus size. Program Humphrey10, testing 56 retinal locations in central 10 degrees with 2° resolution at threshold sensitivities from 0–20 dB, was used, the test spot size was 4 mm2. To compare the sensitivity map directly with AF features, the former was superimposed on the AF image. To ensure that testing points were exactly on the same location in MP 1 and the AF image, superimposition of the blood vessels pattern was done using the Adobe Photoshop 7.0 (Adobe Systems, Mountain View, Calif., USA) program.
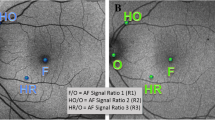
All patients had an abnormal AF in the form of a parafoveal ring of increased AF of different size. To investigate functional implications of areas with increased or decreased AF, patients were divided into two groups. Those from group 1 had no other abnormality seen on AF (inside 30°×30° of view). Patients from group 2 had a ring of increased AF but also focal areas of reduced or non-detectable AF of different distribution and size outside the ring at different eccentricities from the fovea.
In the analysis, the horizontal inner radius of the hyperfluorescent ring and the smallest distance of the areas with reduced AF from the fovea were compared to Octopus MS index and Goldmann II/4e average radius. The ring of increased AF was also studied in relation to PERG P50 and N95 amplitudes and to average P1 amplitudes of the inner three rings of mfERG. The inner radius of increased or reduced AF was measured in degrees assuming that the visual angle between the fovea and the centre of the optic disc subtends 15°. In contrast to outer border of the ring of increased AF the inner border is usually well demarcated and clearly stands out. According to study settings (scaling), P1 amplitude of ring 1 represented the electrical activity of retina between 0 and 2.5°, of ring 2 between 2.5° and 8° and of ring 3 between 8° and 15° eccentricity from the fovea. Figure 1 shows the AF images representative of groups 1 and 2 as well as the retinal region where the AF, PERG and mfERG responses were recorded.
Results
Group 1
There were 19 patients (7 male and 12 female) with classical rod-cone RP. Their age ranged from 13 to 61 (median age 30) years and they had no focal areas of increased or decreased AF inside or outside the hyperfluorescent ring. In addition, the fovea had the AF appearance of a slightly reduced AF that is shown in normals. The inner horizontal radius of the ring varied from 1.5° to 9° of eccentricity and the width of the ring was 1–2°. All patients had high interocular symmetry of typical clinical signs of RP and good visual acuity (group average 0.84±0.1). Peripheral visual field constriction with II/4e isopter varied from 5 to 51 degrees with an average of 22±14°. The average MS index was 10±5 dB. PERGs varied in amplitude from normal to reduced responses according to macular involvement (range 0.50–3.10 μV for P50 and 1.57–4.88 μV for N95 which for the group as a whole represents 0.6±0.3 of the normal mean). There were no interocular differences of amplitudes and P50/N95 ratio was within normal range. Multifocal ERGs were reduced in all patients. According to the nature of the disease, the peripheral rings were affected more than the central one. The mean value of P1 amplitude for a group as a whole was reduced to 0.5±0.18 (range 0.4–0.9) of normal mean in ring 1, to 0.33±0.17 (range 0.2–0.6) in ring 2, and to 0.21±0.13 (from non-detectable to 0.3) in ring 3.
Group 2
Group 2 consisted of 13 (10 male and 3 female) patients with a classical rod-cone RP with hypofluorescent or absent AF areas outside of the hyperfluorescent ring. The ring of increased AF in this group varied between 1.5° and 5° of eccentricity from the fovea, its width was 1–2°. Inside the ring, the AF image of the macula appeared normal. Focal circular lesions of absent AF of different sizes were present, in some cases just around the ring, in others at a greater distance. Visual acuity was good in this group also (0.7±0.1). Visual fields tested with Goldmann II/4e target were constricted to 6°±2, and the average MS index for a group was 4 dB±2. PERGs were significantly reduced or non-detectable in all except four patients. Multifocal ERGs were preserved in ring 1 in some patients (4/13) but were very low (reaching less than 20% of amplitude of the normal mean).
An inverse linear relationship between the age of the patient and the radius of the ring of increased AF (r=−0.49) was found. A summary of clinical, psychophysical and electrophysiology results of patients from groups 1 and 2 are shown in Table 1. Significant differences were found with the Student t-test between the two groups for all tested parameters (MS, average radius II/4e, PERG and mfERG amplitudes, for all P<0.001) except the ring radius of increased AF (P<0.03).
AF image in relation to functional tests
To analyze the functional significance of the high density ring of increased AF, patients from both groups were compared. Since for the patients from group 2 the PERGs and mfERGs were mostly non-detectable, the majority of data comes from patients from group 1.
Patients with more preserved peripheral visual fields had larger radius of the hyperfluorescent ring. In patients who showed field constriction, the AF ring was located closer to the fovea. As shown in Fig. 2, strong correlation was found between the radius of the hyperfluorescent ring and the MS index of static perimetry (r=0.82). The inner radius of the AF ring also correlates highly with Goldmann II/4 average radius (r=0.80).
Comparison of the radius of the ring of increased AF with (a) mean sensitivity index of automated perimetry (Octopus) and (b) average radius of preserved visual fields measured with Goldmann kinetic perimetry (target II/4e). Correlation coefficients (r) are shown on graphs, P-value for both comparisons <0.001
Through comparing PERG amplitudes and the size of the ring of increased AF, a linear relationship was found. Both PERG amplitudes correlate highly with the ring of increased AF: the correlation coefficient for P50 amplitude was 0.72 and for N95 amplitude 0.74 (Fig. 3).
In comparison to multifocal ERG (Fig. 4), no correlation between P1 amplitude of the ring 1 and eccentricity of the increased AF was found (r=0.2). Correlation between P1 amplitude of the mfERG and the radius of increased AF was better for the mfERG ring 2 (r=0.62). As mentioned above, the retinal area stimulated by the central hexagon, i.e. the first mfERG ring, fell within the AF ring area in all cases while that of the second mfERG ring (between 2.5° and 8°) corresponded to the retina with increased AF. The P1 amplitudes of mfERG ring 3 also did not correlate with AF ring radius (r=0.2) - in this case, the mfERG stimulus fell outside the area where the AF rings were measured.
Comparison of the P1 amplitudes of the (a) first, (b) the second and (c) the third ring of mfERG in relation to the radius ring of increased AF. A good correlation was found for the average response in the second ring of mfERG, which corresponds to the retinal eccentricity where higher AF in a form of a ring was detected
The summed response of the inner two mfERG rings correlated well with the eccentricity of increased AF (r=0.62) and PERG P50 amplitude (r=0.72) (Fig. 5). The eccentricity of hypofluorescent AF areas did not correlate with any type of perimetry (r=0.3 for kinetic and r=0.2 for static perimetry).
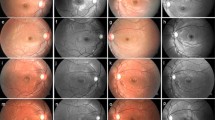
Figure 6 shows the summary of results for three patients with RP; the results of AF imaging, perimetry, and electrophysiology are presented. The reduction of retinal function measured by automated perimetry, PERG, and mfERG in relation to size of the ring of increased AF is clearly demonstrated. The results of fundus-controlled microperimetry with MP1 are superimposed on AF images. Note the correspondence of retinal sensitivity with the ring of increased AF representing the border between functional and dysfunctional retina.
Comparison of autofluorescence images, perimetry, mfERGs and PERGs from three patients in different stages of RP. Results of microperimetry are superimposed on fundus AF images. Note the agreement of the size of high density ring with retinal sensitivity measured by microperimetry (shown in colour coded scale) as well as with both electroretinographic responses. Outside the ring of increased AF the retinal function is lost despite no atrophy is detected with AF (RP1 and RP2)
Figure 7 shows the AF imaging, automated perimetry, PERG and mfERG of two patients with RP. The size of the ring of increased AF as well as PERG and mfERG amplitudes was comparable in both cases, although patient 2 had, in addition to the ring of increased AF, areas of reduced AF just outside the hyperfluorescent ring.
Discussion
In RP, different patterns of abnormal fundus AF have been reported [12, 25, 28]. Based on our experience, almost half of affected individuals demonstrate an area of increased AF ring on the posterior pole during certain stage of their disease. There is no generally accepted opinion about the time changes of the ring of increased AF in RP patients. Considering inverse relationship between the age and the ring radius, we speculate that with progression of the disease the ring of increased AF constricts and moves towards the fovea. Outside this ring, new areas of reduced AF emerge and coalesce as they move towards the centre.
RPE changes in RP are secondary phenomena and result from increased metabolic load for the RPE due to photoreceptor apoptosis [1, 29]. Accumulation of lipofuscin and therefore increased AF intensity is caused by faster turnover of photoreceptors due to genetic causes or through disturbed and ineffective disintegration of their remnants in the RPE [15, 24]. On the other hand, lipofuscin does not form when photoreceptors are absent, when phagocytosis is disturbed or when the RPE is atrophic. In such cases, AF is reduced or absent [7, 12, 26]. Despite recent interest for the study of AF in RP, functional implication of increased or diminished AF intensity at RP is still not fully explained. For successful implementation of future therapeutic applications, information about whether the retina on a certain location is only dysfunctional or also structurally affected is crucial. Robson et al. [20] showed in three members of the family with AD RP that patients with a larger AF ring also had larger central fields. The same group first suggested that the border of the increased AF represented the border of the functional retina [11]. This was supported by linear increase of the PERG amplitude with an increase of the stimulus field size. While the stimulus field was inside the ring of the increased AF, the relationship was linear. Outside this ring, however, there was no additional increase of function as measured by PERG [11, 20]. In addition, it has been shown recently that the ring of increased AF better correlates to photopic than scotopic retinal sensitivity [21].
Our results confirm that the ring of increased AF encircles the area of preserved (photopic) retinal sensitivity. We have shown that the size of this ring correlates well with both kinds of perimetry. Patients with better preserved visual fields had larger ring of increased AF. Despite variability and suprathreshold stimulus of kinetic perimetry in patients with RP [23], we were able to show a somewhat parallel loss of function with the size of the AF ring. Better correlation was observed with the static perimetry (Octopus) where the absolute threshold of the retinal sensitivity showed high correlation with the radius of the AF ring.
We also noticed that in some cases the retinal function may be absent outside of the AF ring regardless of whether the autofluorescence pattern outside the ring looked normal or not. We have illustrated this by using microperimetry. This is demonstrated in Fig. 6, where RP patients in different stages of the disease manifest a ring of increased AF of different size, while the sensitivity map of microperimetry was superimposed directly on the AF image. It is shown clearly that outside of the ring, retinal sensitivity is lost regardless of whether the AF pattern is normal or whether atrophic lesions of the RPE are already present or not. The retinal function is normal inside the ring (green), declines on the ring (yellow) and is absent outside of it (red). Therefore, the border of the AF ring, and not RPE atrophy, is clinically related to preserved retinal function.
We confirmed the results of Robson et al., namely that the relationship between the PERG amplitudes and the distance of the increased AF ring from fovea is linear [20]. Good correlation with objective measure of macular function therefore indicates the functional importance of the AF ring radius. For group 2, where patients had atrophic lesions of the RPE in addition to a hyperfluorescent ring, PERGs were significantly more affected despite comparable AF ring size, visual acuity and visual fields. However, in some cases this may not be so clearly evident (Fig. 7).
We addressed the question on relationship between the AF ring and macular function in RP patients also by using the mfERG, which has not yet been investigated. The relationship between mfERG and fundus-controlled perimetry with another instrument, namely scanning laser ophthalmoscope, has been shown by Rohrschneider et al. [19]. In four patients with advanced RP who had only a central mfERG response, good agreement of both methods was found, but no comparison was made with the pattern of fundus autofluorescence.
PERG is thought to arise predominantly from the inner retina, driven by macular photoreceptors, and was shown to be less affected in RP [9, 10]. Multifocal ERG reflects predominantly outer retinal function [13, 14]. The mfERG responses were accordingly reduced to greater extent than the PERG responses. Our patients had relatively well preserved central mfERG responses, however, and no correlation was found with the size of AF ring. This may be due to the fact that the area of stimulation by the central mfERG hexagon was well within the functional retina and thus similarly preserved in all cases. The second ring of mfERG, however, which was testing retinal function at eccentricities between 2.5 and 8 degrees of visual angle, showed good correlation with the AF ring radius. The stimulated area of these responses corresponds to retinal location where the AF was increased. After summation of the inner two rings of mfERG, the summed response correlated well with the AF ring size and PERG amplitude. The third ring of mfERG that stimulated the retinal area outside the hyperfluorescent ring showed almost no function and no correlation with AF ring size. Overall reduction of mfERG seen in central hexagon showed that the macula is involved to some extent even in cases where the ring of increased AF is still distant. Using mfERG, we obtained topographical functional information inside, across, and outside the AF ring and we found that the highest correlation was displayed over the expected area of hyperfluorescent ring. The summation of function of all contributing cells inside the AF area, namely, the inner two rings of mfERG, showed high correlation both with AF ring and with PERG amplitude.
The results of our study raise several interesting questions. We showed, using clinically available functional tests, that the ring of increased autofluorescence is associated with loss of retinal function in retinitis pigmentosa. It appears that the ring is travelling in concentric fashion towards the macula as the disease progresses. From our results as well as from results of previous studies, it is quite evident that the retina outside the hyperfluorescent ring is not functional even before any RPE atrophy can be observed by AF imaging. This would assume that in patients who show a ring of increased AF this may relate to some kind of functional barrier at the border of the AF ring that may not correspond to morphological appearance and is not readily visible with any other method except AF. It would be interesting to explore further if normally looking but non-functional retina outside of the ring is structurally preserved and may be rescued when new treatment modalities [3] become available. This question was recently discussed in the work of Scholl et al., who also found well preserved autofluorescence patterns in three patients with presumed Leber’s congenital amaurosis and speculated that normal AF would mean that, as disc turnover in the retina appears to be occurring, preserved structure of these photoreceptors may be assumed [27]. Interestingly, no intrinsic autofluorescence was found in patients with RPE 65 subtype of Leber’s congenital amaurosis [16], the animal model of which has been successfully treated by gene replacement therapy. Genetic characterization is important in RP, but our results still show that different inheritance types of this disease exhibit similar ring appearance of autofluorescence pattern. It is not clear whether or not this reflects primary topographical pattern of photoreceptor death or whether it involves common secondary mechanisms; this question would be of some use in developing new treatment strategies. Autofluorescence imaging may become a valuable structural examination able to identify areas of renewed retinal function as well.
In summary, this study addressed the question how the AF imaging, which displays the accumulation of lipofuscin in the retina, is related to retinal function in RP. Results show that the size of a ring of increased AF correlates well with results of psychophysical and electrophysiology tests. The conclusion is based on observed linear decline with the ring size compared to those tests (visual field, as well as PERG and mfERG), and the age of patients. The existence of areas of absent AF outside the ring indicates worse prognosis with the same ring size, greater macular involvement and shows the structural disintegration of photoreceptors and the RPE. Being a non-invasive method, AF can therefore serve as a useful imaging tool in the follow-up of RP patients. This is especially true in the late stages of the disease with macular involvement when all other tests of retinal function fail to produce measurable results.
References
Adler R (1996) Mechanisms of photoreceptor death in retinal degenerations. Arch Ophthalmol 114:79–83
Bach M, Hawlina M, Holder GE, Marmor MF, Meigen T, Vaegan, Miyake Y (2000) Standard for pattern electroretinography. Doc Ophthalmol 99:11–18
Besch D, Zrenner E (2003) Prevention and therapy in hereditary retinal degenerations. Doc Ophthalmol 106:31–35
Delori FC, Dorey CK, Staurenghi G et al. (1995) In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 36:718–729
Delori FC, Staurenghi G, Arend O, Dorey CK et al. (1995) In vivo measurement of lipofuscin in Stargardt’s disease- fundus flavimaculatus. Invest Ophthalmol Vis Sci 36:2327–2331
Delori FC, Goger DG, Dorey CK (2001) Age-related accumulation and spatial distribution of lipofuscin in RPE in normal subjects. Invest Ophthalmol Vis Sci 42:1855–1866
Dorey CK, Wu G, Ebenstein D, Garsd A, Weiter JJ (1989) Cell loss in the aging retina. Relationship to lipofuscin accumulation and macular degeneration. Invest Ophthalmol Vis Sci 30:1691–1699
Hawlina M, Konec B (1992) New noncorneal HK-loop electrode for clinical electroretinography. Doc Ophthalmol 81:253–259
Hawlina M, Jarc M, Popovic P (1998) Pattern ERG is preserved in retinitis pigmentosa—a follow up study. Electroncephalogr Clin Neurophysiol 106:46
Holder GE (2001) Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res 20:531–561
Holder GE, Robson AG, Hogg CR, Kurz-Levin M, Lois N, Bird AC (2003) Pattern ERG: clinical overview, and some observations on associated fundus autofluorescence imaging in inherited maculopathy. Doc Ophthalmol 106:17–23
Holz FG (2001) Autofluorescenz-imaging der Makula. Ophthalmologe: 98:10–18
Hood DC (2000) Assessing retinal function with the multifocal technique. Prog Ret Eye Res 19:607–646
Hood DC, Frishman LJ, Saszik S, Viswanathan S (2002) Retinal origins of the primate multifocal ERG: Implications for the human response. Invest Ophthalmol Vis Sci 43:1673–1685
Kennedy CJ, Rakoczy PE, Constable IJ (1995) Lipofuscin of the retinal pigment epithelium: a review. Eye 9:763–771
Lorenz B, Wabbels B, Wegscheider E, Hamel CP, Drexler W, Preising MN (2004) Lack of fundus autofluorescence to 488 nanometers from childhood on in patients with early-onset severe retinal dystrophy associated with mutations in RPE65. Ophthalmology 111:1585–1594
Marmor MF, Hood DC, Keating D, Kondo M, Seeliger MW, Miyake Y (2003) Guidelines for basic multifocal electroretinography (mfERG). Doc Ophthalmol 106:105–115
Popović P, Jarc-Vidmar M, Brecelj J, Hawlina M (2002) Pattern electroretinography in relation to perimetry and visual acuity in patients with retinitis pigmentosa. Zdrav Vestn 71:119–124
Rohrschneider K, Bültmann S, Kiel R, Weimar P, Krastel H, Blankenagel A (2002) Diagnostik bei Netzhauterkrankungen. Vergleich von multifokalem ERG und funduskontrollierter Perimetrie—Fallstudie. Ophthalmologe 99:695–702
Robson AG, El-Amir A, Bailey C, Egan CA, Fitzke FW, Webster AR, Bird AC, Holder GE (2003) Pattern ERG correlates of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 44:3544–3550
Robson AG, Egan CA, Luong VA, Bird AC, Holder GE, Fitzke FW (2004) Comparison of fundus autofluorescence with photopic and scotopic fine-matrix mapping in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 45:4119–4125
Robson AG, Egan CA, Bird AC, Fitzke FW, Holder GE (2003) Multi-focal ERG, pattern ERG and psychophysical correlates of fundus autofluorescence abnormalities in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 44:E-Abstract 535
Ross DF, Fishmann GA, Gilbert LD (1984) Variability of visual field measurements in normal subjects and patients with retinitis pigmentosa. Arch Ophthalomol 102:1004–1010
von Rückmann A, Fitzke FW, Bird AC (1995) Distribution of fundus autofluorescence with scanning laser ophthalmoscope. Br J Ophthalmol 79:407–412
von Rückmann A, Fitzke FW, Bird AC (1997) In vivo fundus autofluorescence in macular dystrophies. Arch Ophthalmol 115:609–615
von Rückmann A, Fitzke FW, Bird AC (1999) Distribution of pigment epithelium autofluorescence in retinal disease state recorded in vivo and its change over time. Graefe’s Arch Clin Exp Ophthalmol 237:1–9
Scholl HP, Chong NH, Robson AG, Holder GE, Moore AT, Bird AC (2004) Fundus autofluorescence in patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci 45:2747–2752
Wegscheider E, Preising MN, Lorenz B (2004) Fundus autofluorescence in carriers of X-linked recessive retinitis pigmentosa associated with mutations in RPGR, and correlation with electrophysiological and psychophysical data. Graefe’s Arch Clin Exp Ophthalmol 242:501–511
Wong F (1995) Photoreceptor apoptosis in animal models. Implications for retinitis pigmentosa research. Arch Ophthalmol 113:1245–1247
Acknowledgements
This study was supported by the grant of Ministry of Education, Science and Sports of Republic of Slovenia No. J3-4393. and was in part presented at 2004 ARVO meeting, Fort Lauderdale, USA. Authors are grateful to Dr. Jelka Brecelj for valuable comments and advice and to Barbara Klemenc for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Popović, P., Jarc-Vidmar, M. & Hawlina, M. Abnormal fundus autofluorescence in relation to retinal function in patients with retinitis pigmentosa. Graefe's Arch Clin Exp Ophthalmo 243, 1018–1027 (2005). https://doi.org/10.1007/s00417-005-1186-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-1186-x