Abstract
Background
Double filling (DF) with perfluorohexyloctane (F6H8) and silicone oil (SIL) has been recently proposed and tested clinically as a means to improve the tamponade properties of single components. This in vitro study investigated (1) the kinetics of the mixing process of F6H8 with SIL (1,000 mPa s) and (2) the contact and emulsification behaviour of DF as compared with pure liquids, with the aim of assessing the tamponade efficiency and its evolution with time.
Methods
(1) The velocity of the mixing process for F6H8+SIL was estimated by monitoring the position of the interphase in a rectangular cell kept at constant temperature. (2) The surface contact and the tendency to emulsification of DF and of SIL and F6H8 were visually examined by using a Perspex eye model.
Results
(1) The mixing process for F6H8+SIL is slow. In the absence of stirring, equilibrium is reached no earlier than 1 month at 37°C. (2) F6H8 was found to show close contact with the eye model and dispersion into droplets; SIL showed poor contact with the cell surface and no dispersion; DF exhibited poor contact with the superior cell surface and little evidence of dispersion.
Conclusions
F6H8 dissolves slowly in SIL and equilibrium is only reached after 1 month. The final ratio of the DF phases differs from the initial ratio. An initial F6H8/SIL ratio of 70%:30% vol results in 25% vol of pure F6H8 (density, 1.33 g/cm3) and 75% vol of a solution containing F6H8, viz. 60% vol F6H8 in SIL (density 1.17 g/cm3). Because of its density and contact properties, the investigated DF has a tamponade effectiveness better than that of SIL on the inferior retina. Compared with using F6H8 alone, DF reduces emulsification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The combined use of a partially fluorinated alkane and a silicone oil (SIL) has been recently proposed as a method of improving the tamponade effectiveness of the two components used alone [1–3, 7–9]. Two of the authors have previously proposed and used double filling (DF) clinically as a means of producing good inferior tamponade [1, 7, 8].
In this in vitro study, we investigated the rate of mixing of perfluorohexyloctane (F6H8) in SIL and the final ratio of DF obtained from an initial ratio of 70%:30% vol. Moreover, the contact behaviour of DF and its tendency to disperse into droplets were investigated by using a Perspex eye cell similar to that designed by Herbert et al. [2].
The results of the present work were partially communicated at the 18th International Union of Pure and Applied Chemistry (IUPAC) International Conference on Chemical Thermodynamics [4]. In the same communication, the solubility curves of two partially fluorinated alkanes in different SILs were presented. These results are the subject of a paper in preparation.
Materials and methods
Substances
Perfluorohexyloctane (Fluoron, Germany) and silicone oil (SIL, Bausch-Lomb Surgical, Germany) were used. Their physical properties are as follows: F6H8: density, 1.331 g/cm3; viscosity, 2.5 mPa s; interface tension against water, 49.1 mN/m. SIL: density, 0.970 g/cm3; viscosity, 1,000 mPa s; interface tension against water, 23.3 mN/m [5].
Rate of mixing
The kinetics of the mixing process for F6H8+SIL was roughly estimated by placing known amounts of the pure components in a rectangular cell (UV cuvette with a volume of 3 ml) kept at constant temperature in a thermostatic stove. The liquid level at the interphase was monitored over time without stirring. Measurements were carried out at 22°C and 37°C.
Perspex eye model
A cylindrical Perspex (polymethyl methacrylate) chamber with no indents and a volume of about 6 ml, similar to that devised by Herbert et al. [2], was used to simulate clinical conditions. A visual qualitative comparison of surface contact and of dispersion into droplets of F6H8, SIL and DF was carried out. The inner surface of the model eye was made hydrophilic to mimic the retina by using a solution of 0.5% bovine albumin in Ringer lactate (RL) buffer. The liquids were introduced by displacing RL out of the chamber to approximate a 90% fill. Photographs were taken of the examined liquids. The effect of agitation was investigated by manually shaking the cell for half a minute and then for a further 1 min.
Solubility
The solubility of F6H8 in SIL was measured by turbidimetry [6]. F6H8 and SIL were weighed in a 4-ml glass ampoule equipped with a resistance thermometer and a magnetic stirrer.
Results
Rate of mixing
Figure 1 illustrates the evolution, with time, of the liquid level at the interface of F6H8 with SIL at 37°C in the absence of stirring. Since SIL floats on F6H8 and the latter dissolves in SIL, the level of liquid F6H8 lowers as time progresses and the phase volumes change correspondingly. During the dissolution process, both the lower F6H8 layer and the upper SIL+F6H8 phases remained optically clear.
As can be seen from Fig. 1, the rate of decrease of F6H8 volume, or the velocity of the mixing process, is slow. The equilibrium, i.e. the saturation of the upper SIL phase, is reached no earlier than 1 month at 37°C without shaking the cuvette. As expected, the mixing process at 22°C was found to be even slower. In the human eye, the double fill should reach equilibrium more quickly because of head movements.
Surface of contact with the eye model
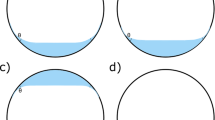
Pure F6H8 liquid adhered perfectly to the inner sides of the Perspex chamber with a large surface of contact. On the contrary, SIL occurred as a bubble floating on water and had a rounded egg-like profile with poor contact with the cell surface.
The DF displayed an SIL phase (a solution of F6H8 in SIL) floating on F6H8. The latter had excellent contact with the inferior sides of the chamber, whereas the SIL phase did not adhere well to the upper cell surface. Only the lower sides of the SIL phase, above the F6H8, were in contact with the chamber wall. Figure 2 shows a representative image of DF in the cylindrical model chamber.
The above findings with regard to the surface of contact confirm those previously made for F6H8 [2, 10], DF of F6H8 with 50-cS SIL [2] and solutions of F6H8 in SIL [10].
Emulsification
When the tamponade liquid with F6H8 alone was stirred, F6H8 dispersed into droplets at the interface with aqueous RL. On the other hand, SIL showed little tendency to emulsify. Similarly, both the DF phases displayed little evidence of dispersion. These observations on dispersion are substantially the same as those of Herbert et al. [2] for F6H8 and DF of F6H8 with 5,000-cS SIL.
Solubility
At 37°C, the solubility of F6H8 in SIL is 60% vol. This solution has the following properties: density, 1.17 g/cm3; viscosity, 70 mPa s; interface tension against water, 31 mN/m [4]. Starting from an initial F6H8/SIL ratio of 70%:30% vol, the equilibrium that is attained is characterized by two phases: 25% of the volume is practically pure F6H8 and the remaining 75% consists of a solution of F6H8 in SIL.
Discussion
Solubility experiments have shown that partially fluorinated alkanes (FALK) and SIL can be used together to give either a homogeneous clear solution, i.e. a heavy silicone oil (HSO), or two separate solutions, i.e. a DF, according to the ratio SIL/FALK employed [4].
The DF investigated here contains F6H8 and SIL at a ratio of 70%:30% vol. The two substances produce two phases of different compositions. The F6H8 phase is practically pure F6H8, whereas the SIL phase, which is a HSO, has a composition at equilibrium of 60% vol F6H8.
Although F6H8 is partially miscible in SIL, the rate of the mixing process, as illustrated in Fig. 1, is slow, and 1 month is required to reach equilibrium without stirring. In Fig. 3, we have represented what is expected to happen inside the vitreous chamber during the time after the introduction of 6 ml of a DF of F6H8 and SIL at a volume ratio of 70%:30%. Initially, 1.8 ml pure SIL will float on 4.2 ml pure F6H8. After a few days (intermediate situation), some of the F6H8 will dissolve in SIL by diffusion and agitation caused by head movements. The amount of the SIL phase will increase to 3 ml, whereas that of F6H8 will decrease to 3 ml. Finally, possibly within a week or up to 1 month, equilibrium will be reached, with 1.5 ml practically pure F6H8 at the bottom and 4.5 ml saturated SIL phase containing 60% vol of F6H8 at the top.
Effect of mixing for DF with F6H8+SIL1000 at an initial volume ratio of 70%:30% in a 6-ml spherical chamber at 37°C. Some of the F6H8 (yellow) will eventually dissolve in SIL to give 3 ml unsaturated solution (green). Finally, 1.5 ml F6H8 and 4.5 ml saturated SIL phase containing 60% vol F6H8 will be acheived. To scale: the liquid level corresponds to the indicated volume
For the sake of simplicity, in Fig. 3, we have ignored the presence of an aqueous phase and assumed that the DF bubble would adhere perfectly to the inner surface of the chamber. In reality, some water forms in the ocular cavity over time and, as we have observed in the eye model (Fig. 2), the SIL phase, in the presence of an aqueous phase, has a rounded shape with poor surface contact, although the lower sides of the SIL half bubble adhere to the cell wall. The aqueous phase, if any, will float on the SIL phase, which becomes increasingly heavier than water.
At all times of the mixing process (Fig. 3), the SIL phase, although increasing in density, floats on the F6H8 layer (density 1.33 g/cm3) and decreases in viscosity. At equilibrium, the presence of the bottom F6H8 layer ensures that the upper SIL solution is constantly saturated with 60% vol F6H8. This solution is an HSO having a density of 1.17 g/cm3 and a viscosity of 70 mPa s. Because of this viscosity value, which is nevertheless markedly lower than that of SIL (1,000 mPa s), the HSO phase has little propensity to disperse into droplets. Moreover, as observed in the Perspex eye experiments, this viscous cap on the top of F6H8 avoids direct contact with the aqueous phase and reduces emulsification. Although the upper HSO of DF adopts an egg-like profile with poor surface contact, the lower sides of HSO and the bottom F6H8 phase adhere well to the inner wall, properly carrying on the tamponade effect on the inferior sides of the chamber, viz. about half of the cell surface. Similar behaviour has been observed for F6H6+SIL double fill, in which the SIL phase has a lower density (1.13 g/cm3) and a higher viscosity (90 mPa s) than F6H8 + SIL [4].
Since both the density and viscosity of the DF SIL phase are unaffected by the initial F6H8/SIL ratio, this can be increased to obtain a higher final layer of F6H8. For instance, by using 6 ml DF with an initial F6H8/SIL volume ratio of 90%:10%, we would have, at equilibrium, 1.5 ml SIL phase and 4.5 ml undissolved F6H8. These figures can be easily calculated from the solubility of F6H8 in SIL. We are considering using F6H8/SIL ratios higher than 70%:30% vol in the case of retina breaks exceeding lower quadrants.
In conclusion, DF with F6H8+SIL at a ratio of 70%:30% vol is a good endotamponade agent for the inferior retina. As the density and surface of contact of the F6H8 phase are adequate, especially during the first few days after addition, the tamponade effect is guaranteed on the inferior fundus, whereas the upper SIL phase strongly slows down emulsification. The best results with such a DF might be obtained during surgery on complicated retina detachments with large inferior retina breaks.
References
Genovesi-Ebert F, Rizzo S, Figus M, Ferretti C, Nardi M (2000) Combination of silicone solvent (F6H8) and PDMS in complex retinal detachment. Invest Ophthalmol Vis Sci 41 (Suppl):663
Herbert E, Stappler T, Wetterqvist C, Williams R, Wong D (2003) Tamponade properties of double-filling with perfluorohexyloctane and silicone oil in a model eye chamber. Graefe Arch Clin Exp Ophthalmol 242:250–254
Hoerauf H, Kobuch K, Dresp J, Menz DH (2001) Combined use of partially fluorinated alkanes, perfluorocarbon liquids and silicone oil: an experimental study. Graefe Arch Clin Exp Ophthalmol 239:373–381
Lepori L, Matteoli E, Spanedda A (2004) Liquid–liquid equilibria for mixtures of a partially fluorinated alkane with a polydimethylsiloxane. 18th IUPAC International Conference on Chemical Thermodynamics, August 17–21, Beijing, China, 01-P-17, p 65
Meinert H, Roy T (2000) Semifluorinated alkanes a new class of with outstanding properties for use in ophthalmology. Eur J Ophthalmol 10:189–197
Novak JP, Matous J, Pick J (1986) Liquid liquid equilibria. Elsevier, Amsterdam
Rizzo S, Zenoni S, Genovesi-Ebert F, Belting C, Borgioli M, Zuccarini S (2002) Long-term vitreous replacement with perfluorohexyloctane (F6H8) and silicone oil. Ophthalmol Sci 5–10
Rizzo S, Genovesi-Ebert F, Belting C (2004) The combined use of perfluorohexyloctane (F6H8) and silicone oil as an intraocular tamponade in the treatment of severe retinal detachment. Graefe Arch Clin Exp Ophthalmol (in press)
Sparrow JR, Jayakumar A, Berrocal M, Ozmert E, Chang S (1992) Experimental studies of the combined use of vitreous substitutes of high and low specific gravity. Retina 12:134–140
Wetterqvist C, Wong D, Williams R, Stappler T, Herbert E, Freeburn S (2004) Tamponade efficiency of perfluorohexyloctane and silicone oil solutions in a model eye chamber. Br J Ophthalmol 88:692–696
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lepori, L., Matteoli, E., Spanedda, A. et al. Combined use of perfluorohexyloctane and silicone oil as intraocular tamponade: an in vitro study. Graefe's Arch Clin Exp Ophthalmo 244, 79–82 (2006). https://doi.org/10.1007/s00417-005-0003-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-005-0003-x