Abstract
Background
Opposite clear corneal incisions (OCCIs) have been reported to reduce pre-existing astigmatism (PEA) during cataract surgery. Our goal was to evaluate the effect of OCCIs on correcting PEA in cataract surgery.
Methods
Non-randomized prospective study. Thirty-four patients with PEA of greater than 1.5 diopters (D) underwent clear cornea phacoemulsification cataract extraction with 3.2-mm OCCIs (OCCI group). The control group consisted of 23 successive patients with PEA <1.5 D who underwent cataract extraction without OCCI. Best-corrected visual acuity, keratometry and refraction were recorded for all patients pre-operatively and post-operatively.
Results
Using keratometric findings, mean astigmatism correction was 1.3 D (±0.9 SD; decreased from 2.6 D pre-operatively to 1.4 D post-operatively) in the OCCI group but only 0.4 D in the control group (P<0.005), 8 months post-operatively. Vector analysis of astigmatism correction showed greater change for OCCI patients (1.8 D vs 1.0 D, P=0.002). Using the Holladay method for calculating surgically induced refractive change (SIRC), the OCCI group showed a higher value of SIRC (−1.6 D vs −0.97 D), but this was not statistically significant. The OCCI patients showed a greater and significant change in refraction spherical equivalent than the controls. No complications related to OCCI or cataract surgery occurred during the follow-up period.
Conclusions
Opposite clear cornea incision seems to be a simple, predictable, safe and effective procedure in reducing pre-existing corneal astigmatism in cataract surgery. It has an enhanced effect in correcting astigmatism compared to a single clear cornea incision when using keratometric findings value but not when using refractive data. Future studies are needed to document the long-term effect of OCCI and to evaluate the correlation between incisions of different size and astigmatism correction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cataract surgery has become one of the most common and successful procedures in ophthalmology. In addition to improving visual acuity (VA), one of the goals of modern cataract surgery is to reduce pre-existing astigmatism (PEA), a factor that may reduce VA and affect the quality of vision [3].
Different factors can affect post-operative astigmatism: incision size and shape [3, 8, 18–20], location relative to the limbus [4, 6], suture technique and material [13]. Small-incision phacoemulsification may be associated with a lower surgically induced astigmatism, although it can lead to long-term flattening along the meridian of incision [7, 10, 18].
Clear corneal incision (CCI) can induce astigmatism of 0.5–1.75 D 1 year post-operatively [16], and many surgeons make the incision in the steepest meridian in order to correct PEA. Recently, it has been suggested by Lever and Dahan [12] that identical CCI opposite to the first incision can enhance the flattening effect of the cornea and further correct PEA.
The purpose of this study was to evaluate the effect of opposite clear corneal incision (OCCI) in correction of pre-operative astigmatism after small-incision clear-cornea phacoemulsification and to compare astigmatism correction and refractive changes to those in patients with CCI phacoemulsification without OCCI.
Material and methods
The study population was a non-randomized group of patients undergoing cataract surgery by one of the authors (H.D.); data were recorded in a prospective manner. Thirty-four patients with PEA of greater than 1.5 D underwent clear-cornea phacoemulsification cataract extraction with OCCIs (OCCI group) to correct pre-operative astigmatism. The control group consisted of 23 successive patients who underwent cataract extraction using the same phacoemulsification technique (Nagahara nucleus cracking and then “stop and chop” nucleus emulsification); the AMO (advanced medical optics) Diplomax phacoemulsification unit (Allergan Optical Microsystems, N. Andover, MA, USA); the same intra-ocular lens [foldable Hanita B lens (Hanita, Kibbutz Hanita, Israel)], except in two cases with IOL of less than 8 D where we used a Corneal foldable IOL (Corneal, Paris, France); and performed by the same surgeon (H.D.). All patients in the control group had PEA of less than 1.5 D. Informed consent was obtained from all patients.
Phaco incision was made in the steepest meridian according to K readings; OCCI was performed 180° from the phaco incision, using a 3.2 keratome blade (Sharpoint, Reading, Pennsylvania, PA, USA) and placed at the limbus creating a tunnel of 1.5 mm in clear cornea. The OCCI was not sutured.
Data regarding patients’ age, gender, surgical details, pre-operative and post-operative refraction, K reading, and best-corrected VA were recorded and analyzed. Patients were examined 1 day, 1 week, 1 month, 3 months and every 6 months thereafter. Computerized videokeratometry or manual (Javal) keratometry readings were obtained 3 months to 1 year post-operatively.
Statistical analysis
Statistical analysis was performed using the paired-samples t test to evaluate pre-operative and post-operative data within each group such as VA, keratometry reading, astigmatism and refraction. Independent-sample t test was used to evaluate differences in astigmatism correction between OCCI and CCI groups. Vector analysis of astigmatism correction was calculated as previously described by Alpins [1, 2].
Surgically induced refractive change (SIRC) was calculated using the ten steps method described by Holladay and co-workers [9]. Spherical equivalent (SE) of SIRC, magnitude of astigmatism, with-the-wound change (delta WTW), against-the-wound change (delta ATW), coupling ratio and axis of the SIRC were plotted as bar chart in the OCCI and control groups using the Holladay [9] method of reporting and graphing aggregate results.
Statistical analysis was carried out with Microsoft Excel XP and SPSS programs.
Results
Thirty-four patients (22 males, 12 females) with a mean (±SD) age of 72 (±11) years underwent clear-cornea phacoemulsification cataract extraction with OCCIs. The control group consisted of 23 patients (9 males, 14 females, mean age of 66±9 years). Pre-operative and post-operative data are summarized in Table 1.
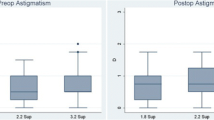
Using keratometric findings, in the OCCI patients, the mean pre-existing corneal astigmatism decreased from 2.6 D (±1.2) to 1.4 D (±0.9) post-operatively (P=0.000, (95% CI 0.9–1.6) (Fig. 1). The corresponding change was not significant in the control group [from 0.9 D (±0.4) pre-operatively to 0.7 D (±0.5) post-operatively]. Mean astigmatism correction by vector analysis was significantly higher for OCCI patients than for controls (1.8 D vs 1.0 D, P=0.002).
Using refractive data and analyzing the results with the ten steps method described by Holladay et al. [9], similar spherical equivalent change is found in both groups (non-significant statistical difference by independent-samples t test). Magnitude of astigmatism (absolute value of the cylinder in SIRC) is higher for OCCI patients (P<0.001). With-the-wound change (delta WTW) and against-the-wound change (delta ATW) were similar in both groups (Fig. 2). Coupling ratio (delta ATW/delta WTW) was 1.0 for the OCCI and 0.9 for the control group (not significant), and the average axis of SIRC was 35.6 and 41.4, respectively (not significant).
Spherical equivalent refractive change after cataract surgery in the OCCI and control groups. Bar columns represent the spherical equivalent of surgically induced refractive change (SE-SIRC), magnitude of astigmatism (Mag-Ast), with-the-wound change (delta WTW) at surgical meridian and against-the-wound change (delta ATW) at 180-surgical meridian for both groups. ***P<0.006, independent-samples t test
VA improved from a pre-operative value of 20/150 to 20/40 in the OCCI group and from 20/90 to 20/30 in the controls. OCCI patients showed a better improvement in VA, [delta logMAR(VA) of 0.54 (±0.3) vs 0.39 (±0.2) in controls; P=0.04, 95% CI 0.005–0.3].
The OCCI patients had higher pre-operative and post-operative absolute K difference than the controls (Table 1), but mean astigmatism correction was greater [1.3 D (±0.9) vs 0.4 D (±0.4), P=0.000].
Refraction spherical equivalent (seq) decreased in both groups, but the change was statistically significant only in the OCCI group (Table 1, Fig. 3). Post-operative estimated refraction (target refraction) was lower than actual refraction in OCCI patients (−1 D and −1.5 D, respectively, P=0.016).
All patients (OCCI and control groups) were satisfied with the results of surgery,except for one patient in the OCCI group who developed vitreous hemorrhage post-operatively (see next paragraph).
Complications included one case of vitreous hemorrhage in a high-risk proliferative diabetic retinopathy patient (vitreous hemorrhage resolved spontaneously)—whether this complication was related to the OCCI procedure or the cataract surgery is uncertain. No cases of post-operative wound leak, hypotony or endophthalmitis occurred in either group.
Discussion
This study supports previous observations [12] that OCCIs can correct PEA in cataract surgery. It also evaluates the extent of astigmatism correction by the procedure, compared with small- incision cataract surgery with single CCI.
Small-incision phacoemulsification has become the more common cataract surgery, and many surgeons perform the clear-cornea phaco incision in the steepest corneal meridian [9, 18]. This may reduce astigmatism by 1–2 D, depending on factors that have been extensively investigated [3, 6, 8, 19, 20].
The recently reported method described by Lever and Dahan [12], using a simple OCCI at 180° to the phaco incision (usually 12 and 6 o’clock for with-the-rule astigmatism, 9 and 3 o’clock for against-the-rule astigmatism) has been reported to correct PEA by a mean of 2.06 D. With the same OCCI technique, our patients achieved a mean astigmatic correction of 1.3 D. The differences in astigmatism reduction among different surgeons using on-axis surgery stem from difficulty in placing the surgery exactly in the correct meridian; even a small degree of deviation may greatly reduce the astigmatism-neutralizing effect. Mean astigmatism correction by vector analysis, which may be a more accurate method of evaluating astigmatism change, was 1.8 D in our work compared with 2.25 D in the work of Dahan and Lever [12].
Although astigmatism in the control group was reduced by 0.2 D (mean pre-operative value of 0.9D, post-operative 0.7 D) this change was not statistically significant, suggesting that the OCCI technique may correct PEA to a greater extent than a single CCI. It may also be more difficult to correct astigmatism in corneas that are less steep (the patients in the control group had PEA of less than 1.5 D), but this remains to be examined.
These changes in PEA are reflected also by change in refraction. Spherical equivalent decreased from −2.9 D to −1.5 D in OCCI patients (P=0.02) while the change in the control group was not statistically significant (−2.1 D to −1.4 D). This may imply that most PEA in our cataract patients was corneal rather than lens induced.
One may argue that we should have chosen patients with PEA of greater than 1.5 D as controls in order to accurately evaluate the effect of OCCI in correcting PEA. This may be true, but still we showed that OCCI can reduce astigmatism to a greater extent than a single CCI—as one would expect from the results of Lever and Dahan [12].
Patients in the OCCI group had a lower pre-operative VA (20/147 vs 20/88), which may explain the greater improvement they achieved with cataract surgery [delta logMAR(VA) of 0.54 vs 0.39, P=0.04]. Relaxing incisions, transverse or arcuate keratotomies are also used to correct PEA [17–19] but these are more skilled techniques than OCCI, require special instruments, and may have a long-term flattening effect on the cornea [21]. Although others advocate the former because OCCI is a penetrating procedure, and recommend that longer OCCI incisions should be sutured [14], the effect of same-length incision on correcting astigmatism is much greater for OCCI than for arcuate keratotomy [5]. The long-term effect of OCCI was not examined in this study, since all patients had a single post-operative K reading (by computerized videokeratometry or manual measurement) ranging from 3 months to 1 year after surgery. Dahan and Lever [22] use OCCIs not only to correct PEA in cataract surgery but also in congenital astigmatism and in astigmatism following trauma or keratoplasty. They also recommend suturing any OCCIs longer than 3.2 mm.
Interestingly, surgically induced keratometric astigmatism was higher for OCCI patients than controls based on keratometric findings but similar based on refractive data. Because keratometry was performed using different methods—manual keratometers and videokeratometers—and at different time points, we believe that re-evaluation of the results using refractive data may be a more accurate method of estimating SIRC [9]. Thus, OCCI failed to show a greater refractive change than single CCI.
Our study might have benefited from: (1) use of a control group with similar pre-operative astigmatism; (2) a longer follow-up to evaluate the long-term effect of OCCI on correcting PEA; (3) repeat keratometry measurements (preferably computerized video-keratometry) at different intervals, to ascertain the astigmatic changes over time; and (4) use of different sizes of OCCI incisions (3.2–4.2 mm) to establish the effect of incision size on astigmatism correction. Still, we found OCCI to be a simple, predictable, safe and effective method of correcting PEA in cataract surgery. Further studies are needed to evaluate this technique originally described by Lever and Dahan.
References
Alpins N (1993) A new method of analyzing vectors for changes in astigmatism. J Cataract Refract Surg 19:524–533
Alpins N (1997) Vector analysis of astigmatism changes by flattering, steepening, and torque. J Cataract Refract Surg 23:1503–1514
Beltrame G, Salvetat ML, Chizzolini M, Driussi G (2001) Corneal topographic changes induced by different oblique cataract incisions. J Cataract Refract Surg 27:720–727
Cravy T (1991) Routine use of a lateral approach to cataract extraction to achieve rapid and sustained stabilization of post operative astigmatism. J Cataract Refract Surg 17:415–423
Dahan E, Lever J (2001) Opposite clear cornea incision—authors’ reply. J Cataract Refract Surg 27:8
Dam-Johansen M, Olsen T, Theodorsen F (1994) The long-term course of the surgically induced astigmatism after a scleral tunnel incision. Eur J Implant Refract Surg 6:337–342
Ernest P, Tipperman R, Eagle R et al (1998) Is there a difference in incision healing based on location? J Cataract Refract Surg 24:4822–4826
Hayashi K, Hayashi H, Nakao F, Hayashi F (1995) The correlation betweem incision size and corneal shape changes in sutureless cataract surgery. Ophthalmology 102:550–556
Holladay JT, Cravy TV, Koch DD (1992) Calculating the surgically induced refractive change following ocular surgery. J Cataract Refract Surg 18:429–443
Kondroft E (1991) Keratometric cylinder and visual recovery following phacoemulsification and intraocular lens implantation using a self-sealing cataract incision. J Cataract Refract Surg 17:731–733
Krumeich JH, Knulle A, Daniel J (1998) Improved technique of circular keratotomy for the correction of corneal astigmatism. J Cataract Refract Surg 24:765–771
Lever J, Dahan E (2000) Opposite clear corneal incisions to correct pre-existing astigmatism in cataract surgery. J Cataract Refract Surg 26:803–805
Masket S (1988) Comparison of suture materials for closure of the scleral pocket incision. J Cataract Refract Surg 14:548–551
Muller-Jensen K, Fischer P, Siepe U (1999) Limbal relaxing incisions to correct astigmatism in clear corneal cataract surgery. J Refract Surg 15:586–589
Nichamin L (2001) Opposite clear cornea incision—letter to the editor. J Cataract Refract Surg 27:7–8
Nielsen P (1995) Prospective evaluation of surgically induced astigmatism and astigmatic keratotomy effects of various self-sealing small incisions. J Cataract Refract Surg 21:43–48
Pfleger T, Skorpik C, Menapace R et al (1996) Long-term course of induced astigmatism after clear cornea incision cataract surgery. J Cataract Refract Surg 22:72–77
Rosen E (1998) Clear corneal incisions and astigmatism. In: Fine IH (ed) Clear corneal lens surgery. Slack, Thorofare, pp 21–42
Samuelson SW, Koch D, Kuglen CC (1991) Determination of maximal incision length for true small-incision surgery. Ophthalmic Surg 22:204–207
Singer J (1991) Frown incision for minimizing induced astigmatism after small incision cataract surgery with rigid optic intraocular lens implantation. J Cataract Refract Surg 17:677–688
Thornton S (1990) Astigmatic keratotomy: a review of basic concepts with case reports. J Cataract Refract Surg 16:430–435
Waring GO, Lynn MJ, Nizam A et al (1991) Results of the prospective evaluation of radial keratotomy (PERK). Study five years after surgery. Ophthalmology 98:1164–1176
Acknowledgement
The authors wish to thank Elie Dahan, MD, for teaching and assistance in analyzing the data and for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Simon, G.J., Desatnik, H. Correction of pre-existing astigmatism during cataract surgery: comparison between the effects of opposite clear corneal incisions and a single clear corneal incision. Graefe's Arch Clin Exp Ophthalmol 243, 321–326 (2005). https://doi.org/10.1007/s00417-004-1035-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-1035-3