Abstract
Background
Recent studies have suggested that the relationship between elevated plasma homocysteine (Hcy) and increased risk of vascular disease holds also for certain diseases of the eye with vascular aetiology. Elevated plasma Hcy levels have been noted among patients with exfoliation syndrome (XFS). The purpose of this study was to establish whether subjects with XFS have higher plasma and aqueous humour Hcy levels values than non-XFS subjects, particularly in relation to vitamin B status.
Methods
Using a cross-sectional study design, 36 subjects with XFS and 36 non-XFS subjects with intraocular pressure (IOP) lower than 23 mmHg, matched by age and gender, were first selected. The participant exclusion criteria included parameters known to alter Hcy metabolism. In the XFS group, 11 subjects had a concurrent diagnosis of exfoliative glaucoma (XFG). Fasting plasma and aqueous humour Hcy samples were collected, along with erythrocyte folate (E-Fol) and serum vitamin B6 and B12 samples. The Hcy samples were analysed using a fluorescence polarization immunoassay method.
Results
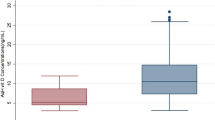
Plasma Hcy level was significantly higher (P=0.020, after Bonferroni correction for multiple testing) in the XFS group than in the controls. The Hcy concentrations in the aqueous humour did not differ statistically between the two groups. Plasma and aqueous humour Hcy concentrations were not statistically significantly correlated within the groups of exfoliation-positive and -negative subjects. E-Fol, and serum vitamin B6 and B12 levels did not differ statistically between the XFS group and the control group.
Conclusions
The finding that subjects with XFS are more prone to elevated plasma Hcy emphasizes exfoliation as a clinical sign and a marker of thromboembolic vasculopathies induced by hyperhomocysteinaemia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Raised plasma level of homocysteine (Hcy), an intermediary sulfur-containing amino acid formed during the conversion of the amino acid methionine to cysteine, is reportedly a risk factor for retinal arterial and venous occlusive disorders [1, 6, 7, 47], although some studies do not support this for the aetiology of central retinal vein occlusion (CRVO) [5, 20, 29]. In addition, positive correlation has been noted between plasma Hcy levels and non-arterial ischaemic optic neuropathy [17, 29]. Three recent studies found a correlation between open-angle glaucoma and Hcy, one in both chronic primary open-angle glaucoma (POAG) and exfoliation glaucoma (XFG) [4], the other two in XFG alone [21, 46].Vessani and co-workers also noted that plasma Hcy levels were significantly higher in non-glaucomatous patients with XFS [46].
Hyperhomocysteinaemia can be genetic in origin or mediated by nutritional factors. Because the metabolism of methionine via Hcy to cysteine is a complex pathway involving enzymes dependent on cobalamin (vitamin B12), pyridoxal phosphate (derived from vitamin B6) and folic acid, among other co-factors, genetically inherited defects or deficiencies in these rate-limiting enzymes are probably the most important determinants of elevated levels of Hcy.
Hcy appears to have a prominent role in vascular pathogenesis. It promotes endothelial injury, myointimal hyperplasia, hypertrophy, deposition of sulfated glycosaminoglycans, collagen synthesis, and fibrosis and calcification of atherosclerotic plaques [24]. Within the vessel wall Hcy is bound to extracellular matrix (ECM) components [42, 45], and in hyperhomocysteinaemia Hcy is thought to induce adverse changes in turnover and remodelling of ECM [10, 43, 44]. On the other hand, the vascular damage reported in serious hyperhomocysteinaemia might result from the oxidative metabolism of Hcy to Hcy thiolactone [15, 24], or to other sulfated compounds such as Hcy sulfinic acid and homocysteic acid [28].It has been suggested that Hcy thiolactone, by increasing free radical oxidants, induces endothelial cell apoptosis in a concentration-dependent manner [25] and makes alterations to mitochondrial function resulting in hyperplasia and fibrosis of smooth muscle cells [24].
Exfoliation syndrome (XFS) is known to be the most common identifiable cause of open-angle glaucoma worldwide [33]. Although XFS manifests clinically as small whitish deposits of fibrillar–granular flecks in the anterior segment of the eye, this ECM material may also be found in extraocular tissues, including skin and the connective tissue portions of various visceral organs, e.g. heart, lung, liver, kidney, gall bladder, and cerebral meninges [34, 40]. Nevertheless, despite its wide prevalence in the older population [11, 41], the pathogenic mechanism of XFS and its clinical importance remain poorly understood.
XFS and hyperhomocysteinaemia-related vascular diseases have some characteristics in common. Exfoliation is significantly associated with a history of coronary disease or arterial hypertension, or a combined history of angina and acute myocardial infarction or stroke [26]. Schumacher and co-workers observed an association between aneurysms of the abdominal aorta and ocular exfoliation [36]. On the other hand, later, a high incidence of elevated Hcy levels was found in patients who suffered an abdominal aortic aneurysm [38, 50].
Regarding the role of vascular pathology in disturbances of ocular haemodynamics, some studies have indicated lowered orbital blood flow in patients with XFS [31, 52], suggesting probable vascular or flow abnormalities in the development of exfoliation. Repo et al. described generalized peripheral iris transluminance in XFS, suggesting a possible role of iris hypoxia in the development of XFS [30]. Later, vasculopathy of iris blood vessels leading to obliteration of the vascular lumen with concurrent iris hypoperfusion was observed in XFS eyes [13]. In a further study, Repo et al. observed in iris specimens of XFS eyes strongly fibrotized muscle tissue devoid of normal morphology with fibrillar material around the blood vessels, as well as vacuolization and significantly decreased amounts of mitochondria [32]. In contrast, a histopathological study on eyes with simultaneous anterior segment XFS and ischaemic CRVO found no morphologically evident XFS vasculopathy in the central retinal vessels within or immediately behind the lamina cribrosa [8].
The literature contains no clear documentation on the amino acid content of the human aqueous humour. The blood–aqueous barrier (BAB) restricts protein access from the circulation into the aqueous humour, but in experimentally induced anterior segment ischaemia, disruption of BAB could be detected with laser flare photometry [14]. There are divergent opinions on the likelihood that XFS enhances the aqueous protein concentration. Some studies have shown that impairment of BAB with increased aqueous protein concentration is a feature of XFS [18, 49], but one report failed to support this [3].
Assuming that Hcy metabolism influences the intracellular synthesis of exfoliation material and its transition into ECM, we set out to measure and compare the plasma and aqueous humour concentrations of Hcy in subjects with exfoliation in the anterior segment of the eye. Subjects with no sign of XFS served as controls. In addition, erythrocyte folate (E-Fol) and serum vitamins B6 and B12 were determined in both groups.
Materials and methods
Subjects and controls
The study participants were patients attending the University Hospital, Kuopio, and the North Karelia Central Hospital, Joensuu, both in eastern Finland, for routine cataract surgery.
The use of these volunteers followed the tenets of the declaration of Helsinki and received approval from the local research ethics committees. All the enrolled subjects signed written consent forms and were told in detail about the purpose of the investigation and the method used to obtain aqueous humour for research purposes. The diagnosis of XFS was confirmed in 36 patients prior to the cataract operation, using biomicroscopic examination in mydriasis; these subjects formed the study group. The same number of patients with no sign of exfoliation material in the anterior segment of the eye, and matched by age and gender, were chosen as controls. In the XFS group, 11 patients had a diagnosis of XFG. The controls had an IOP below 23 mmHg, and they had no medical history or clinical sign of any other eye disease than cataract. All the participants had the anterior segment of the eye examined by biomicroscope in mydriasis, and the posterior segment with Volk lenses of 60 and 90 dioptres. Otherwise, the exclusion criteria for parameters known to interfere with Hcy metabolism were: medical history of major systemic illness (diabetes, vasculitis, renal and hepatic disease), thromboembolic diseases (including stroke, myocardial infarction and peripheral arterial or venous occlusions) during the past half year, evidence of chronic alcohol abuse, anticonvulsant and immunosuppressive therapy, hormone substitution, cholesterol-lowering agents (statin drugs), antidepressants, recent antimicrobial therapy and intake of vitamin supplements, natural products or fish oil.
In the study population, both the XFS/XFG group and the control group comprised 28 female and 8 male patients. The mean age of the XFS/XFG group was 77.4 years (SD 6.0) and of the controls 77.2 years (SD 5.4).
Variables examined
Blood samples to test for plasma Hcy and E-Fol and serum vitamins B6 and B12 were collected in the fasting state just before the patients were prepared for cataract surgery. The samples were taken to the laboratory immediately, centrifuged within 1 h and stored at −70°C. The aqueous humour samples were taken at the onset of the surgical procedure via corneal paracentesis, and an amount of 100–200 μl (depending on the structural volume) was aspirated from the anterior chamber and injected into a specimen container (Microtainer, UCSD Healthcare, CA, USA). These tubes were immediately frozen and stored at −70°C. Serum vitamin B6 was analysed using a HPLC system and a Chromsystems Vitamin B6 assay (Munich, Germany) (reference range 15–73 nmol/l). The levels of plasma and aqueous Hcy were measured using a fluorescence polarization immunoassay (Abbott IMx Homocysteine Assay, Abbott Laboratories, USA). For this assay the normal values of the plasma Hcy are 5–15 μmol/l according to the manufacturer. In contrast, the literature gives no information on Hcy contents of the aqueous humour for reference value. E-Fol and serum vitamin B12 concentrations were measured using an AutoDelfia Folate Assay and AutoDelfia B12 Vitamin Assay, based on a time-resolved fluoroimmunoassay method (Wallac, Perkin Elmer, Turku, Finland).
Statistics
A matched-pairs study design with cases and controls of the same gender and age was applied. Because the variables were not normally distributed in either of the main groups (tested by Kolmogorov–Smirnov and Shapiro–Wilk methods), the Wilcoxon matched-pairs signed-ranks test was used to perform the analysis in comparisons between the groups with regard to the above-mentioned variables. For the subgroups, the Mann–Whitney U test for continuous variables with a skewed distribution was applied. Associations between parameter concentrations in various environments were calculated with Spearman’s rank-order correlation. As testing a lot of variables interferes with some probable correlations among them, we considered a simple Bonferroni correction of the P values. The significance level for comparisons between parameters was set at α=0.05.
Results
Homocysteine
As shown in Table 1, significantly higher plasma Hcy levels were found in patients with XFS/XFG (17.8±6.7 μmol/l, range 9.6–38.2 μmol/l, median 16.8 μmol/l) compared with the control group (15.8±6.5 μmol/l, range 8.3–35.7 μmol/l, median 13.9 μmol/l) (P=0.020, Wilcoxon matched-pairs signed ranks test, after making a Bonferroni-type correction). In contrast, the Hcy concentrations in the aqueous humour were almost identical, with no statistically significant difference between the two groups (P=0.995, Wilcoxon matched-pairs signed-ranks test). Nor did the plasma Hcy levels show any correlation with the aqueous Hcy concentrations in the groups of exfoliation-positive or -negative subjects (P=0.976 and P=0.731, respectively, Spearman’s rank-order correlation). Regarding the XFS and XFG subgroups, the plasma Hcy levels were found to be higher in XFG than non-glaucomatous XFS patients (20.3±8.0 μmol/l, range 12.4–38.2 μmol/l, median 17.7 μmol/l vs 16.7±5.9 μmol/l, range 9.6 – 37.7 μmol/l, median 14.8 μmol/l), though the difference was not statistically significant, at least partly due to the small number of patients (n=11 and n=25, respectively; P=0.112, Mann–Whitney U test).
Erythrocyte folate and serum vitamins B6 and B12
Table 1 shows the measured levels for E-Fol and serum vitamins B6 and B12 in the XFS/XFG and control groups. There were no statistical differences in these three parameters between the two main study groups (respective P values: 0.441, 0.600 and 0.911, Wilcoxon matched-pairs signed-ranks test).
When the XFS and XFG subgroups were compared, the mean serum vitamin B12 concentration was lower in the subjects with XFG (303±158 pmol/l, range 166–747 pmol/l, median 255 pmol/l vs 318±77 pmol/l, range 121–560 pmol/l, median 325 pmol/l). The difference remained slightly below the level of significance (P=0.069, Mann–Whitney U test), again at least partly due to the small number of individuals in this subgroup. Neither were there any statistical differences in E-Fol and serum vitamin B6 between the subgroups.
The influence of gender on homocysteine level
The influence of gender on plasma and aqueous Hcy was examined within the subgroups of XFS-positive and XFS-negative subjects (Table 2). In neither case was the difference statistically significant, though the mean plasma Hcy levels were slightly higher in males than females. In the XFS- positive group, the mean plasma Hcy concentration was 2.7 μmol/l higher in males than females (median 18.9 μmol/l vs 15.6 μmol/l), but, due at least partly to the small number of individuals in this subgroup, the difference did not reach statistical significance (P=0.069, Mann–Whitney U test). Nor was there any difference in the aqueous Hcy levels between males and females in the XFS-positive and XFS-negative subgroups (P=0.094 and P=0.987, respectively, Mann–Whitney U test).
Discussion
The results of the present study demonstrate a statistically significant association between the presence of raised plasma Hcy and XFS. However, in terms of aqueous humour levels of Hcy, and measurements of E-Fol and serum vitamins B6 and B12, no statistically significant differences could be found between the index and control patients. The mean aqueous concentrations of Hcy were about 1/20 those in plasma (Table 1), which implies an active functioning of the BAB and a balancing role regarding amino acid access into the intraocular space. According to our results, there was no correlation between the plasma and aqueous concentrations of Hcy, which again suggests an active BAB function.
In vascular pathogenesis, endothelial dysfunction in conduit and resistance vessels may underlie the associations between Hcy and atherosclerosis, and increased oxidative stress contributes a pathophysiological role to the deleterious endothelial effects of Hcy [16, 51]. In addition, Hcy has several harmful effects on haemodynamics. It enhances thromboxane A2 formation and platelet aggregation [9], impairs the ability of endothelial cells to inhibit platelet aggregation and, by forming S-nitrosothiols, reduces nitric oxide (NO) bioavailability [39] and has procoagulant effects [22]. These facts could help explain the increased risk of ocular thromboembolism, but also suggest how the eye becomes predisposed to slowly developing ischaemic alterations.
In iris vasculopathy among patients with XFS, there is a gradual and persistent degeneration of the cells in the affected vessel walls associated with the production of excess ECM, including exfoliative fibres, and these alterations apparently develop under hypoxic conditions [13, 31]. This hypothesis is supported by an observation that oxygen partial pressure (pO2) values measured in the anterior chamber in patients with XFS were lower than in those without XFS [13]. In the present study, however, the fact that Hcy was not elevated in the aqueous humour in XFS contradicts its direct relationship with the pathobiology of the abnormal ECM formation process, which involves all aqueous-bathed ocular structures, and implies a relationship only with XFS-associated systemic and ocular vasculopathy.
In the pathogenesis of exfoliation material, there may be an association between Hcy and matrix metalloproteinases (MMPs). Hcy decreases endothelial NO availability by generating nitrotyrosine [39]. In an experimental induction study, a progressive rise in nitrotyrosine levels was shown at 4 and 8 weeks after Hcy administration, and the consequent decrease in NO concentration resulted in increased MMP activity [37]. In another experimental study, a homocysteinic medium increased elastinolytic gelatinase A and B (MMP-2 and -9) in the vessel wall and, in addition, provided more marked labelling for caspase, a cytoplasmic protease involved in mediating some forms of apoptotic degradation [27]. In a recent report by Schlötzer-Schrehardt et al. [35], the aqueous humour of patients with XFS or XFG had significantly higher concentrations of MMP-2 and -3 than control eyes. Simultaneously, TIMP-1 and -2, as representatives of tissue inhibitors of MMPs, were also detected at higher level. In XFG patients, the ratio of MMP-2 to its principal inhibitor TIMP-2 was decreased, resulting in an excess of TIMP-2 over MMP-2. The authors suggest that complex changes in the local MMP–TIMP balance and reduced MMP activity in the aqueous humour may promote the abnormal matrix accumulation characteristic of XFS with evident implication in the pathogenesis of XFG. Both MMPs and TIMPs are widely expressed in the anterior uvea, and their differential localization in the ciliary body in particular suggests they may have a physiologic role in maintaining homeostasis in the uveal tract [19].
As it has been shown that Hcy-induced mitochondrial abnormalities follow the toxic exposure to hydrogen peroxide (H2O2) generated during oxidative reactions [2], it is evident that Hcy and H2O2 act synergistically to promote mitochondrial damage. A novel observation is that H2O2 increases MMP-2 activity, too, leading to stimulation of Ca2+ ATPase during the oxidation process [23]. This finding is parallel with the observation that the aqueous humour of patients with XFS or XFG had significantly higher concentrations of MMP-2 than that in control eyes [35].
In the present study, plasma Hcy concentrations were found to be rather high in both cases and controls, i.e. 17.8 μmol/l (SD 6.7) in patients with XFS and 15.8 μmol/l (SD 6.5) in controls; this is somewhat above the upper reference limit of 15 μmol/l. In a study population of 326 eastern Finnish men aged 42–60 years, the mean plasma Hcy concentration was 11.2 μmol/l (SD 3.1) [48]. However, under normal physiologic conditions, Hcy blood concentrations increase gradually with age and, in addition, men have plasma Hcy levels more than 20% higher than women [12]. The fact that our study population comprised mainly elderly subjects (median age of cases 78.5 years and controls 78.0 years) would explain the high basic plasma Hcy levels. Furthermore, the study design was not modelled to use any cut-off values in evaluating hyperhomocysteinaemia.
Among previous studies there are a few reports of elevated plasma Hcy associated with XFS, implying that hyperhomocysteinaemia may have an important influence on the pathogenesis of glaucomatous optic neuropathy and may also partly explain the increased risk of vascular diseases among patients with XFS [4, 21, 46]. However, the basic mechanism by which these two clinical findings are interlinked remains unclear, and more detailed investigations are warranted to resolve the underlying associations.
References
Abu El-Asrar AM, Abdel Gader AG, Al-Amro SA, Al-Attas OS (2002) Hyperhomocysteinemia and retinal vascular occlusive disease. Eur J Ophthalmol 12:495–495
Austin RC, Sood SK, Dorward AM, Singh G, Shaughnessy SG, Pamidi A, Outinen PA, Weitz JI (1998) Homocysteine-dependent alterations in mitochondrial gene expression function and structure. Homocysteine and H2O2 act synergistically to enhance mitochondrial damage. J Biol Chem 273:30808–30817
Berlau J, Lorenz P, Beck R, Makovitzky J, Schlötzer-Schrehardt U, Thiesen HJ, Guthoff R (2001) Analysis of aqueous humour proteins of eyes with and without pseudoexfoliation syndrome. Graefes Arch Clin Exp Ophthalmol 239:743–746
Bleich S, Jünemann A, von Ahsen N, Lausen B, Ritter K, Beck G, Naumann GO, Kornhuber J (2002) Homocysteine and risk of open-angle glaucoma. J Neural Transm 109:1499–1504
Boyd S, Owens D, Gin T, Bunce K, Sherafat H, Perry D, Hykin PG (2001) Plasma homocysteine, methylene tetrahydrofolate reductase C677T and factor II G20210A polymorphism, factor VIII, and VWF in central retinal vein occlusion. Br J Ophthalmol 85:1313–1315
Cahill M, Karabatzaki M, Meleady R, Refsum H, Ueland P, Shields D, Mooney D, Graham I (2000) Raised plasma homocysteine as a risk factor for retinal vascular occlusive disease. Br J Ophthalmol 84:154–157
Cahill MT, Stinnett SS, Fekrat S (2003) Meta-analysis of plasma homocysteine, serum folate, serum vitamin B(12), and thermolabile MTHFR genotype as risk factors for retinal vascular occlusive disease. Am J Ophthalmol 136:1136–1150
Cursifien C, Hammer T, Küchle M, Naumann GO, Schlötzer-Schrehardt U (2001) Pseudoexfoliation syndrome in eyes with ischemic central vein occlusion. A histopathologic and electron microscopic study. Acta Ophthalmol Scand 79:476–478
Durand P, Lussier-Cacan S, Blache D (1997) Acute methionine load-induced hyperhomocysteinemia enhances platelet aggregation, thromboxane biosynthesis, and macrophage-derived tissue factor activity in rats. FASEB J 11:1157–1168
Forrester JS, Fishbein M, Helfant R, Fagin J (1991) A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. Am J Coll Cardiol 17:758–769
Forsius H (1988) Exfoliation syndrome in various ethnic populations. Acta Ophthalmol Scand 184:71–85
Ganji V, Kafai MR (2003) Demographic, health, lifestyle, and blood vitamin determinants of serum total homocysteine concentrations in the National Health and Nutrition Examination Survey 1988–1994. Am J Clin Nutr 77:826–833
Helbig H, Schlötzer-Schrehardt U, Noske W, Kellner U, Foerster MH, Naumann GO (1994) Anterior-chamber hypoxia and iris vasculopathy in pseudoexfoliation syndrome. Ger J Ophthalmol 3:148–153
Inoue M, Shirabe H, Yamamoto M (1999) Blood-aqueous barrier disruption in experimental anterior segment ischemia in rabbit eyes. Ophthalmic Res 31:213–219
Jakubowski H (2000) Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr 130 [Suppl]:377S–381S
Kanani PM, Sinkey CA, Browning RL, Allaman M, Knapp HR, Haynes WG (1999) Role of oxidant stress in endothelial dysfunction produced by experimental hyperhomocyst(e)inemia in humans. Circulation 100:1161–1168
Kawasaki A, Purvin VA, Burgett RA (1999) Hyperhomocysteinaemia in young patients with non-arteric anterior ischaemic optic neuropathy. Br J Ophthalmol 83:1287–1290
Küchle M, Ho TS, Nguyen NX, Hannappel E, Naumann GO (1994) Protein quantification and electrophoresis in aqueous humor of pseudoexfoliation eyes. Invest Ophthalmol Vis Sci 35:748–752
Lan J, Kumar RK, Di Girolamo N, McCluskey P, Wakefield D (2003) Expression and distribution of matrix metalloproteinases and their inhibitors in the human iris and ciliary body. B J Ophthalmol 87:208–211
Larsson J, Hultberg B, Hillarp A (2000) Hyperhomocysteinemia and the MTHFR C677T mutation in central retinal vein occlusion. Acta Ophthalmol Scand 78:340–343
Leibovitch I, Kurtz S, Shemesh G, Goldstein M, Sela BA, Lazar M, Loewenstein A (2003) Hyperhomocystinemia in pseudoexfoliation glaucoma. J Glaucoma 12:36–39
Ling Q, Hajjar KA (2000) Inhibition of endothelial cell thromboresistance by homocysteine. J Nutr 130:373S–376S
Mandal M, Das S, Chakraborti T, Mandal A, Chakraborti S (2003) Role of metalloprotease-2 in oxidant activation of Ca2+ ATPase by hydrogen peroxide in pulmonary vascular smooth muscle plasma membrane. J Biosci 28:205–213
McCully KS (1996) Homocysteine and vascular disease. Nat Med 2:386–389
Mercie P, Garnier O, Lascoste L, Renard M, Closse C, Durrieu F, Marit G, Boisseau RM, Belloc F (2000) Homocysteine-thiolactone induces caspase-independent vascular endothelial cell death with apoptotic features. Apoptosis 5:403–411
Mitchell P, Wang JJ, Smith W (1997) Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol 124:685–687
Mujumdar VS, Aru GM, Tyagi SC (2001) Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem 82:491–500
Omori S, Kodama H, Ikegami T, Mizuhara S, Oura T (1972) Unusual sulphur-containing amino acids in the urine of homocystinuric patients. III. Homocysteic acid, homocysteine sulfinic acid, S-(carboxymethylthio)homocysteine, and S-(3-hydroxy-3-carboxy-n-propyl). Physiol Chem Phys 4:286–294
Pianka P, Almog Y, Man O, Goldstein M, Sela BA, Loewenstein A (2000) Hyperhomocysteinemia in patients with nonarteric anterior ischemic optic neuropathy, central retinal artery occlusion, and central retinal vein occlusion. Ophthalmology 107:1588–1592
Repo LP, Teräsvirta ME, Tuovinen EJ (1990) Generalized peripheral iris transluminance in the pseudoexfoliation syndrome. Ophthalmology 97:1027–1029
Repo LP, Suhonen MT, Teräsvirta ME, Koivisto KJ (1995) Color Doppler imaging of the ophthalmic artery blood flow spectra of patients who have had a transient ischemic attack. Correlations with generalized iris transluminance and pseudoexfoliation syndrome.Ophthalmology 102:1199–1205
Repo LP, Naukkarinen A, Paljärvi L, Teräsvirta ME (1996) Pseudoexfoliation syndrome with poorly dilating pupil: a light and electron microscopic study of the sphincter area. Graefes Arch Clin Exp Ophthalmol 234:171–176
Ritch R (1994) Exfoliation syndrome: the most common identifiable cause of open-angle glaucoma. J Glaucoma 3:176–178
Schlötzer-Schrehardt U, Koca MR, Naumann GO, Volkholz H (1992) Pseudoexfoliation syndrome: ocular manifestation of a systemic disorder? Arch Ophthalmol 110:1752–1756
Schlötzer-Schrehardt U, Lommatzsch J, Küchle M, Konstas AG, Naumann GO (2003) Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci 44:1117–1125
Schumacher S, Schlötzer-Schrehardt U, Martus P, Lang W, Naumann GO (2001) Pseudoexfoliation syndrome and aneurysms of the abdominal aorta. Lancet 357:359–360
Sood HS, Cox MJ, Tyagi SC (2002) Generation of nitrotyrosine precedes activation of metalloproteinases in myocardium of hyperhomocysteinemic rats. Antioxid Redox Signal 4:799–804
Spark JI, Laws P, Fitridge R (2003) The incidence of hyperhomocysteinaemia in vascular patients. Eur J Vasc Endovasc Surg 26:558–561
Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, Loscalzo J (1993) Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest 91:308–318
Streeten BW, Li ZY, Wallace RN, Eagle RC Jr, Keshgegian AA (1992) Pseudoexfoliative fibrillopathy in visceral organs of a patient with pseudoexfoliation syndrome. Arch Ophthalmol 110:1757–1762
Tarkkanen AH (1986) Exfoliation syndrome. Trans Ophthalmol Soc U K 105:233–236
Tyagi SC (1998) Homocysteine redox receptor and regulation of extracellular matrix components in vascular wall. Am J Physiol 274:396–405
Tyagi SC (1999) Homocyst(e)ine and heart disease: pathophysiology of extracellular matrix. Clin Exp Hypertens 21:181–198
Tyagi SC, Kumar SG, Katwa L (1997) Differential regulation of extracellular matrix metalloproteinase and tissue inhibitor by heparin and cholesterol in fibroblast cells. J Mol Cell Cardiol 29:391–404
Tyagi SC, Smiley LM, Mujumdar VS, Clonts B, Parker JL (1998) Reduction-oxidation (Redox) and vascular tissue level of homocyst(e)ine in human coronary atherosclerotic lesions and role in extracellular matrix remodeling and vascular tone. Mol Cell Biochem 181:107–116
Vessani RM, Ritch R, Liebmann JM, Jofe M (2003) Plasma homocysteine is elevated in patients with exfoliation syndrome. AJO 136:41–46
Vine AK (2000) Hyperhomocysteinemia: a risk factor for central retinal vein occlusion. Am J Ophthalmol 129:640–644
Voutilainen S, Lakka TA, Hämelahti P, Lehtimäki T, Poulsen HE, Salonen JT (2000) Plasma total homocysteine concentration and the risk of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. J Intern Med 248:217–222
Wang L, Yamasita R, Hommura S (1999) Corneal endothelial and aqueous flare intensity in pseudoexfoliation syndrome. Ophthalmologica 213:387–391
Warsi AA, Davies B, Morris-Stiff G, Hullin D, Lewis MH (2004) Abdominal aortic aneurysm and its correlation to plasma homocysteine, and vitamins. Eur J Vasc Endovasc Surg 27:75–79
Xu D, Neville R, Finkel T (2000) Homocysteine accelerates endothelial cell senescence. FEBS Lett 470:20–24
Yuksel N, Karabas VL, Arslan A, Demirci A, Caglar Y (2001) Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology 108:1043–1049
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was not sponsored by any party or organization
Rights and permissions
About this article
Cite this article
Puustjärvi, T., Blomster, H., Kontkanen, M. et al. Plasma and aqueous humour levels of homocysteine in exfoliation syndrome. Graefe's Arch Clin Exp Ophthalmol 242, 749–754 (2004). https://doi.org/10.1007/s00417-004-0918-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-0918-7