Abstract
Purpose
To evaluate the histological changes in the retina after experimental vitreous substitution with various amounts of perfluorophenanthrene (PFPH).
Methods
Thirty-two rabbit eyes were mechanically vitrectomized and filled up with 0.8 cc or 0.2 cc highly purified PFPH. The substance remained for 1 week (4 eyes), 2 weeks (8 eyes), 4 weeks (10 eyes) or 8 weeks (10 eyes). Eight eyes underwent the same surgical procedure without PFPH exchange to serve as a control group. A histological comparison of corresponding areas in the center and in the periphery of the inferior retina ensued.
Results
After 2 weeks, nuclear drop-downs and irregularities of the outer plexiform layer and of both nuclear layers were observed centrally in the eyes with a 0.8 cc substitution. The changes proceeded to irregularities and cell loss of all retinal layers with focal areas of complete destruction of the retinal architecture after 8 weeks. In contrast, single nuclear drop-downs, wrinkling of the outer nuclear layer and cell loss in the photoreceptor layer were observed in the peripheral retina at the end of the observation period. In those eyes where 0.2 cc PFPH was exchanged nuclear drop-downs were found after 2 weeks, leading to focal thinning of the outer plexiform layer and irregularities of the outer nuclear layer after 4 weeks with an insignificant increase after 8 weeks. At this time these histological alterations were comparable with those that we observed after 2 weeks in the eyes with a 0.8 cc tamponade in a corresponding area.
Conclusions
Even high purification of PFPH does not prevent retinal damage. The different results in different areas after vitreous substitution with 0.2 cc and with 0.8 cc demonstrate that the high specific gravity of the substance may also play a role in the development of histological changes after extended tamponade.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perfluorocarbon liquids (PFCLs) and heavy fluorocarbon liquids (HFCLs) are routinely used in vitreoretinal surgery for various indications [1, 5, 6, 7, 9, 11, 15, 17, 18, 22, 23, 27] due to the ability to stabilize the retina on the posterior pole by their high specific gravity. In special situations like breaks in the inferior retina they might be suitable for postoperative temporary tamponade [17]. However, retinal damage after short-term and extended substitution with different heavy perfluorocarbon liquids has been demonstrated experimentally [4, 8, 10, 12, 24, 30, 31, 32]. The better tolerance of highly purified substances has been shown clearly in animal experiments [31, 32], but the effect of the weight of perfluorocarbon liquids has never been discussed. To date, complete vitreous exchange was aimed for in all reported studies on experimental perfluorocarbon testing [4, 8, 10, 12, 14, 24, 25, 30, 31, 32].
The present study was set up to evaluate histologically the role of the specific gravity of heavy perfluorocarbon liquids, exemplified by perfluorophenanthrene (PFPH).
Methods
A total of 40 eyes of albino rabbits weighing between 2.8 and 3.1 kg was involved in the study. Initially the animals were scheduled to be killed at 1, 2, 4, or 8 weeks of follow-up. Institutional guidelines regarding animal experimentation were followed.
Surgery was performed with the animal under general anesthesia. Preoperative sedation comprised an intramuscular injection of 0.4 ml/kg ketamine hydrochloride (100 mg/ml) and 0.15 ml/kg xylazine hydrochloride (2%). The intraoperative anesthesia with 1.0 ml ketamine hydrochloride (100 mg/ml) was given slowly via the auricular vein. The pupils were dilated with 0.5% tropicamide and 2.5% phenylephrine. After preparation of the conjunctiva a sclerotomy for the infusion canula was performed 1.5 mm behind the limbus in the inferior nasal quadrant for right eyes and in the temporal quadrant for left eyes. The infusion fluid consisted of Ringer’s solution. Thereafter, a mechanical vitrectomy (Ocutome) followed through a second sclerotomy on the opposite side under the coaxial illumination of an operative microscope (Zeiss OPMI2). The vitreous was removed as completely as possible also in the eyes where a small amount of PFPH was planned as substitution to exclude different preconditions. At the end of surgery the sclerotomy site for the vitrectomy probe was partially closed with 7.0 Vicryl sutures. PFPH was injected intravitreally using a syringe and irrigation fluid was allowed to escape through the sclerotomy. We used a commercially available product (Vitreon, Vitrophage, Illinois). Sixteen eyes respectively received 0.8 cc or 0.2 cc PFPH. Eight eyes where Ringer’s solution was left served as comparative group. Wound closure was completed and antibiotic ointments were administered. Surgery was uneventful in all cases.
During the observation period clinical controls were carried out daily for the first week after surgery, thereafter weekly. They included a biomicroscopic examination of the eyes and fundoscopy under dilated pupils using an indirect ophthalmoscope. Three rabbits (six eyes) were followed up for 1 week (two eyes with 0.8 cc, two eyes with 0.2 cc, two control eyes), five rabbits (10 eyes) for 2 weeks (four eyes with 0.8 cc, four eyes with 0.2 cc, two control eyes), 6 rabbits (12 eyes) for 4 weeks (five eyes with 0.8 cc, five eyes with 0.2 cc, two control eyes), and 6 rabbits (12 eyes) for 8 weeks (five eyes with 0.8 cc, five eyes with 0.2 cc, two control eyes) (Table 1).
After these periods the eyes were enucleated with the animals under deep anesthesia. The globes were dissected circumferentially close to the limbus. The globes were shortly fixated with glutaraldehyde to cut horizontal sections with razor-blades 5–9 mm below the medullary ray measured with compasses (Fig. 1) to allow a histological comparison of corresponding areas.
In these sections changes were judged in the middle part and in eyes with 0.8 cc also in the periphery (Fig. 1) where the weight of the overlying PFPH was less. The samples were immediately fixed in a 2.5% formalin solution buffered with phosphate, embedded in paraffin, and stained with hematoxylin–eosin.
Results
Clinical observations
In all eyes examination of the anterior and posterior segments showed no abnormalities during follow-up. No inflammatory reaction, pucker formation, or lens opacification was observed.
The 0.8 cc bubble reached the level of the medullary ray in the rabbit eye, whereas the 0.2 cc bubble was only covering the inferior retina. Irrespective of the volume injected, dispersion of the PFPH was observed after 2 weeks. After 4 weeks the view of the inferior fundus was limited due to the increasing number of smaller droplets particularly in eyes with a 0.8 cc tamponade.
Histological results
After 1 week of PFPH tamponade we found no changes in the inferior retina, neither in the eyes with a 0.8 cc tamponade (two eyes) nor in the eyes with a 0.2 cc substitution (two eyes) (Table 2).
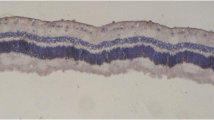
Two weeks postoperatively we observed single nuclear drop-downs into the photoreceptor layer, irregularities in the outer plexiform layer, and reduction of cells in both nuclear layers in the center of the sections of the eyes with a 0.8 cc tamponade (four cases) (Fig. 2). The changes were classified as mild. No abnormalities were found in the periphery of the sections. In those eyes where 0.2 cc PFPH was exchanged (four eyes) only a small number of nuclear drop-downs were present in the inferior retina at this time, which was classified as none to mild changes.
Four weeks’ tamponade with 0.8 cc PFPH (five eyes) caused cell loss in both nuclear layers and the photoreceptor layer, focal areas of thinning of the outer plexiform layer, wrinkling of the outer retinal layers, an increased number of nuclear drop-downs, and bump like protrusions through the outer limiting membrane into the photoreceptor interspace caused by Müller cell hypertrophy in the middle part of the inferior retina (Fig. 3). The changes were classified as moderate. In the periphery of these eyes, where the effect of weight was less, single nuclear drop-downs were present. The nuclear layers showed normal density and thickness, but in the photoreceptor layer areas of decreased cell density were found which was classified as mild changes (Fig. 4).
Periphery of the same histological section as Fig. 3 (hematoxylin and eosin, ×400)
A quantity of 0.2 cc PFPH over a 4-week period (five eyes) led to thinnings in the outer plexiform layer, irregularities of the outer nuclear layer, and occasionally to nuclear drop-downs in the middle of the samples (Fig. 5). The changes were classified as mild and were comparable to those which were found after 2 weeks’ tamponade with 0.8 cc PFPH in the corresponding area (Fig. 2).
Eight weeks postoperatively, in the eyes which had a 0.8 cc tamponade (five eyes) we observed irregularity of all retinal layers with cell loss in both nuclear layers, an increasing number of nuclear drop-downs and bump-like protrusions in the middle part of the sections. The number of photoreceptor cells was reduced. There were large areas of complete destruction of the normal retinal architecture (Fig. 6). In one sample macrophages invaded at the internal limiting membrane. The changes were classified as severe. In contrast, the changes in the periphery were less severe: nuclear drop-downs, wrinkling of the outer retinal layers, and cell loss in the photoreceptor layer were present (Fig. 7).
Periphery of the same histological section as Fig. 6 (hematoxylin and eosin, ×400)
At the same time point the eyes with a 0.2 cc tamponade (five eyes) showed centrally a narrowing of the outer plexiform layer, slight wrinkling of the outer retinal layers with cell loss in the outer nuclear layer in some areas, and nuclear drop-downs (Fig. 8). The changes were classified as mild because the damage was of similar severity to that we found in corresponding areas after 4 weeks’ tamponade with the same amount (Fig. 5) or after 2 weeks’ tamponade with 0.8 cc PFPH (Fig. 2).
In control eyes normal retinal anatomy was preserved during the whole observation period (Fig. 9). It was evident that in all eyes with PFPH tamponades the thickness of the retina was reduced to about half that in the control eyes.
Discussion
Heavier-than-water fluorinated or semi-fluorinated liquids facilitate vitreoretinal surgery in a variety of settings. These include proliferative vitreoretinopathy [1, 5, 9, 11, 17], ocular trauma [6], diabetic retinopathy [23], giant retinal tears [3, 4, 5, 6, 8, 9], and dislocated lenses or lens implants [1, 7, 15, 18, 22, 27]. Together with their high contact angle, an intraocular tamponade with perfluorocarbon liquid results in a larger contact area than an intraocular silicone oil tamponade or gas bubble may provide (Bacon AS, Lavin MJ. Perfluorocarbon liquids: potential interactions during vitreoretinal surgery. Presented as a poster at the Annual Meeting of the College of Ophthalmologists of UK, Glasgow,1991). In an upright position of the patient, perfluorocarbon liquids therefore offer an effective tamponade for eyes with inferior retinal breaks [2, 3, 33].
Animal experiments with different perfluorocarbon substances, however, demonstrated histological damage of the retina after various observation periods in most of the studies. Miyamoto et al. [24] describes gliosis of the retina 1 month after intravitreous injection of perfluoroether. Chang et al. [8] found moth-eaten defects in the superior and inferior retina as early as 2 days after vitreous replacement with perfluorotributylamine. Nahib et al. [25] observed an unchanged retinal anatomy after 6 weeks’ tamponade with PFPH. Eckardt et al. reported that perfluorooctane and perfluoropolyether caused hypertrophy of Müller cells with bump-like protrusions into the photoreceptor interspaces at 6 days after surgery [12]. Similar changes were found by Chang et al. [10] and Sparrow et al. [30] when perfluoro-n-octane and perfluoroethylcyclohexane respectively were left in the eye for more than 1 week. Bryan et al. [4] proved perfluorotri-n-propylamine to be safe for a duration of 2 weeks. Velikay et al. [32] compared the histological effect of perfluorodecalin and perfluorooctylbromide on the retina and described similar lesions in the outer retinal layers after 1 and 2 weeks, more pronounced in perfluorooctylbromide-filled eyes. Flores et al. [14] observed no adverse reactions in the retina after perfluorooctylbromide exchange up to 6 months.
The histological results are controversial due to the unclear mechanism of retinal intolerance. The importance of inertness of vitreous substitutes was emphasized in 1990 [29] in a fibroblast model. Toxic reactions due to chemical impurities of perfluorocarbon liquids, resulting mainly from incomplete fluorination of hydrocarbons, have since been demonstrated clearly in animal experiments [31, 32].
Perfluorocarbon liquids are 1.76–2.03 times heavier than water and are thus heavier than aqueous, subretinal fluid, or irrigation solutions. This raises the question of the effect of weight on the inferior retina, to which the damage has frequently been limited [4, 8, 10, 24, 30]. In all studies [4, 8, 10, 12, 21, 24, 25, 30, 31, 32] a perfluorocarbon exchange as complete as possible was aimed for.
For the histological evaluation of the effect of various amounts of perfluorocarbon liquids in the present study we used PFPH because its specific weight is the highest among perfluorocarbon liquids (2.03 g/cc); it has been suggested to be an excellent candidate for experimental vitreous replacement [25, 29]. The available product is 99.9% pure. The quantity of 0.2 cc PFPH as the smaller tamponade was chosen because perfluorocarbon volumes less than 0.1 ml were found to have no adverse effect on the retina even in the long term [10, 30].
In the eyes with a 0.8 cc PFPH tamponade we observed retinal damage in the center of the inferior sections after 2 weeks with increasing severity thereafter, ending up in complete destruction of the normal retinal anatomy. In the periphery of the samples, distinct changes occurred after 4 weeks and increased after 8 weeks to irregularities in the outer nuclear layer and cell loss in the photoreceptor layer. Our observations correspond well with the histological findings reported earlier from studies where comparable amounts of other perfluorocarbon liquids were injected [4, 8, 10, 30, 31, 32]. They are inconsistent with the preliminary results after PFPH substitution in a previous study [25]. However, there has never been an explanation for the better tolerance of PFPH.
In contrast, in eyes with a replacement of 0.2 cc PFPH the histological changes which were present in the center of the sections after 2 weeks showed a much lesser severity. They proceeded after 4 weeks in a smaller extent than in the eyes with a 0.8 cc substitution and did not increase in the later course. After 4 and 8 weeks the effect of a 0.2 cc PFPH volume on the middle inferior retina is comparable to that of a 0.8 cc quantity after 2 weeks on a corresponding area. This area represents the deepest point of the rabbit’s eye in an upright position of the animal. In our experimental model the upper level and probably the weight of the overlying perfluorocarbon bubble in this area was about 3 times higher in eyes with a 0.8 cc tamponade.
Our results suggest that even highly purified PFPH causes retinal damage. The comparison of histological changes in central and peripheral areas in eyes with small and large amounts of PFPH substitution showed different severity, different time of appearance, and different progression. The high specific gravity of perfluorocarbon liquids is a potential factor in the mechanism of retinal intolerance.
References
Binder S, Velikay M, Wedrich A, Stolba U, Datlinger P (1992) Die klinische Anwendung flüssiger Perfluorocarbone in der Netzhautchirurgie. Spektrum Augenheilkd 6:4–7
Blinder KJ, Peyman GA, Desai UR, Norman NC, Alturki W, Paris CL (1992) Vitreon, a short-term vitreoretinal tamponade. Br J Ophthalmol 76:525–528
Bottoni F, Sborgia M, Arpa P, De Casa N, Bertazzi E, Monticelli M (1993) Perfluorocarbon liquids as postoperative short-term vitreous substitutes in complicated retinal detachment. Graefes Arch Clin Exp Ophthalmol 231:619–628
Bryan JS, Friedman SM, Mames RN, Margo CE (1994) Experimental vitreous replacement with Perfluorotri-n-Propylamine. Arch Ophthalmol 112:1098–1102
Carroll BF, Peyman GA, Mehta NJ, Millsap CM, Greve MDJ, Dunlap WA (1994) Repair of retinal detachment associated with proliferative vitreoretinopathy using perfluoro-perhydrophenanthrene (Vitreon). Can J Ophthalmol 29:66–69
Chang S, Reppucci V, Zimmerman NJ, Heinemann MH, Coleman DJ (1981) Perfluorocarbon liquids in the management of traumatic retinal detachments. Ophthalmology 96:785–792
Chang S, Lincoff H, Zimmerman NJ, Fuchs W (1981) Giant retinal tears. Surgical techniques and results using Perfluorocarbon liquids. Arch Ophthalmol 107:761–766
Chang S, Zimmerman NJ, Iwamoto T, Ortiz R, Faris D (1987) Experimental vitreous replacement with Perfluorotributylamine. Am J Ophthalmol 103:29–37
Chang S, Ozmert E, Zimmerman NJ (1988) Intraoperative Perfluorocarbon liquids in the management of proliferative vitreoretinopathy. Am J Ophthalmol 106:668–674
Chang S, Sparrow JR, Iwamoto T, Gershbein A, Ross R, Ortiz R (1991) Experimental studies of tolerance to intravitreal Perfluoro-n-octane. Retina 11:367–374
Coll GE, Chang S, Sun J, Wieland MR, Berrocal MH (1995) Perfluorocarbon liquid in the management of retinal detachment with proliferative vitreoretinopathy. Ophthalmology 102:630–639
Eckardt C, Nicolai U, Winter M, Knop E (1991) Experimental intraocular tolerance to liquid Perfluorooctane and Perfluoropolyether. Retina 11:375–384
Fanous MM, Friedman SM (1992) Ciliary sulcus fixation of a dislocated posterior chamber intraocular lens using liquid perfluorophenanthrene. Ophthalmic Surg 23:551–552
Flores-Aguilar M, Munguia D, Loeb E, Crapotta JA, Vuong C, Shakiba S (1995) Intraocular tolerance of Perfluoro-octylbromide (Perflubron). Retina 15:3–13
Glaser BM, Carter JB, Kuppermann BD, Michels RG (1991) Perfluoro-octane in the treatment of giant retinal tears with proliferative vitreoretinopathy. Ophthalmology 98:1613–1621
Greve MDJ, Peyman GA, Mehta NJ, Millsap CM (1993) Use of Perfluoroperhydro-phenanthrene in the management of posteriorly dislocated crystalline and intraocular lenses. Ophthalmic Surg 24:593–597
Kirchhof B, Wong D, Van Meurs J, Hilgers RD, Macek M, Lois N, Schrage NF (2002) Use of perfluorohexyloctane as a long-term internal tamponade agent in complicated retinal detachment surgery. Am J Ophthalmol 133:95–101
Le Mer Y, Kroll P (1991) Die Verwendung von flüssigem Perfluorocarbon bei Riesenrissen. Klin Mb Augenheilkd 198:264–267
Lewis H, Sanchez G (1993) The use of perfluorocarbon liquids in the repositioning of posteriorly dislocated lenses. Ophthalmology 100:1055–1059
Lewis H, Blumenkranz MS, Chang S (1992) Treament of dislocated crystalline lens and retinal detachment with perfluorocarbon liquids. Retina 12:229–304
Liu KR, Peyman GA, Chen M, Cheng K (1991) Use of high density vitreous substitutes in the removal of posteriorly dislocated lenses or intraocular lenses. Ophthalmology 22:503–507
Mathis A, Pagot V, Gazague C, Malecaze F (1992) Giant retinal tears. Surgical techniques and results using perfluorodecaline and silicone oil tamponade. Retina 12(Suppl):7–10
Mathis A, Pagot V, Idder A, Malecaze F (1993) Utilisation de la perfluorodecaline au cours de la vitrectomie chez le diabetique. J Fr Ophtalmol 16(11):584–590
Miyamoto K, Refojo MF, Tolentino FI, Fournier GA, Albert DM (1984) Perfluoroether liquid as a long-term vitreous substitute. An experimental study. Retina 4:264–268
Nahib M, Peyman GA, Clark LC, Hoffman RE, Miceli M, Abou-Steit M (1989) Experimental evaluation of Perfluoro-phenanthrene as a high specific gravity vitreous substitute: a preliminary report. Ophthalmic Surg 20(4):286–293
Rowson NJ, Bacon AS, Rosen PH (1992) Perfluorocarbon heavy liquids in the management of posterior dislocation of the lens nucleus during phakoemulsification. Br J Ophthalmol 76:169–170
Schulman JA, Peyman GA, Blinder KJ (1993) Management of giant retinal tears with perfluoroperhydrophenanthrene (Vitreon). Jpn J Ophthalmol 37:70–77
Shapiro MJ, Resnik KI, Kim SH, Weinberg A (1991) Management of the dislocated crystalline lens with a Perfluorocarbon liquid. Am J Ophthalmol 112:401–405
Sparrow JR, Ortiz R, MacLeish PR, Chang S (1990) Fibroblast behaviour at aqueous interface with Perfluorocarbon, Silicone, and Fluorosilicone liquids. Invest Ophthalmol Vis Sci 31:638–646
Sparrow JR, Matthews GP, Iwamoto T, Ross R, Gershbein A, Chang S (1993) Retinal tolerance to intravitreal Perfluoro-ethylcyclohexane liquid in the rabbit. Retina 13:56–62
Velikay M, Wedrich A, Stolba U, Datlinger P, Li Y, Binder S (1993) Experimental long-term vitreous replacement with purified and non purified Perfluorodecaline. Am J Ophthalmol 116:565–570
Velikay M, Stolba U, Wedrich A, Li Y, Datlinger P, Binder S (1995) The effect of chemical stability and purification of Perfluorocarbon liquids in experimental extended-term vitreous substitution. Graefes Arch Clin Exp Ophthalmol 223(1):26–30
Verma LK, Peyman GA, Wafapor H, Greve MD, Millsap CM, Adile SL (1995) An analysis of posterior segment complications after vitrectomy using the perfluorocarbon perfluoroperhydrophenanthrene (Vitreon). Ophthalmic Surg 26:29–33
Wallace RT, McNamara JA, Brown G, Benson W, Belmont J, Goldberg R (1993) The use of perfluorophenanthrene in the removal of intravitreal lens fragments. Am J Ophthalmol 116:196–200
Acknowledgement
The authors thank the Center of Biomedical Research Vienna (chairman: Prof. Dr. U. Losert) for the support they received.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author states that she has no proprietary interest in the development or marketing of products mentioned in the article or competing drugs
Rights and permissions
About this article
Cite this article
Stolba, U., Krepler, K., Velikay-Parel, M. et al. The effect of specific gravity of perfluorocarbon liquid on the retina after experimental vitreous substitution. Graefe's Arch Clin Exp Ophthalmol 242, 931–936 (2004). https://doi.org/10.1007/s00417-004-0916-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-0916-9