Abstracts
Background
The purpose of this study is to investigate the effect of fibrinogen on angiogenesis in vitro formed by cultured bovine choroidal endothelial cells (BCECs) and the involvement of vascular endothelial growth factor (VEGF) in this mechanism.
Methods
For in vitro tube formation assay, BCECs were seeded on collagen gel containing fibrinogen (0–1.5 mg/ml). After 3 days of cultivation, the total length of the tubular structure was measured using Macscope Analyzer. Total RNA and conditioned media were collected after fibrinogen treatment and subjected to Northern and Western blot analyses, respectively. Transcription factor HIF-1α was also analyzed by Western blot analysis using cytosolic and nuclear fraction of BCECs. Involvement of VEGF in fibrinogen-dependent in vitro tube formation was evaluated using anti-VEGF neutralizing antibody or VEGF receptor 2-selective inhibitor (SU5416).
Results
Formation of the tubular structure was enhanced 20~50 times in fibrinogen-containing gel in a concentration-dependent manner. The treatment of BCECs with fibrinogen resulted in a significant increase in VEGF gene and protein expression. Accumulation of HIF-1α protein in the nuclear fraction was also detected after the treatment with fibrinogen. Finally, fibrinogen-induced tube formation was significantly inhibited in the presence of anti-VEGF-neutralizing antibody (52.0% inhibition at the concentration of 1 μg/ml, P<0.05) or SU5416 (54.8% inhibition at the concentration of 3 μΜ, P<0.05).
Conclusions
Extravasated fibrinogen might play an important role in the development of choroidal neovascularization associated with age-related macular degeneration, at least in part, through the function of VEGF in an autocrine manner. Transcription factor HIF-1 appears to be involved in fibrinogen-induced VEGF expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Age-related macular degeneration (AMD) is the most common cause of severe visual acuity loss in patients over the age of 60 in developed countries [17]. Subfoveal choroidal neovascularization (CNV) is the major pathological feature of AMD that leads patients to the more severe stage of the disease. However, the precise mechanism of choroidal neovascularization is not fully clarified to date.
Neovascularization, the formation of new blood vessels from pre-existing vessels, plays a critical role during many physiological and pathological processes, such as inflammation, wound repair and tumor growth [11]. Folkman and Haudenschild [10] demonstrated that cultured capillary endothelial cells form tubular structures, and these tubes formed in vitro were also shown to be ultrastructurally similar to capillaries in vivo [31, 21]. It was also revealed that angiogenesis is regulated not only by the angiogenic growth factors such as basic fibroblast growth factor (b-FGF) and vascular endothelial growth factor (VEGF), but also by the composition of the extracellular matrix (ECM) [12, 20]. Many investigators [16, 28, 36, 44] have shown that the ECM of endothelial cells is capable of influencing several aspects of cellular behavior including cell-to-cell and cell-to-ECM attachment, cell growth and migration.
The Blue Mountain Eye Study [37] found there to be a significant increased risk of AMD associated with increasing plasma fibrinogen levels. Additionally, it is reported that fibrinogen extravasates and deposits around the newly formed choroidal vessels of eyes with AMD [26, 35, 23]. The temporarily deposited fibrin matrix acts as a sealing matrix as well as the scaffolding for invading leukocytes and endothelial cells during the process of angiogenesis [42]. However, the molecular mechanisms underlying the association between extravasated fibrinogen and AMD are incompletely understood.
VEGF is known to be an endothelial cell-specific mitogen and an angiogenesis inducer released from a variety of cells [25]. The heterodimeric protein hypoxia-inducible factor consists of the constitutively expressed HIF-1β and the inducible protein HIF-1α [43]. HIF-1 is activated in response to several stimuli such as thrombin [15], advanced glycation end products [40] and insulin [41] in addition to hypoxic conditions [13]. However, to our knowledge, the association between fibrinogen and HIF-1 is still unknown.
VEGF could also cause vascular leakage, by which fibrinogen extravasates and forms a fibrinous bed providing additional scaffolding for the invasion process. Immunohistochemical analysis of surgically excised CNV membranes from eyes with AMD showed that the CNV area was strongly immunoreactive to VEGF, especially in transdifferentiated retinal pigment epithelial (RPE) cells [27]. We previously demonstrated increased expression of VEGF in CNV membranes in an experimental CNV model by immunohistochemical examination and in situ hybridization [22]. Considering these previous reports, it is reasonable to assume some association between fibrinogen, VEGF and the development of CNV in AMD.
In the present study, we demonstrated the promoting effect of fibrinogen on angiogenesis in vitro formed by choroidal endothelial cells, and possible involvement of VEGF in this effect. We also addressed the possible transcription factor mediating fibrinogen-induced VEGF gene expression. These in vitro studies will contribute to a better understanding of the role of extravasated fibrinogen on the progression of CNV membrane in AMD.
Materials and methods
Reagents
Human fibrinogen (fraction I, type IV) was purchased from Sigma (St. Louis, Mo.), and contaminating plasminogen was removed by lysine-Sepharose 4B column chromatography (Pharmacia Biotech, Uppsala, Sweden). A VEGF receptor-2 (VEGFR2)-selective inhibitor SU5416 was synthesized in our laboratory as previously described [2].
Antibodies
Agarose-conjugated rabbit anti-human VEGF (A-20), mouse anti-human VEGF (C-1) and rabbit anti-human HIF-1α (C19) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Goat anti-human VEGF-neutralizing antibody and non-immunized control goat IgG were purchased from R&D Systems (Minneapolis, Minn.). Anti-phospho-specific ERK1(p44)/ERK2(p42) monoclonal antibody was from New England Biolabs (Beverly, Mass.). Non-phosphorylation-specific anti-ERK1 antibody was from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated secondary antibody was from Amersham Corp. (Arlington Heights, Ill.).
Cell cultures
Primary cultures of bovine choroidal endothelial cells (BCECs) were prepared by homogenization and a series of filtration step as previously described for the isolation of retinal capillary endothelial cells [18]. Cells were maintained in Dulbecco’s modified eagle medium (DMEM, Gibco, Grand Island, N.Y.) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco), penicillin G (100 IU/ml) and streptomycin sulfate (50 mg/ml). Passage 5–10 cultures of BCECs were used in the experiments. In our research, we conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Capillary tube formation assay
Collagen gel containing 1 mg/ml of type I collagen (Koken Co., Ltd., Tokyo) was prepared in a 24-well plate (Nunc, Roskilde, Denmark) as previously described [18]. Fibrinogen plus collagen gel was prepared by adding various concentrations of fibrinogen to the control gel instead of distilled water. After gelatinization, BCECs (2×105 cells/1 ml DMEM) were seeded on each gel. The tubular structure was photographed under a phase-contrast microscope 3 days after seeding. Five fields per gel were randomly selected, recorded and the total length of capillary tubes per unit area (mm/mm2) was measured using Macscope Analyzer (Mitani Corporation, Fukui, Japan).
Northern blot analysis
Subconfluent monolayers of BCECs in a 9-cm dish were starved with DMEM containing 1% FBS for 6 h and then incubated with various concentrations (0, 0.5, 1 and 1.5 mg/ml) of fibrinogen for 16 h. Total cellular RNA was isolated using the acid guanidinium thiocyanate-phenol-chloroform extraction method [3] and subjected to Northern blot analysis for VEGF gene expression. Radioactive VEGF cDNA probe [38] was generated using Amersham Multiprime labeling kits and [α-32P]dCTP (NEN)]. The radioactivity of the hybridized signal was detected and quantified using a Fujix BAS2000 Bioimage Analyzer (Fuji Photo Film Co., Tokyo). Lane loading differences were normalized by rehybridization with a radiolabeled 36B4 cDNA probe [38].
Preparation of conditioned media and cytosolic/nuclear protein
Subconfluent BCECs were starved with DMEM containing 1% FBS for 6 h. After starvation, the medium was changed to fresh DMEM containing 1% FBS with either vehicle or fibrinogen at a concentration of 1 mg/ml. Forty-eight hours after stimulation, supernatants were collected and concentrated five-fold using a centrifugal filter device (Millipore, Bedford, Mass.) with a 10,000-MW cutoff. Cytosolic and nuclear protein was isolated as previously described [19] after 12 h stimulation with fibrinogen or hypoxic condition (1% O2).
Immunoprecipitation and Western blot analysis
Five hundred microliters of 5-fold concentrated conditioned media were immunoprecipitated with agarose-conjugated rabbit anti-human VEGF antibody (A-20) and subjected to 15% SDS-PAGE after the treatment with 2-mercaptoethanol. Proteins were transferred to nitrocellulose filters (BioRad, Hercules, Calif.). The membrane was incubated with mouse monoclonal anti-human VEGF antibody (C-1), followed by horseradish peroxidase-conjugated secondary antibody (Amersham Corp.). Cytosolic and nuclear protein was subjected to 6% SDS-PAGE and analyzed for HIF-1α protein expression and localization. Visualization was performed using Amersham’s enhanced chemiluminescence (ECL) detection system according to the manufacturer’s instructions.
Statistical analysis
All experiments were repeated at least three times with similar findings, and results are expressed as means +SD. The Mann-Whitney U test was used, and a P value of less than 0.05 was considered significant.
Results
In vitro tube formation enhanced by fibrinogen
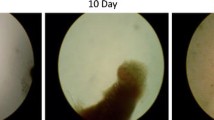
First, we examined the effect of fibrinogen on angiogenesis in vitro formed by cultured BCECs. The total length of the tubular structure was enhanced 20–50 times in fibrinogen-containing gels compared with that in gels without fibrinogen (Fig. 1). The promoting effect of fibrinogen on the tubular structure was dose dependent (0.5–1.5 mg/ml).
The effect of fibrinogen on tube formation by BCECs. After 3 days cultivation with various concentrations of fibrinogen (Fg), the tubular structure was photographed under a phase-contrast microscope, and the total length of capillary tubes per unit area (mm/mm2) was measured. The length of the tubular structure was enhanced 20–50 times in fibrinogen-containing gels compared with control. The effect was dose dependent (n=5, **P<0.05)
Effect of fibrinogen on VEGF mRNA expression by BCECs
Although the angiogenic effect of fibrinogen has been reported previously, the exact mechanism is not fully defined. Thus, to make certain whether fibrinogen induces VEGF mRNA expression in BCECs, starved BCECs were stimulated with 0, 0.5, 1 or 1.5 mg/ml fibrinogen for 16 h. As shown in Fig. 2, fibrinogen-enhanced VEGF mRNA expression by BCECs increased in a dose-dependent manner (3.9-fold increase at 1 mg/ml fibrinogen, P<0.05; 5.3-fold increase at 1.5 mg/ml fibrinogen, P<0.05). These results suggest that VEGF, at least in part, mediates fibrinogen-dependent angiogenesis.
The effect of fibrinogen on VEGF mRNA expression by BCECs. Subconfluent BCECs in a 9-cm dish were starved with DMEM containing 1% FBS for 6 h and then stimulated with 0, 0.5, 1 or 1.5 mg/ml fibrinogen (Fg) for 16 h. Total RNA was isolated and subjected to Northern blot analysis for VEGF mRNA expression. Fibrinogen significantly increased VEGF expression in BCECs in a dose-dependent manner (n=5, *P<0.01, **P<0.05)
Effect of fibrinogen on VEGF protein secretion by BCECs
To determine whether VEGF mRNA overexpression by BCECs was accompanied by an increase in protein synthesis and secretion into the conditioned media, the supernatants were collected and analyzed by Western blotting. Immunoprecipitates were electrophoresed after the treatment with 2-mercaptoethanol. As a result of immunoblotting, a single band of approximately 20 kDa appearing to correspond to the VEGF monomer was detected in immunoprecipitates collected from fibrinogen-stimulated BCECs, but not those from unstimulated BCECs (Fig. 3).
Fibrinogen-dependent VEGF protein secretion by BCECs. Subconfluent BCECs were stimulated with vehicle (control: C) or 1 mg/ml fibrinogen (Fg) for 48 h, and then supernatants were collected. Samples were subjected to immunoprecipitation and Western blot analysis after treatment with 2-mercaptoethanol. A single band of approximately 20 kDa, which corresponds to the VEGF monomer, was detected in immunoprecipitates of fibrinogen-stimulated BCECs, but not in unstimulated BCECs
Nuclear accumulation of transcription factor HIF-1α
Transcription factor HIF-1α is known to be one of the key regulators of VEGF gene expression. We thus examined whether HIF-1α was activated in BCECs in response to fibrinogen treatment. As shown in Fig. 4, fibrinogen treatment resulted in increased accumulation of HIF-1α into the nuclear fraction. As a positive control, we also confirmed the effect of hypoxic cultivation on nuclear accumulation of transcription factor HIF-1α.
Fibrinogen-induced nuclear accumulation of HIF-1α. BCECs were stimulated with vehicle (control: C), 1 mg/ml fibrinogen (Fg) or hypoxic condition (Hy) for 12 h. Isolated cytosolic or nuclear faction was subjected to 6% SDS-PAGE. Increased accumulation of transcription factor HIF-1α was detected in samples stimulated with fibrinogen or hypoxia as compared with control
Involvement of VEGF in fibrinogen-induced tube formation
To confirm the participation of VEGF in fibrinogen-induced in vitro tube formation, we assessed the effect of anti-VEGF-neutralizing antibody. BCECs were seeded on the collagen gels (without or with fibrinogen) and cultivated in the absence or presence of anti-VEGF-neutralizing IgG (0.5 and 1 μg/ml) or non-immunized control IgG (1 μg/ml). As shown in Fig. 5, fibrinogen-induced tube formation was significantly prohibited by anti-VEGF neutralizing IgG (51.9% inhibition at a concentration of 0.5 μg/ml, P<0.05; 52.0% inhibition at a concentration of 1 μg/ml, P<0.05). However, the tube formation in collagen gel only was not influenced by anti-VEGF-neutralizing IgG, suggesting that VEGF protein in the medium was newly secreted VEGF from fibrinogen-stimulated BCECs. Non-immunized control IgG did not show any inhibitory effects on the tube formation in fibrinogen containing gel or in control gel.
Involvement of VEGF in fibrinogen-induced tube formation. Anti-human VEGF antibody (1 μg/ml) added to the medium did not have any effect on tube formation by BCECs in the absence of fibrinogen (Fg), while anti-human VEGF antibody (0.5, 1 µg/ml) added to the medium of fibrinogen-containing gels significantly inhibited the tube formation of BCECs. Non-immunized control IgG did not show any inhibition (n=5, **P<0.05 )
Effect of SU5416 on fibrinogen-induced tube formation
Previous studies have shown that SU5416, a potent and a selective inhibitor of VEGFR-2, inhibited VEGF-driven mitogenesis of HUVECs [2]. To determine the receptor responsible for VEGF signaling in fibrinogen-induced tube formation by BCECs, we tested the effect of SU5416. To determine an appropriate concentration of SU5416, the inhibitory effect of VEGF-induced p44/p42 MAP kinase, one of the downstream targets of VEGFR-2, was examined. As shown in Fig. 6A, VEGF caused dramatic phosphorylation of p44/p42 MAP kinase, while SU5416 almost totally abrogated this effect at a concentration of 3 μM. SU5416 treatment also resulted in a dose-dependent inhibition of fibrinogen-induced tube formation in vitro at the corresponding concentrations, which inhibited MAP kinase phosphorylation (Fig. 6B).
The effect of SU5416 on tube formation by BCECs. The effect of a potent VEGFR-2 selective inhibitor SU5416 was assessed by its inhibitory effect on VEGF-dependent MAP kinase activation, a downstream target of VEGFR-2 (A). BCECs were stimulated with VEGF (25 ng/ml) for 5 min. Total cell lysates were immunoblotted with anti-phospho-p44/p42 MAPK antibody (top) and re-probed with anti-non-phospho-specific ERK1 antibody (bottom). The inhibitory effect of SU5416 on fibrinogen (Fg)-induced tube formation by BCECs was also analyzed (B), (n=5, **P<0.05 )
Discussion
Fibrinogen has been considered as one of the factors that contribute to neovascularization in the areas of wound healing and tumor growth [4, 7]. An elevated serum fibrinogen level is associated with many diseases, including diabetes mellitus [14, 6], arthritis [24], cardiovascular risk factors [5] and AMD [37]. Fibrinogen deposit around the newly formed choroidal vessels of eyes with AMD has been reported [26, 35, 23].
In the present experiments, we clearly demonstrated that fibrinogen/fibrin enhanced in vitro angiogenesis formed by BCECs at concentrations of 0.5–1.5 mg/ml. Although many authors have shown that fibrinogen/fibrin induces in vitro [32, 33, 39] and in vivo [8] angiogenesis, this is the first report, to our knowledge, demonstrating the angiogenic effect of fibrinogen/fibrin using ocular vascular endothelial cells.
While the detailed mechanism of the angiogenic effect of fibrinogen is not fully clarified to date, angiogenesis is known to be regulated by angiogenic growth factors including b-FGF, VEGF and epidermal growth factor (EGF), as well as by the composition of ECM. Takei et al. [39] showed that the angiogenic effects of fibrin in vitro are mediated by b-FGF released from the endothelial cells in an autocrine manner. In the case of wound healing, it was also reported that platelet-derived growth factor (PDGF) released from platelets and macrophages might contribute to the proliferation of endothelial cells in the fibrin clot [30]. Accumulating studies, however, revealed VEGF as the most critical angiogenic growth factor because of its pattern of spatial and temporal expression that establishes its pivotal role both in physiological and pathological angiogenesis [9]. Our Northern and Western blot analyses demonstrated VEGF overexpression by BCECs under stimulation with fibrinogen, and the addition of anti-VEGF-neutralizing antibody or SU5416 in the media resulted in a strong suppression of fibrinogen-dependent tube formation by BCECs. These findings indicated that the angiogenic effect of fibrinogen on BCECs appeared to be mainly mediated through VEGF in an autocrine manner. Additionally, SU5416 suppressed fibrinogen-induced elongation of tube formation by BCECs as potently as VEGF-neutralizing antibody. SU5416 has an inhibitory effect on the autophosphorylation of receptor tyrosine kinase selectively for VEGFR-2 and is less potent for PDGF receptor β, but does not affect that of the bFGF receptor [2]. So this result is in agreement with the results obtained by other researchers using endothelial cells from non-ocular organ origin, demonstrating that the VEGF-dependent angiogenic property of endothelial cells is mainly mediated through VEGFR-2.
Hypoxia-inducible factor 1 (HIF-1) is a transcription factor involved in normal mammalian development and in the pathogenesis of several disease states. Activated HIF-1 induces the expression of genes involved in angiogenesis, erythropoiesis and glucose metabolism. We investigated the effect of fibrinogen on the nuclear accumulation of HIF-1 protein using BCECs. Although we could not exclude the involvement of transcription factors other than HIF-1, this is the first report demonstrating the possible role of HIF-1 mediating fibrinogen-induced VEGF gene expression.
The pathological features of CNV in AMD also have been studied to date. Considering the accumulating reports, diffuse thickening of Bruch’s membrane seems to cause the degeneration of RPE cells and the occurrence of chronic inflammation in the eyes with AMD. As a result of this inflammation, growth factors including VEGF are considered to be produced by RPE cells, macrophages, retinal glial cells and choroidal endothelial cells [1, 29, 34]. This process could be the mainstream of the development of CNV. Additionally, extravasated fibrinogen/fibrin because of the local hyperpermeability would further increase the expression of VEGF by the cells, including choroidal endothelial cells. VEGF secreted from choroidal endothelial cells would act in an autocrine manner, and these phenomena could result in a vicious circle of CNV formation.
In summary, we partly approached the elucidation of the molecular mechanisms underlying the association between extravasated fibrinogen and AMD. Our studies may allow a better understanding of the role of extravasated fibrinogen/fibrin in the development of CNV, at least in part, through the function of VEGF in an autocrine manner.
References
Aiello LP, Avery RL, Arrigg PG, et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Annie T, Fong T, Shawver LK, Sun L, Tang C, App H, et al (1999) SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59:99–106
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid gunidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159
Clark RAF, Dellapella P, Manseau E, et al (1982) Blood vessel fibrinogen increases in conjunction with endothelial cell proliferation and capillary ingrowth during wound healing. J Invest Dermatol 79:269–276
Cushman M, Yanez D, Psath BM, et al, for the Cardiovascular Health Study Investigators (1996) Association of fibrinogen and coagulation factors VII and VIII with cardiovascular risk factors in the elderly: the cardiovascular health study. Am J Epidemiol 143:665–676
De Silva SR, Shawe JE, Patel H, Cudworth AG (1979) Plasma fibrinogen in diabetes mellitus. Diabetes Metab 5:201–206
Dvorak HF, Dvorak AM, Manseau E, Wiberg L, Churchill WH (1979) Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis and fibroplasia in Guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage and infarction in line 1 tumor regression. J National Cancer Institute 62:1459–1472
Dvorak HF, Harvey VS, Estrella P, et al (1987) Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest 57:673–686
Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18:4–25
Folkman J, Haudenschild C (1980) Angiogenesis in vitro. Nature 288:551–556
Folkman J (1985) Tumor angiogenesis. Adv Cancer Res 43:175
Form DM, Pratt BM, Madri JA (1986) Endothelial cell proliferation during angiogenesis: in vitro modulation by basement membrane components. Lab Invest 55:521–530
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Ganda OP, Arkin CF (1992) Hyperfibrinogenemia: an important risk factor for vascular complications in diabetes. Diabetes Care 15:1245–1250
Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, Brandes RP, Kietzmann T, Busse R (2001) Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Cir Res 89:47–54
Gospodarowicz D, Gonzales R, Fuji D (1983) Are factors originating from serum, plasma or cultured cells involved in the growth promoting effect of the extracellular matrix produced by cultured bovine corneal endothelial cells? J Cell Physiol 114:191–202
Group MPS (1991) Argon laser photocoagulation for neovascular maculopathy: five year results from randomized clinical trials. Arch Ophthalmol 109:1109–1114
Hata Y, Nakagawa K, Ishibashi T, Inomata H, Ueno H, Sueishi K (1995) Hypoxia-induced expression of vascular endothelial growth factor by retinal glial cells promotes in vitro angiogenesis. Virchows Arch 426:479–486
Hata Y, Duh E, Zhang K, Robinson GS, Aiello LP (1998) Transcription factors Sp1 and Sp3 alter vascular endothelial growth factor receptor expression through a novel recognition sequence. J Biol Chem 273:19294–19303
Ingber DE, Folkman J (1989) How does extracellular matrix control capillary morphogenesis? Cell 58:803-805
Ishibashi T, Murata T, Sueishi K, et al (1993) Morphologic study of angiogenesis in vitro. In Vitro Cell Dev Biol 29A:91–93
Ishibashi T, Hata Y, Yoshikawa H, Nakagawa K, Sueishi k, Inomata H (1997) Expression of vascular endothelial growth factor in experimental choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 235:159–167
Lafaut BA, Bartz-Schmidt KU, Broecke CV, Aisenbrey S, De Laey JJ, Heimann K (2000) Clinicopathological correlation in exudative age related macular degeneration: histological differentiation between classic and occult choroidal neovascularisation. Br J Ophthalmol 84:239–243
Lanier JD, McCrary JA III, Justice J (1976) Autosomal recessive retinitis pigmentosa and Coats disease: a presumed familial incidence. Arch Ophthalmol 94:1737–1742
Leumg DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309
Lopez PF, Grossniklaus HE, Lambert HM, Aaberg TM, Capone A Jr, Sternberg P Jr, L’Hernault N (1991) Pathologic features of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol 112:647–656
Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR (1996) Transdifferentiated retinal pigment epithelial cells are immunoreactive for vascular endothelial growth factor in surgically excited age-related macular degeneration-related choroidal neovascular membranes. Invest Ophthalmol Vis Sci 37:855–868
Madri JA (1982) Endothelial cell-matrix interactions in hemostasis. Prog Hemostasis Thorombosis 3:1–24
Malecaze F, Clamens S, Simorre-Pinatel V, et al (1994) Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol 112:1476–1482
Martinet Y, Bitterman PB, Mornex J, et al. (1986) Activated human monocyte express the c-sis proto-oncogene and release a mediator showing PDGF-like activity. Nature 319:158–160
Montesano R, Vasalli JD, Baird A, Guillemin R, Orci L (1986) Basic fibroblast growth factor induces angiogenesis in vitro. Proc Natl Acad Sci USA 83:7297–7301
Nicosia RF, Ottinetti A (1990) Modulation of microvascular growth and morphogenesis by reconstituted basement membrane gel in three dimensional cultures of rat aorta: a comparative study of angiogenesis in matrigel, collagen, fibrin, and plasma clot. In Vitro Cell Dev Biol 29:119–128
Nicosia RF, Ottinetti A (1990) Growth of microvessels in serum-free matrix culture of rat aorta: a quantitative assay of angiogenesis in vitro. Lab Invest 63:115–122
Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH (1995) Vascular endothelial growth factor/ vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 92:905–909
Schaft TL, Mooy CM, Bruijn WC, Jong PTVM (1993) Early stages of age-related macular degeneration: an immunofluorescence and electron microscopy study. Br J Ophthalmol 77:657–661
Schor AM, SL Schor, S Kumar (1979) Importance of a collagen substratum for stimulation of capillary endothelial cell proliferation by tumour angiogenesis factor. Int J Cancer 24:225–234
Smith W, Mitchell P, Leeder SR, Wang JJ (1998) Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy. Arch Ophthalmol. 116:583–587
Suzuma I, Hata Y, Clermont A, et al (2001) Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes 50:444–454
Takei A, Tashiro Y, Nakashima Y, Sueishi K (1995) Effects of fibrin on the angiogenesis in vitro of bovine endothelial cells in collagen gel. In Vitro Cell Dev Biol 31:467–472
Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E (2001) Regulation of vascular endothelial growth factor expression by advanced glycation end products. J Biol Chem 276:43836–43841
Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E (2002) Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J Biol Chem 277:27975–27981
van Hinsbergh VW, Collen A, Koolwijk P (2001) Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci 936:426–437
Wenger RH (2000) Mammalian oxygen sensing, signalling and gene regulation. J Exp Biol 203:1253–1263
Young WC, IM Herman (1985) Extracellular matrix modulation of endothelial cell shape and motility following injury in vitro. J Cell Sci 73:19–32
Author information
Authors and Affiliations
Corresponding author
Additional information
The study was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan [grant-in-aid for scientific research (A) (2) no. 09307040 and grant-in-aid for exploratory research no. 11877300].
Rights and permissions
About this article
Cite this article
Shiose, S., Hata, Y., Noda, Y. et al. Fibrinogen stimulates in vitro angiogenesis by choroidal endothelial cells via autocrine VEGF. Graefe's Arch Clin Exp Ophthalmol 242, 777–783 (2004). https://doi.org/10.1007/s00417-004-0910-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-0910-2