Abstract
Background
Subretinal implants intend to replace photoreceptor function in patients suffering from degenerative retinal disease by topically applying electrical stimuli from the subretinal space. This study intended to prove the feasibility of a newly developed transchoroidal surgery and, furthermore, of a subretinal electrode array, which closely resembles envisioned human implants to electrically stimulate the visual system in rabbits.
Methods
Five rabbits (ten eyes) were implanted with a 4×2-electrode array via a transchoroidal access to the subretinal space. The electrodes were connected to an arbitrary stimulus generator to apply voltage pulses. Retinae were accessed by light microscopy after stimulation with various intensities.
Results
The stimulating foil could be introduced into the subretinal space in all eyes. In seven of ten eyes electrically evoked cortical potentials following subretinal electrical stimulation could be elicited. Threshold voltages ranged from less than 0.1 to 2.38 V with a corresponding threshold charge of approximately 1.0 nC per electrode or 10 µC/cm2. Histology revealed localized retinal damage over some of the electrodes succeeding stimulation strengths of 2 V and consistent damage over all electrodes succeeding voltages of 3 V.
Conclusions
The study demonstrates the feasibility of the transchoroidal surgical access to place subretinal implants in rabbit eyes and provides proof of successful cortical activation following subretinal electrical stimulation by an electrode array envisioned for human implantations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many degenerative retinal diseases, such as retinitis pigmentosa (RP) and age-related macular degeneration (ARMD), retinal cell death and re-organization ultimately lead to irreversible blindness. There is little debate that the majority of cell loss occurs in the outer nuclear layer (ONL) containing the photoreceptors (PRs) [19, 23, 30]. The inner nuclear layer (INL) and the ganglion cell layer (GCL) in comparison stay relatively intact preserving 20–40% of their neurons depending on the type and stage of the disease and on the eccentricity on the retina. From these results it seems reasonable to expect surviving cell populations in the inner retinal layers which could receive, transform, and transmit electrical signals from the prosthesis via the optic nerve to the brain.
Consistently many studies [2, 3, 4, 6, 7] have demonstrated the possibility to elicit phosphenes by electrical stimulation in healthy persons and in patients blinded by degenerative retinal disease. These studies ultimately raised the concept of a visual prosthesis designed to electrically stimulate the visual system with multi-electrode arrays thereby restoring pattern vision by topically correct excitation at various locations along the visual pathway.
Four main locations for electrical stimulation of the visual system have been proposed: (a) the visual cortex; (b) the optic nerve and the lateral geniculate nucleus (LGN); (c) the ganglion cell layer (GCL) via an epiretinal prosthesis; and (d) the retinal network via a subretinal prosthesis.
The visual cortex has been activated by variable numbers of electrodes in animals and also humans [3, 4, 8, 9, 10, 11, 24, 25, 29, 31]. The LGN has been accessed [40] as well as the optic nerve [36]. Most experiments with successful electrical stimulation of the visual system have been performed with epiretinal prostheses in animals and humans in acute and chronic experiments [13, 15, 16, 17, 18, 20, 21, 28, 38, 39]. Furthermore, studies have clearly demonstrated the principal feasibility of a subretinal prosthesis [5, 13, 14, 27, 32, 34, 43, 44, 45, 46].
One of the advantages of a subretinal prosthesis is the possibility to utilize the remaining retinal network to process the information initiated by the prosthesis. Furthermore, electrical multisite stimulation from the subretinal space (SRS) comes closest to physiological conditions in terms of stimulation site and would, in the ideal case, not require external cameras and image processing devices to compensate for off-site stimulation, as may be the case in epiretinal prosthesis where GC-fibers from far away in the visual field might be stimulated.
Chow et al. [5] have been able to evoke cortical responses following subretinal electrical stimulation in rabbits by placing a single large electrode under the retina which was connected to a photocell outside of the eye that was illuminated by a bright light flash; however, no studies have thus far examined the exact conditions which are necessary to successfully evoke visual perceptions from the SRS in vivo. To make use of the possible advantages of subretinal prostheses the stimulus characteristics have to be defined more clearly. This prompted us to determine voltage and charge, respectively, by using electrical stimulation with regularly arranged electrodes which more closely resemble future implants. The electrodes were stimulated in a controlled manner by an external stimulus generator to find thresholds of voltage and charge for successful cortical activation as proven by electrically evoked cortical potentials (EECPs) within a range that does not harm the cells stimulated.
Materials and methods
Animals
Five adult Chinchilla Bastard rabbits were included in the study (Charles River GmbH, Germany) with experiments performed on ten eyes. All animal experiments adhered to the “Principles of laboratory animal care” (NIH publication no. 85–23, revised 1985), the OPRR Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 1986), and the U.S. Animal Welfare Act, as amended, as well as the local commission for animal welfare.
Anesthesia
Anesthesia was achieved by intramuscular injection of ketamine hydrochloride 10% (Ketavet 10%) and Xylazine 2% (Rompun 2%) in a mixing ratio of 2:1. Initial dose was 50 mg ketamine hydrochloride per kilogram body weight.
Electrophysiological recording electrodes
One epidural stainless steel screw was placed as active electrode epidurally 4 mm anterior and 5 mm lateral on each side of lambda, and the reference electrode was placed epidurally on the midline 25 mm anterior to lambda. A subcutaneous needle electrode at the tip of the nose served as ground electrode.
Surgical technique: transchoroidal implantation of the subretinal foil strip
For implantation, a scleral flap (3×3 mm) was prepared about 6 mm away from the limbus in the upper lateral quadrant of the eye. After local drug-induced vasoconstriction with Suprarenin 1:1000 (Aventis Pharma, Bad Soden, Germany) the choroid was incised along the great vessels with a 27-G needle taking great care not to injure the underlying retina.
The stimulating foil strip was introduced along a previously introduced, custom-made slide rail to the desired position. Time for a single operation was approximately 1 h. After the surgery, the eye was immediately stimulated electrically followed by the same procedure in the other eye within one session before killing and enucleation for histology.
Electrophysiological recordings
For all recordings an Espion Console was used (Diagnosys LLC, Littleton, Mass.). The Pentium-based system provides five 28-bit DC coupled opto-isolated differential input channels. All data were stored on the hard disk for offline analysis. Bandpass filter settings were 0.03 Hz as low and 300 Hz as high cut-off frequency (except for rabbits I and II: 0–1000 Hz). Cortical activity was recorded in response to stimulation of the contralateral retina. For objective assessment of the response to subretinal electrical stimulation the area under the curve (AUC) was calculated for the time period in which a physiological response from the visual cortex was expected (25–200 ms after onset of stimulation). To adjust for the noise level the AUC of the response (25–200 ms) was divided by the AUC of periods without stimulation which yielded an AUC ratio for each stimulus condition. The AUC ratio provides an objective and quantifiable measure of response strength and is independent of the waveform. It is also an objective parameter in all recordings without any discernible waveform (i.e., sub-threshold stimulation). Similar techniques have extensively been used to evaluate ERG and cortical responses (e.g., [12, 13]).
For assessment of the retina and the visual pathway to control effects of surgery or electrical stimulation ganzfeld visual evoked potentials (VEPs) were recorded before surgery and after electrical stimulation of the retina.
Electrostimulation of the retina
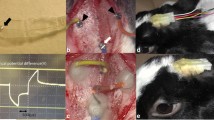
For subretinal stimulation an array of thin-film platinum electrodes on a flexible polyimide foil (manufacturer: Fraunhofer Institute for Biomedical Engineering, St. Ingbert, Germany; model RS8–50; [35]; Fig. 1) was used. The flexible foil strip is made of 50-µm thin polyimide with 8 substrate integrated, insulated golden connection lanes terminating in a 2×4 array at the end of the strip. Rectangular openings (100×100 µm2) in the insulation layer at the terminals of the lanes define the size of the stimulation electrodes. The gold electrodes are covered with a thin layer of platinum to enhance the safe charge injection capability. At the other end a plug is soldered to the contact pads to allow connection with the computer-controlled stimulator device (either STG 1008, Multi Channel Systems, Reutlingen, Germany, or function generator HP33120A, Hewlett-Packard, Boeblingen, Germany). Generally biphasic, anodic-first voltage pulses were applied between the subretinally implanted electrodes and a gold-ring contact lens electrode (ERGJet, Universo, La Chaux-de-Fonds, Switzerland) on the cornea serving as large-sized return electrode.
Flexible foil strip for acute subretinal electrical stimulation. a The polyimide foil strip (thickness 50 µm, length 53 mm) carries eight platinum electrodes on its tip (left side). Each electrode was connected individually through the plug on the right side to an external stimulus generator. The foil strip was used for acute stimulation up to 8 h in final experiments. The tip of the foil was placed in the subretinal space with electrodes facing the overlying retina, the plug outside of the eye. b The subretinal foil stimulator with electrode array and contact pads (dimensions in millimeters)
Measurement of charge transfer
The physiologically most common measure of strength of stimulation is “charge injected per pulse”, often standardized to the geometric area of the stimulation electrode. To measure the charge transfer through each electrode we estimated in three experiments (animals III, IV, V) the injected charge per voltage pulse and per electrode by measuring the current and subsequent off-line integration of the current transient in each electrode (Fig. 2).
Electrical stimulation with voltage impulses and estimation of injected charge. a The current (bold line, left scaling) resulting from a voltage pulse (top trace) is measured with a current-to-voltage converter connected between corneal return electrode and ground. By integration of the current trace, the injected charge is estimated and standardized to the electrode area (broken line, right scalingsKluwer Academic /Plenum Publishers, New York). b Peak current at the raising edge of the voltage pulse and maximum charge reached at the end of the pulse (pulse duration 500 µs) of 8 electrodes of a foil in animal V, plotted against the applied voltage
Monophasic voltage pulses (increasing amplitudes up to 3.4 V, pulse duration 500 µs) were applied against ground by a function generator (HP33120A, Hewlett Packard, Böblingen, Germany). The resulting current was measured by means of a current-to-voltage converter that was connected between the corneal return electrode and ground [34].
Preparation for histology
The posterior part of the enucleated eye was immersed in Karnovsky’s fixating solution (2% glutaraldehyde, 3% formaldehyde) overnight. Tissue blocs of retina, retinal pigment epithelium (RPE), choroid, and sclera were dissected representing the area of the implantation channel and the area overlying the stimulation electrodes. The specimens were rinsed in phosphate buffer, postfixed in 2% OsO4 for 2 h at 4°C, dehydrated in graded alcohol, and embedded in epoxy-resin (Epon 812, Science Services, Munich, Germany). Semi-thin sections were stained with Toluidine Blue O (Sigma, Taufkirchen, Germany) for light microscopy.
Results
Implantation of the foils
By using an ab-externo transchoroidal access, a stable subretinal implantation of the electrodes in the area of the visual streaks could be achieved in all ten eyes. Complications from choroidal bleeding did not occur in any case. The retina remained well attached at the area of the electrodes as shown by ophthalmoscopy. In two eyes retinal perforation occurred during and at the site of the incision of the choroid. In these cases, the protruding vitreous was pushed back by an additional epiretinal foil providing good access to the SRS. Thereafter, the subretinal implantation could be performed according to the procedure described above without hampering the subsequent investigations.
Visual and electrically evoked cortical potentials
The major positive peak of the VEP culminated usually at 90 ms implicit time with some inter-individual variation and did not show significant differences before and after electrical stimulation or surgery.
Cortical recordings following subretinal electrical stimulation of the right and left eye of rabbit III are given illustratively in Fig. 3a,b, respectively. The amplitudes of the major positive peak at the tested stimulation amplitudes are shown in Fig. 3c. At 0.1 V a clearly discernible waveform was detected with an amplitude growing up to 2 V stimulation strength. 0.1 V correspond to a charge transfer value of approximately 1.0 nC per electrode or 10 µC/cm2 (Fig. 2). The AUC ratios of rabbit III are shown in Fig. 3d. In the right eye a linear regression analysis was performed to calculate the intersection with the null-stimulus line. This intersection has been used to determine thresholds for electrical stimulation before (e.g. [13]). It is necessary to determine the threshold in order to be able to use the entire dynamic range which was at least 1.3 log units (0.1–2 V); however, to find a clearly detectable response we decided to use a higher AUC ratio of greater than 2.0 as a threshold which corresponded well with clearly discernible waveforms throughout our study.
Tracings of electrically evoked cortical potentials (EECPs) following subretinal electrical stimulation in rabbit III and evaluation of amplitudes and the area under the curve (AUC). a In the right eye (first operated eye) a graded response was recorded at 0.1 V (threshold) increasing in amplitude up to 2 V. b Even stimulation with 3 V did not lead to discernible cortical responses in the left eye. c Relationship of amplitude of EECP and stimulation strength (rabbit III OD; in OS no discernible waveforms were recordable). The amplitude of the cortical response increased with increasing stimulation strength, starting with 25 µV at 0.1 V and reaching 116 µV at 2 V. d Relationship of the AUC ratio and stimulation strength in rabbit III (OD and OS). For quantification of cortical responses the AUC ratio was calculated by dividing the AUC of the time where a response was expected (25–200 ms) by the AUC of the noise
The stimulation strengths at the point where the linear regression exceeded AUC ratio=2.0 for all experiments are given in Table 1. Thresholds ranged from 0.09 to 2.38 V, corresponding to approximately 1 nC per electrode or 10 µc/cm2 to 17 nC per electrode or 170 µC/cm2, respectively (Fig. 2; Table 1). Average stimulation threshold for the seven successful eyes was 0.94 V, corresponding to less than 5 nC per electrode or 50 µC/cm2. Three eyes did not show discernible waveforms at the highest voltage used (3 V) and were thus classified as non-successful. The amplitude of the cortical responses was in average 28±27 µV with an average implicit time of 73±19 ms.
Histology
Histology showed some abrasion of the PRs’ outer segments and partial disruption of the monolayered RPE in the area of the implantation channel. After repeated stimulation with more than 2 V, the retinal area adjacent to some of the electrodes showed local destruction starting in the outer retinal layers with vacuolization of the PRs’ outer segments (Fig. 4a). Stimulation strengths of more than 3 V consistently led to a destruction of all retinal layers (Fig. 4b).
Histological preparation (magnification ×40, Toluidine Blue staining) of a retina after extended stimulation with 2 V (a) and 3.4 V (b). a After repeated stimulation with 2 V, a local retinal lesion developed in the area above an electrode. The photoreceptors and their outer segments are partially destructed and vacuolization of the outer retinal layers is evident (arrow), whereas the inner retinal surface and the surrounding retina appear intact. b In contrast, 3.4 V-stimulation led to a destruction of all retinal layers. GCL ganglion cell layer, INL inner nuclear layer, ONL outer nuclear layer, RPE retinal pigment epithelium
Discussion
In all ten rabbit eyes electrode foils could be implanted into the SRS via the transchoroidal access. Even in eyes with an iatrogenic retinal break at the site of the choroidal incision the electrode could be implanted into the SRS by use of an additional guiding foil to keep back the vitreous. In the setting of the current acute experiments no long-term complications had to be considered; however, close attention will have to be paid to minimize and treat such an adverse event in human eyes. The position of the foils remained stable and the retina well attached during the complete duration of all experiments. Synthetic vitreous substitutes for acute apposition of the retina or for long-term vitreous substitution, which might influence retinal integrity, were not required. No previous detachment of the retina by a liquid bleb was necessary, ensuring the closest possible contact between retina and electrode array in acute experiments. This surgical technique may offer advantages over the classical, transvitreal three port-pars plana surgical technique by possibly avoiding many of the risks and late complications of an intraocular procedure such as endophthalmitis, proliferative vitreoretinopathy, etc. Certainly, more experience has to be gained in other animal models to be able to judge possible use of this method in humans. The surgical technique presented here may be part of the final, complex surgical procedure for long-term implantation of subretinal devices in humans, especially with external epi- or subscleral components which might be necessary for energy supply or larger electronic parts. The unchanged amplitudes and latencies of the VEP before and after surgery and stimulation indicate that the whole procedure did not harm the global retinal and visual pathway function.
Amplitudes of cortical responses were generally much smaller in response to topical electrical stimulation as compared with ganzfeld light stimulation (VEP). This can be attributed to the total stimulated area: with ganzfeld light stimuli approximately 1000 mm2 of retina are stimulated simultaneously, whereas the electrodes on the foil strip comprised an area of less approximately 0.5 mm2.
The dynamic range in our experiments for electrical stimulation of the retina ranged from 0.1 to 1.3 log units (0.09–2 V and 2.38–3 V, respectively; see Table 1). Possible reasons for the intra- and inter-individual difference in dynamic range include the different position of the electrodes on the retina, the different localization of cortical electrodes, differing cortical foldings of the rabbit brain, and differing susceptibility of retinal areas to electrical stimulation as well as saturation effects. We intentionally decided not to exceed 3 V because harm to the retina through physico-chemical effects was expected, thereby possibly reducing the calculated dynamic range. Some saturation effects can already be observed at 2 V (Fig. 3d). A linear regression fit was used nevertheless in order to calculate thresholds. The dynamic ranges of our experiments with electrical stimulation correspond well to reports of VEP V-log I functions [42] showing that subretinal electrical stimulation can make use of a similar dynamic range to produce phosphenes.
After repeated stimulation with more than 2 V the retina adjacent to some of the electrodes suffered from local destruction in the outer retinal layers with vacuolization of the PRs’ outer segments (Fig. 4a). Stimulation strengths of more than 3 V consistently led to a destruction of all retinal layers (Fig. 4b). Usage of the entire dynamic range has to be questioned again under this impression. Studies on different animal models will have to elucidate the upper threshold for long-term electrical stimulation of retinal tissue as it is intended in retinal prostheses in human clinical use. The choice to use a voltage source was driven by the fact that the final subretinal implant will contain thousands of stimulation electrodes with individually voltage-controlled output and because currently there is no realistic technological concept available for fabrication of microchip with low-power consumption for thousands of current sources with individually controlled current outputs.
The average voltage threshold in the present study was less than 0.94 V, corresponding to less than 5 nC per electrode or 50 µC/cm2 (Fig. 2; Table 1). This threshold charge density was approximately three magnitudes higher than the threshold in the study by Chow et al. [5] which provides the only other available measurements for in vivo subretinal electrical stimulation experiments. One possible reason is the extreme difference in size of the stimulation device in the SRS: in the present study the area covered by all 8 electrodes was 0.5 as compared with 36 mm2 in the study by Chow et al. [5]. A smaller implant as used in our study comes closer to clinically useful devices and previous results [5] therefore do not shed much light on implants in these terms. In consideration of the size of the stimulating devices used in this study we consider the results to be of higher clinical importance, although it may imply higher thresholds as measured by VEPs/EECPs. Additionally, we prefer small densely arranged electrodes to achieve optimal spatial resolution.
The threshold in epiretinal stimulation ranges from 3 to 40 mC/cm2 (Table 2). The average threshold charge density in our study of 50 µC/cm2 falls in the lower part of this range. Two of the cited studies were performed in humans without objective proofs of visual perception, where perceptual thresholds should be lower. It could be expected that the threshold in acute subretinal stimulation are higher than that of acute epiretinal stimulation because of the following reasons: firstly, some injury and subsequent edema of the retina is inevitable in subretinal surgery and is certainly connected with accumulation of some interstitial fluid in the SRS and between the electrodes and the retina. Topical edema of the retina was clearly found transiently with optical coherence tomography in vivo [37]. Small amounts of retinal edema will interfere with signal transduction [41]. Moreover, in vitro experiments have shown that even minute amounts of separation in the micrometer range from electrode to tissue will decrease signal transduction enormously. From this point of view, it seems reasonable to expect that the excitation threshold will be significantly lower in chronic implantation when the retina had enough time to recover from surgical trauma. Secondly, according to the “size principal” [1], which states that larger neurons can be more easily excited by electrical current, the smaller distant neurons of the retina should show a higher threshold than the larger retinal GCs. Contrary to this expectation, our results showed that the other way is the case, i.e., the threshold charge density in our study was in the lower part of the range which epiretinal implant experiments yielded. This fact could point to a higher excitability of the distal retina from the SRS.
The disadvantages of acute experiments, such as transient retinal edema, incomplete retinal attachment over the electrodes, and temporary disturbances of the retinal metabolic state, could be overcome by chronic implantation of devices. Different animal models, however, with holoangiotic retina which could stand the blockage of nutrients from the RPE better would have to be chosen. Cats and Yucatan micropigs have been examined by our group. It has been shown that these retinae tolerate a chronic implant without obvious damage [14, 22, 32]. In contrast to epiretinal implants, where the problem of permanent stability arises, experience in subretinal implantations has shown that the implant remains at the original position for years [14, 22, 32, 33, 43, 44, 45, 46, 47].
Conclusion
In conclusion, this study has proven the principal feasibility of eliciting cortical responses (EECPs) by subretinal electrical stimulation with electrode arrays. The novel transchoroidal approach has been highly successful in all experimental animals of this study in providing a way to the SRS aside of the classical, transvitreal access. Although many studies have been successfully performed by our consortium [13, 14, 26, 32, 33, 34, 44, 45, 46, 47], many questions, such as the shape of the phosphenes elicited by the implants, their interpretation by the cortex, andtheir color will have to be answered by volunteers who can report on their perceptions.
References
Baratta R, Ichie M, Hwang SK, Solomonow M (1989) Orderly stimulation of skeletal muscle motor units with tripolar nerve cuff electrode. IEEE Trans Biomed Eng 36:836–843
Brindley GS (1955) The site of electrical excitation of the human eye. J Physiol127:189–200
Brindley GS (1973) Sensory effects of electrical stimulation of the visual and para-visual cortex in man. In: Jung R (ed) Handbook of sensory physiology, vol 3. pp 583–594
Brindley GS, Lewin WS (1968) The sensations produced by electrical stimulation of the visual cortex. J Physiol Lond 196:479–493
Chow AY, Chow VY (1997) Subretinal electrical stimulation of the rabbit retina. Neurosci Lett 225:13–16
Crapper DR, Noell WK (1963) Retinal excitation and inhibition from direct electrical stimulation. J Neurophysiol 26:924–947
Dawson WW, Radtke ND (1977) The electrical stimulation of the retina by indwelling electrodes. Invest Ophthalmol Vis Sci 16:249–252
Dobelle WH (2000) Artificial vision for the blind by connecting a television camera to the visual cortex. ASAIO J 46:3–9
Dobelle WH, Mladejovsky MG, Evans JR, Roberts TS, Girvin JP (1976) “Braille” reading by a blind volunteer by visual cortex stimulation. Nature 259:111–112
Dobelle WH, Mladejovsky MG, Girvin JP (1974) Artificial vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science 183:440–444
Dobelle WH, Turkel J, Henderson DC, Evans JR (1979) Mapping the representation of the visual field by electrical stimulation of human visual cortex. Am J Ophthalmol 88:727–735
Drasdo N, Aldebasi YH, Chiti Z, Mortlock KE, Morgan JE, North RV (2001) The SCone PhNR and Pattern ERG in Primary Open Angle Glaucoma. Invest Ophthalmol Vis Sci 42:1266–1272
Eckhorn R, Stett A, Schanze T, Gekeler F, Schwahn H, Zrenner E, Wilms M, Eger M, Hesse L (2001) Physiological functional evaluation of retinal implants in animal models. Ophthalmologe 98:369–375 [in German]
Gekeler F, Schwahn HN, Stett A, Kohler K, Zrenner E (2001) Subretinal microphotodiodes to replace photoreceptor-function: a review of the current state. In: Doly M, Droy M-T, Christen Y (eds) Vision, sensations et environment, Irvinn, Paris, pp 77–95
Hesse L, Schanze T, Wilms M, Eger M (2000) Implantation of retina stimulation electrodes and recording of electrical stimulation responses in the visual cortex of the cat. Graefes Arch Clin Exp Ophthalmol 238:840–845
Humayun MS, de Juan EJ (1998) Artificial vision. Eye 12:605–607
Humayun MS, de Juan EJ, Dagnelie G, Greenberg RJ, Propst RH, Phillips DH (1996) Visual perception elicited by electrical stimulation of retina in blind humans. Arch Ophthalmol 114:40–46
Humayun MS, de Juan EJ, Weiland JD, Dagnelie G, Katona S, Greenberg R, Suzuki S (1999) Pattern electrical stimulation of the human retina. Vision Res 39:2569–2576
Humayun MS, Prince M, de Juan EJ, Barron Y, Moskowitz M, Klock IB, Milam AH (1999) Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci 40:143–148
Humayun MS, Propst RH, de Juan EJ, McCormick K, Hickingbotham D (1994) Bipolar surface electrical stimulation of the vertebrate retina. Arch Ophthalmol 112:110–116
Humayun MS, Weiland JD, de Juan EJ (1999) Electrical stimulation of the human retina. In: Hollyfield JG, Anderson RE, LaVail MM (eds) Retinal degenerative diseases and experimental therapy. Kluwer Academic /Plenum Publishers, New York, p 479
Kohler K, Hartmann JA, Werts D, Zrenner E (2001) Histological studies of retinal degeneration and biocompatibility of subretinal implants. Ophthalmologe 98:364–368
Milam AH, Li ZY, Fariss RN (1998) Histopathology of the human retina in retinitis pigmentosa. Prog Retin Eye Res 17:175–205
Normann RA, Maynard EM, Guillory KS, Warren DJ (1996) Cortical implants for the blind. IEEE Spectrum 33:54–59
Normann RA, Maynard EM, Rousche PJ, Warren DJ (1999) A neural interface for a cortical vision prosthesis. Vision Res 39:2577–2587
Pardue MT, Stubbs EB Jr, Perlman JI, Narfstrom K, Chow AY, Peachey NS (2001) Immunohistochemical studies of the retina following long-term implantation with subretinal microphotodiode arrays. Exp Eye Res 73:333–343
Peyman G, Chow AY, Liang C, Chow VY, Perlman JI, Peachey NS (1998) Subretinal semiconductor microphotodiode array. Ophthalmic Surg Lasers 29:234–241
Rizzo JF, Loewenstein J, Wyatt JL (1999) Development of an epiretinal electronic visual prosthesis: the Harvard Medical School–Massachusetts Institute of Technology Research Program. In: Hollyfield JG, Anderson RE, LaVail MM (eds) Retinal degenerative diseases and experimental therapy. Kluwer Academic /Plenum Publishers, New York, p 463
Ronner SF, Foote WE, Feldon SE (1980) Activation of single cells in cat visual cortex by electrical stimulation of the cortical surface. Exp Neurol 70:47–64
Santos A, Humayun MS, de Juan EJ, Greenburg RJ, Marsh MJ, Klock IB, Milam AH (1997) Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch Ophthalmol 115:511–515
Schmidt EM, Bak M, Hambrecht FT, Kufta CV, O’Rourke DK, Vallabhanath P (1996) Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain 119:507–522
Schwahn HN, Gekeler F, Kohler K, Kobuch K, Sachs HG, Schulmeyer F, Jakob W, Gabel VP, Zrenner E (2001) Studies on the feasibility of a subretinal visual prosthesis: data from Yucatan micropig and rabbit. Graefes Arch Clin Exp Ophthalmol 239:961–967
Schwahn HN, Gekeler F, Kohler K, Zrenner E (2001) Stimulation des visuellen Systems durch subretinale Elektrostimulation: Tierexperimentelle Daten zur Machbarkeit einer subretinalen Netzhautprothese. Nova Acta Leopoldina NF 84:209–221
Stett A, Barth W, Weiss S, Haemmerle H, Zrenner E (2000) Electrical multisite stimulation of the isolated chicken retina. Vision Res 40:1785–1795
Stieglitz T, Blau C, Beutel H, Keller R, Meyer JU (1997) Conception and development of flexible stimulator structures within a retinal implant system] Konzeption und Entwicklung von flexiblen Stimulatorstrukturen innerhalb eines Retina Implantat Systems. Biomed Tech Berl 42 (Suppl):458–459 [in German]
Veraart C, Raftopoulos C, Mortimer JT, Delbeke J, Pins D, Michaux G, Vanlierde A, Parrini S, Wanet-Defalque MC (1998) Visual sensations produced by optic nerve stimulation using an implanted self-sizing spiral cuff electrode. Brain Res 813:181–186
Volker M, Gekeler F, Shinoda K, Sachs H, Gmeiner H, Kohler K, Inhoffen W, Bartz-Schmidt KU, Zrenner E (2002) Implantation of Microphotodiode Arrays (MPDA) in cats: OCT and fluorescein angiography. Invest Ophthalmol Vis Sci (abstract)
Walter P, Heimann K (2000) Evoked cortical potentials after electrical stimulation of the inner retina in rabbits. Graefes Arch Clin Exp Ophthalmol 238:315–318
Walter P, Szurman P, Vobig M, Berk H, Ludtke-Handjery HC, Richter H, Mittermayer C, Heimann K, Sellhaus B (1999) Successful long-term implantation of electrically inactive epiretinal microelectrode arrays in rabbits. Retina 19:546–552
Yagi T, Ito Y, Kanda H, Tanaka S, Watanabe M, Uchikawa Y (1994) Hybrid retinal implant: fusion of engineering and neuroscience. IEEE 4:382–385
Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S (2001) Morphological and functional analyses of diabetic macular edema by optical coherence tomography and multifocal electroretinograms. Graefes Arch Clin Exp Ophthalmol 239:96–101
Zrenner E The spectral properties of the human visual Ssystem as revealed by visually evoked cortical potentials/VECP) and psychophysical investigations. In: Braitenberg V, Barlow HB, Bullock H, Florey E, Grüsser OJ, Peters A (eds) Neurophysiological aspects of color vision in primates. Springer, Berlin Heidelberg New York, pp 112–172
Zrenner E (2002) Will retinal implants restore vision? Science 295:1022–1025
Zrenner E, Gekeler F, Gabel VP, Graf HG, Graf M, Guenther E, Haemmerle H, Hoefflinger B, Kobuch K, Kohler K, Nisch W, Sachs H, Schlosshauer B, Schubert M, Schwahn H, Stelzle M, Stett A, Troeger B, Weiss S (2001) Subretinal microphotodiode array as replacement for degenerated photoreceptors?. Ophthalmologe 98:357–363
Zrenner E, Miliczek K-D, Gabel V-P, Graf HG, Guenther E, Haemmerle H, Hoefflinger B, Kohler K, Nisch W, Schubert M, Stett A, Weiss S (1997) The development of subretinal microphotodiodes for replacement of degenerated photoreceptors [see comments]. Ophthalmic Res 29:269–280
Zrenner E, Stett A, Weiss S, Aramant RB, Guenther E, Kohler K, Miliczek K-D, Seiler MJ, Haemmerle H (1999) Can subretinal microphotodiodes successfully replace degenerated photoreceptors? Vision Res 39:2555–2567
Zrenner E, Weiss S, Stett A, Brunner B, Gabel V-P, Graf M, Graf HG, Haemmerle H, Hoefflinger B, Kobuch K, Miliczek K-D, Nisch W, Sachs H, Stelzle M (1999) Are subretinal microphotodiodes suitable as a replacement for degenerated photoreceptor? In: Hollyfield JG, Anderson RE, LaVail MM (eds) Retinal degenerative diseases and experimental therapy. Kluwer Academic /Plenum Publishers, New York, pp 497–506
Acknowledgements
Support for this study was provided by the German Federal Ministry of Education and Research (BMBF; grant no. 01KP0008). We gratefully acknowledge the help of our project partners in this work (Institute for Microelectronics, Stuttgart; Institute for Physical Electronics, Stuttgart; Natural and Medical Sciences Institute, Reutlingen; Fraunhofer-Institute for Biomedical Techniques, St. Ingbert; all Germany). E. Eckert was extremely helpful in surgery and animal care. The Ewald+Karin Hochbaum-Stiftung generously supported our work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gekeler, F., Kobuch, K., Schwahn, H.N. et al. Subretinal electrical stimulation of the rabbit retina with acutely implanted electrode arrays. Graefe's Arch Clin Exp Ophthalmol 242, 587–596 (2004). https://doi.org/10.1007/s00417-004-0862-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-004-0862-6