Abstract
Up to 10% of people living to 80 years of age have one or more seizures; and many will not require anti-seizure medication (ASMs). In 85% of patients, the diagnosis comes from the history of the index event. One-third of patients with an apparent “first seizure” have previous events, changing their diagnosis to epilepsy. Targeted investigations are important for classification and risk prediction. Patients with a low risk of seizure recurrence are not usually offered ASM treatment. High-risk patients have multiple seizures, neurological deficits, intellectual disability and/or relevant abnormal investigations; and are offered ASMs. Individual factors modulate this decision-making. Future integrated technologies offer the game-changing potential for seizure monitoring and prediction, but are not yet robust, convenient or affordable. Therapeutic drug monitoring in patients taking ASMs may confirm ASM toxicity, or when non-adherence, malabsorption, or rapid metabolism are suspected causes of breakthrough seizures. They are less useful when these factors are intermittent or irregular. Current evidence does not favour routine monitoring of serum levels, as it neither reliably predicts control, relapse, or adverse effects. The decision to discontinue ASM should follow a full discussion with the patient of risks and benefits. Along with population risk factors for seizure recurrence, the patient’s lifestyle and preferences must be considered. ASM are usually discontinued in a slow step-wise fashion, one at a time, after at least two years of remission. Seizure recurrence risk plateaus only after 2 years following ASM discontinuation, and patients need access to specialist follow-up over that period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite their many shortcomings, anti-seizure medications (ASMs) remain the main therapy for seizure control. Decisions on their use are central to a clinician’s practise. This article discusses the evidence for starting, monitoring and stopping ASMs in adults, and approaches to the areas where evidence is lacking. The newer term ASMs replaces antiepileptic drugs (AEDs). This emphasises the unfortunate reality that treatment is usually of the symptoms rather than the underlying cause of epileptic seizures. Questions facing the patient and clinician about ASM use are summarised in Table 1.
Starting ASMs
Introduction
In general, ASMs are recommended if the patient has epilepsy, or an epileptic seizure with a significant recurrence risk. The first apparently simple point is whether the diagnosis is correct. Next questions are whether the seizures will recur without medication, the potential risks from another seizure, the risks of treatment and effectiveness of alternative treatments. Analysing these factors allows for evidence-based recommendations to inform patient decisions, but there are multiple lacunes in evidence.
Accuracy of diagnosis
25% of episodes of transient loss of consciousness presenting to secondary care are epileptic seizures, 50% are syncope and approximately 10% are dissociative seizures [1, 2]. With a life-time cumulative incidence of > 35% [3], most episodes of syncope do not reach medical attention. Accurate differentiation of an epileptic seizure from mimics occurs in about two-thirds of patients seen by non-specialists [4].
With no established biomarkers, epilepsy remains a clinical diagnosis in 85% of cases [1, 4] aided sometimes by an eyewitness account if available and more recently, a smartphone recording. In 94 consecutive patients, a hospital study found post event confusion with disorientation increased the likelihood of events being epileptic five-fold (p < 0.001) [5], and along with lateral tongue biting and cyanosis, are the most important discriminators [1, 5]. Preceding déjà vu or jamais vu, confirmed unresponsiveness, head or eye deviation to one side, and rhythmic limb shaking or dystonic posturing are strong seizure markers. Incontinence and injury do not discriminate between seizure, syncope, and dissociative events [1, 4, 5]. Myoclonic jerks are common in syncope and may be misinterpreted as a seizure [1].
Previous subtle seizures with retained consciousness such as brief aura or myoclonus are often overlooked [1, 6]. Nocturnal incontinence or tongue biting, early morning headache, and daytime fatigue alert clinicians to consider nocturnal seizures. These important features convert the diagnosis from “single seizure” to epilepsy in 1/3 of cases, based on a study in 208 outpatients [7].
Half of the cases of misdiagnosis are due to incomplete or misinterpreted history, with the remainder due to misinterpretation of investigations, particularly over-calling of findings on the electroencephalogram (EEG) such as non-specific slowing [8]. With expert interpretation, the diagnostic yield of EEG is 50% in people shown over time to have epilepsy. This yield increases by 11% with provocation (photic stimulation, hyperventilation, sleep deprivation) [9].
Risk of seizure recurrence
It is estimated that 5–10% of people reaching the age of 80 years will have a seizure, and many of these are isolated events (most commonly febrile convulsions aged < 2 years) not requiring treatment [10]. Previous formulations of epilepsy were simply two or more unprovoked seizures more than 24 h apart [11]. The 2014 definition emphasises recurrence risk, by including single unprovoked seizures and > 60% recurrence risk over the next 10 years, or a recognised epilepsy syndrome [12]. This allows the definitive diagnosis of epilepsy after a single event in some patients, usually resulting in recommendation of ASMs [1, 2]
Provoked seizures have a recurrence rate estimated at 3–10% [13]. They are most commonly due to alcohol withdrawal, drugs, acute metabolic disturbance, brain or systemic infection, all of which are also triggers for seizures in people with epilepsy. Provocations may be recurrent, such as hypoglycaemia, and may have secondary effects such as infarction or gliosis associated with head injury, increasing seizure risk. Provoking factors may also unmask pre-disposing factors, making expert evaluation essential [1].
The definition of a single seizure currently includes multiple seizures within a 24-h period, although some authors argue that these should be re-classified as epilepsy. In children, multiple seizures in one day carry twice the recurrence risk compared to a lone seizure, suggesting they should be treated as epilepsy [14]. In an adult series, patients with multiple seizures in the first 24 h had the same recurrence rate [15] but higher inpatient mortality in this group affects interpretation of long-term outcome. Some of the patients with multiple seizures in one day have status epilepticus. 16% of patients with a first episode status epilepticus patients die during their inpatient stay, while the immediate mortality from a lone seizure is low [16]. Of those surviving the first 30 days, the long-term standardised mortality rate for first seizure status epilepticus is double that for a brief single first seizure [17]. Given these factors, ASMs should be considered in patients with multiple seizures over 24 h.
Risk factors of an underlying neurological disorder including intellectual disability and/or relevant EEG and imaging abnormalities allow the estimation of recurrence risk [18]. Recurrence risk increases with multiple risk factors. Equivocal risk factors are photosensitivity, nocturnal seizures, family history, Todd’s paresis and previous febrile seizures [1, 7].
Recognising epilepsy syndromes allows a definitive diagnosis of epilepsy. For adult neurologists, the most common is juvenile myoclonic epilepsy, carrying a > 90% recurrence risk without medication. A tonic–clonic seizure, triggered frequently by alcohol or sleep and/or food deprivation, is the usual prompt for medical attention. These triggers may result in a spurious diagnosis of a provoked seizure and no offer of treatment. Specific questioning about preceding absences or myoclonic jerks (sometimes mistaken for early morning clumsiness), often starting months or years before, is vital in adolescents presenting with a major seizure [1, 7].
Severity of seizures
Treatment should also be considered for a severe first seizure, occurring with injury, if the person lives alone or if the first presentation is status epilepticus [1]. These judgement calls involve a close dialogue with the patient.
Risks of ASMs
Adverse events of ASMs are myriad, and interpretation complicated by the effects of epileptic seizures themselves, the underlying cause of the epilepsy, co-morbidities, and other medical treatments. Actual and perceived adverse effects are a major contributor to the 26–79% non-adherence rates reported with ASMs [19].
Alternative better treatments
While ASMs are currently the usual treatment for epilepsy, this may change in the future. Primary prevention has the potential to reduce late-onset epilepsy as well as strokes and dementia, and reduction in neuro-infections such as malaria and cysticercosis would have a major world-wide effect [2].
Increasing understanding of auto-immune encephalitides are shifting treatment paradigms for this group, as associated seizures as well as neuropsychiatric manifestations are responsive to immune treatments and may be resistant to ASMs [2]. As the spectrum of these diseases is characterised, early recognition offers the hope of targeted immunotherapy and prevention of chronic epilepsy for these patients. Developments in neurostimulation and gene therapy will also modulate the landscape of treatment options for drug-resistant patients [2].
Informed patient choice
Patient empowerment to make informed decisions depends on a careful risk benefit analysis and discussion of options [20]. This must include a discussion of the risks of avoiding ASMs, including Sudden Unexplained Death in EPilepsy (SUDEP) and other morbidities and mortalities of uncontrolled seizures [21].
Choosing the right ASM
Choosing the most suitable of the more than 25 ASMs, the primary therapy for epilepsy, depends upon various patient-specific factors, including age, sex, seizure type, co-morbidities, and co-medications. Long-term retention and efficacy rates differ between the variously prescribed drugs [22, 23], but overall approximately two-thirds of patients attain at least 12 months seizure freedom at the last evaluation when followed for an extended period [24]. These studies have led some to conclude that certain agents are superior to others, and that medications fail to induce seizure remission in many patients. However, such studies are generally performed targeting standard doses, albeit with some flexibility in later dose adjustments, and seizure recurrence constitutes therapeutic failure when taking the prescribed dose.
MONITORING ASMs
Long-term seizure freedom is affected by multiple factors, including pseudo-resistance—drug failure due to inappropriate drug or dose selection—and non-adherence [25, 26]. The former can be avoided by ensuring that appropriately titrated broad spectrum agents (such as valproate, levetiracetam, lamotrigine or topiramate) are chosen for genetic generalised epilepsies (GGE), or those with uncertain seizure classification [22, 23]. For focal epilepsy the potential choice of ASMs is wider [2, 22].
Non-adherence, with a reported range of 26–79%, is multifactorial and more difficult to tackle [19]. Clinicians rely largely on patient reports to monitor therapeutic response. Self-reported seizure diaries are widely used but their accuracy is limited [27]. Therapeutic drug monitoring is the most commonly available method of monitoring, and the potential benefits and limitations are discussed below.
Establishing therapeutic ranges
Breakthrough seizures in some individuals might be caused by a failure to achieve a therapeutic drug concentration in the brain. As the brain concentration of an ASM can be extrapolated from its serum level, some have advocated that serum levels be monitored so that doses may adjusted; ensuring that suitable brain levels are maintained presumably enhances the odds of attaining seizure control [25]. Therapeutic ranges are typically established during clinical trials, establishing both the level at which improvement in seizure frequency is noted and the levels at which adverse effects tend to appear. The lower limit is presumably that below which improvement is unlikely, and the upper limit denotes a level above which further improvement in seizure control is unlikely and tolerability is compromised.
Monitoring might further be justified due to individual variability in metabolism and elimination of drugs. Some people might require high doses to attain a particular level whereas others might achieve that same level with a fraction of the dose; this is determined by genetic and other factors that influence drug clearance. However, individuals also differ in their need for a medication. Relatively “low” levels might be adequate to prevent seizures in some people, while others may require much higher levels for similar effect. Sensitivity to side effects also differs from one person to the next, and what constitutes an easily tolerated dose and level for one patient might cause intolerable side effects in another. Moreover, therapeutic ranges are derived from investigational trials that usually recruit drug-resistant subject, who may have different needs and tolerances than new onset or more easily controlled individuals. Wide therapeutic ranges exist for many drugs (Table 2) [25] and questions remains as to the usefulness of therapeutic drug monitoring (TDM).
Evidence for TDM
Both individual studies and systematic reviews have attempted to determine the value of TDM. A recent systematic review [26] found 16 studies that met criteria for inclusion in an analysis. This included 4 randomised controlled trials, 1 meta-analysis, and 11 quasi-experimental (non-randomised) studies. Some quasi-experimental (QE) studies reported better results in patients who had TDM, but this was not found in higher quality randomised studies. While seven QE studies using historical controls reported seizure reduction after implementation of TDM, the results were statistically significant in only three. Three of the four randomised trials found no effect of TDM on seizure control; the remaining one showed a benefit but only assessed patients taking phenytoin. Conflicting results were found for adverse effects as well, with no consistent benefit noted for TDM. Among the many problems noted in studies, including varying definitions of outcome and assessment measures, failure of TDM might relate to its incorrect use. Some studies cited in the systematic review found no difference in serum concentrations between those dosed, based on TDM results and those whose doses were not altered. Moreover, a significant proportion of patients in the TDM arm, up to 40%, had sub-therapeutic levels. Lastly, it is estimated that 26–79% of patients have incomplete adherence to their medication regimens [19] and scheduled TDM might not detect intermittent non-adherence, which could account for relapse.
A recent single-centre randomised controlled trial also failed to find benefit for TDM [28]. In this trial, 151 patients were randomised to TDM or rescue TDM (use of serum levels only when therapy failure occurred). Outcome was assessed by retention rate, which was similar in the two arms. Those assigned to regular TDM had a one-year retention rate of 58% whereas those assigned to rescue TDM had a 53% one-year retention rate (p = 0.6). The proportion of patients requiring dose adjustment was similar in the two arms as well (55% vs. 53%).
Situations where TDM may be helpful
While large scale studies fail to provide convincing support for TDM, perhaps certain situations might be suitable, though lacking randomised controlled trial evidence. A serum level once a patient is well controlled to establish a therapeutic level for that individual might be useful if relapse later occurred, to assess whether the level has changed and perhaps explain the recurrence. A physician can certainly assess patient adherence by measuring serum levels. If impaired gastrointestinal tract absorption is suspected, TDM might help determine whether that is the case. Lastly, individuals who have genetic polymorphisms associated with slow or rapid metabolism, hepatic or renal co-morbidities, or special situations such as pregnancy might be more accurately dosed by use of TDM. Trough TDM for suspected toxicity can be useful, particularly when there are other potential confounding factors [25]. While these situations provide an inherently attractive rationale for monitoring serum levels, proof must be provided by studies at some point to justify the expense and inconvenience of TDM.
Therapeutic drug monitoring in pregnancy
Pregnancy is associated with alterations in drug clearance and protein binding. During pregnancy, phenytoin levels fall 55–61%, carbamazepine by 0–42%, lamotrigine by 66%, levetiracetam by 50%, and oxcarbazepine by approximately 50% [29]. TDM is commonly advocated by experts during pregnancy. However, a randomised controlled trial failed to show a beneficial effect of seizure control during pregnancy in a study of 263 women with epilepsy [30]. Women were randomised to either monthly serum level monitoring or management based on clinical features. Approximately, two-thirds of both monitored and unmonitored women remained seizure-free while pregnant, with no observed significant difference in relapse rate. Serum levels were significantly higher in umbilical cord blood of neonates born to women on TDM, suggesting that maternal serum levels were higher, though daily drug exposure was similar for women in the two groups. Hence, evidence fails to support a significant role for TDM during pregnancy, though clinical acumen and expertise may have compensated for the lack of knowledge of levels in patients.
Future developments
Despite numerous theoretical advantages to performing TDM in selected patients, there is insufficient evidence currently that large scale use in general or specific epilepsy populations affords benefit. TDM certainly provides useful information for some patients, for example, when non-adherence, malabsorption, or rapid metabolism are suspected causes of seizure recurrence. Further studies in special populations, or perhaps for specific drugs, might demonstrate utility. TDM is insensitive to intermittent non-adherence, a key problem [19]. Existing evidence does not favour routine use of monitoring of serum levels while managing the typical patient with epilepsy, as it neither reliably predicts control, relapse, or adverse effects.
Future therapeutic refinements in TDM, incorporating individualised pharmacogenomics might change the situation. Automated seizure monitoring devises are currently being investigated but are not in widespread use. They offer the possibilities of monitoring and predicting seizures. Areas of development include non-invasive and minimally invasive subscalp EEG electrodes and non-invasive wearable sensors. The latter measures are indirect and most sensitive to major seizures. All the technologies are challenged by dealing with the vast amount of data acquired and problems related to reliability, convenience, and cost [27]. Systems incorporating automated seizure monitoring and TDM may become cost effective in the future and adapted for everyday use, starting with application for tonic–clonic seizures in drug-resistant patients (Table 2).
Stopping ASMs
Risks and benefits of discontinuation
Epilepsy is resolved for those with an age-dependent epilepsy syndrome who are past the applicable age, or who have remained seizure free for 10 years and off ASM for at least 5 years. This period of time comes from epidemiological series showing that seizure-free patients after ASM withdrawal reached a plateau of seizure recurrence after 5 years [31].
The reported risk of seizure recurrence after ASM discontinuation ranges widely between 12 and 66% in various studies. Reinstitution of ASM was effective in 64–91% and seizure control was regained within one year in half of these patients, but in some it took 5–12 years [32]. It is important for each patient to evaluate the burden of seizure recurrence and active epilepsy. A Canadian survey of 713 adult patients found active epilepsy significantly impaired education, employment, sleep, usual activities, and working status; and increased stigma [33]. Driving is a major issue for many people, prohibited if epilepsy is active, and in some countries, during discontinuation of ASMs. On the other hand, taking ASMs comes at a cost. Considering the benefits of discontinuing them, there is no evidence that withdrawal of ASM in selected cases increases mortality. Benefits include reduced adverse effects and the stigma of ASMs, decreased pregnancy malformations in women not taking ASM and reduced economic costs of ASM [34, 35].
The risks and consequences of ASM withdrawal have been evaluated in a randomised, double-blind study in patients who had been seizure-free for at least 24 months. The seizure relapse rate was evaluated over a 12-month period in groups of ASM withdrawal (79 patients) and ASM continuation (81 patients) and in a subsequent open follow-up period (all patients off medication). Seizure relapse at 12 months occurred in 15% of the withdrawal group and 7% of the continuation group (p = 0.095). Cognitive outcomes based on a battery of 15 neuropsychological tests, found normal scores increased significantly from 11 to 28% post-withdrawal. Withdrawal had no significant effects of on quality of life [36]. Recurrence of seizures is most likely to happen in the first 6 months, but plateaus only after 2 years [37, 38].
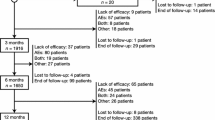
Risk factors for seizure recurrence after ASM withdrawal (Fig. 1).
A recent meta-analysis evaluated seizure recurrence and long-term outcomes after withdrawal of ASM in a total of 1769 pooled patients [31]. This highlighted predictors of seizure recurrence. These are epilepsy duration and seizure frequency before remission, seizure freedom for < 2 years underlying neurological disorder or intellectual disability, and epileptiform changes on EEG before withdrawal [31, 39]. A meta-analysis including a total of 2349 participants reported a relative risk ratio associated with an abnormal EEG of 1.37 (p < 0.0001) [40]. With regard to neurological disorders, the relative risk for remote symptomatic epilepsy was 1.55 compared to GGE, 1.66 for intellectual disability and 1.79 for associated motor deficit [41].
Other prognostic factors, less consistently associated with seizure recurrence after treatment discontinuation, are the number of ASM before discontinuation, focal seizures, gender and a family history [42]. Age at onset of epilepsy had a U-shaped relationship to seizure recurrence. There is an elevated risk at birth, falling by age 3–4 years rising again until age 10 years and plateauing until age 25 years. Subsequently, the risk rises after the age of 25 [42].
Predictors of successful withdrawal are presence of a self-limiting epilepsy syndrome, such as patients with benign epilepsy with centrotemporal spikes and in many with GGE [42]. The exception amongst GGE is juvenile myoclonic epilepsy—which is usually well controlled on medication, but recurs in > 90% if ASMs are withdrawn [1, 2, 38].
Discontinuation after epilepsy surgery
There is no consensus on post-surgical ASM withdrawal in adults as definitive evidence is lacking [38]. A mixed methods review found that 12–32% of post-surgical patients had seizure recurrence after ASM withdrawal. 45–92% of these regained seizure control, the wide range reflecting variability of risk. The most consistent risk factors were age > 30 years at the time of surgery, early ASM tapering, normal MRI, longer pre-operative duration of epilepsy, absence of hippocampal sclerosis, and the presence of interictal discharges on EEG after surgery. Persistent auras and seizure recurrence before ASM reduction are cited as risk factors, but simply reflect that the patient is not seizure free at the time of reduction [43].
Optimal discontinuation rates for ASMs
Although an important clinical question, there is only one randomised trial examining optimal ASM withdrawal rates in adults. This 2022 study in 48 (of an intended 350) patients found no difference in outcomes for slow withdrawal (160 days) or “rapid” withdrawal (60 days) over 12 months, with a low overall seizure recurrence rate (8%) [44]. A Cochrane review in 2019 found no adult studies, and two observational studies in children with insufficient data to make recommendations [45]. Consensus statements and common sense favour slow step-wise withdrawal, particularly for medications such as phenytoin with steep dose response curves where initial reductions should be small (25–50 mg) [46]. There is no definitive evidence regarding patients taking multiple ASMs, but it is sensible to discontinue one at a time, especially as multiple ASMs may be a marker for more severe epilepsy [38].
Guidelines recommendations on discontinuing ASMs
Most guidelines emphasise that the decision to discontinue ASM should be individualised and taken after a full discussion of risks and benefits between specialist and patients. In addition to risk factors for recurrence, this discussion should consider other factors such as: adverse effects of ASM, disease burden, cost, risk of physical injury, status epilepticus or death, quality of life, psychosocial factors and driving. If after consideration the patient wishes to stop ASMs, they should be slowly discontinued after a remission of at least two years. A patient discontinuing treatment should ideally be followed for no fewer than 2 years [38].
Conclusions
Up to 10% of people living to old age have one or more seizures; and many will be isolated events, not requiring ASMs. In 85% of patients, the diagnosis comes from a detailed history. One-third of patients with an apparent “first seizure” have had other minor seizures, changing their diagnosis to epilepsy. Although the diagnosis is clinical, targeted investigations aid classification and risk prediction. Low risk patients with an isolated seizure, no neurological deficits, normal magnetic resonance imaging and electroencephalography, have a 35% risk of recurrence at 5 years and are not usually offered treatment. High-risk patients have neurological deficit, pertinent magnetic resonance imaging and/or electroencephalography abnormalities, and a 70% risk of recurrence at 5 years; they are offered ASMs.
Future integrated technologies offer the potential for seizure monitoring and prediction, but there is a long way to go before they are convenient and affordable. Therapeutic drug monitoring of patients on ASMs provides useful information for some patients, for example, when non-adherence, malabsorption, or rapid metabolism are suspected causes of seizure recurrence; or when ASM toxicity is suspected. Further studies in special populations, or perhaps for specific drugs, might demonstrate utility. Existing evidence does not favour TDM while managing the typical patient with epilepsy, as it neither reliably predicts control, relapse, or adverse effects.
The decision to discontinue ASM should be individualised and taken after a full discussion with the patient of risks and benefits. If ASM are discontinued, this should be done slowly after at least 2 years of remission. Risk factors for recurrence after ASM withdrawal include underlying neurological disease and an abnormal EEG. Other factors for patients and clinicians to consider are the adverse effects of ASM, disease burden, cost, risk of physical injury, status epilepticus or death, quality of life, psychological factors and driving. A patient discontinuing treatment for seizure freedom should have specialist access for at least 2 years.
References
Angus-Leppan H (2014) First seizure in adults. BMJ 348:g2470
Harris L, Angus-Leppan H (2020) Epilepsy: diagnosis, classification and management. Medicine. https://doi.org/10.1016/j.mpmed.2020.05.001
Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, van Dijk N (2006) Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol 17(11):1172–1176. https://doi.org/10.1111/j.1540-8167.2006.00595.x
Angus-Leppan H (2008) Diagnosing epilepsy in neurology clinics: a prospective study. Seizure 17:431–436. https://doi.org/10.1016/j.seizure.2007.12.010
Hoefnagels W, Padberg G, Overweg J, Van der Velde E, Roos R (1991) Transient loss of consciousness: the value of the history for distinguishing seizure from syncope. J Neurol 238:39–43
Angus-Leppan H, Parsons LM (2008) Epilepsy: epidemiology, classification and natural history. Medicine (Baltimore) 36:571–578
Hauser WA, Rich SS, Annegers JF, Anderson VE (1990) Seizure recurrence after a 1st unprovoked seizure: an extended follow-up. Neurology 40:1163–1170
Smith D, Defalla BA, Chadwick DW (1999) The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. QJM 92(1):15–23. https://doi.org/10.1093/qjmed/92.1.15
Angus-Leppan H (2007) Seizures and adverse events during routine scalp electroencephalography: a clinical and EEG analysis of 1000 records. Clin Neurophysiol 118(1):22–30. https://doi.org/10.1016/j.clinph.2006.08.014
Hauser WA, Annegers JF, Rocca WA (1996) Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 71:576–586
Fisher RS, van Emde BW, Blume W, Elger C, Genton P, Lee P, Engel J Jr (2005) Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 46(4):470–472. https://doi.org/10.1111/j.0013-9580.2005.66104.x
Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr, Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshé SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S (2014) ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55(4):475–482
Pohlmann-Eden B, Beghi E, Camfield C, Camfield P (2006) The first seizure and its management in adults and children. BMJ 332(7537):339–342. https://doi.org/10.1136/bmj.332.7537.339
Camfield P, Camfield C (2000) Epilepsy can be diagnosed when the first two seizures occur on the same day. Epilepsia 41:1230–1233
Kho LK, Lawn ND, Dunne JW, Linto J (2006) First seizure presentation: do multiple seizures within 24 hours predict recurrence? Neurology 67:1047–1049
Rossetti A, Hurwitz S, Logroscino G, Bromfield E (2006) Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry 77:611–615
Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA (2002) Long-term mortality after a first episode of status epilepticus. Neurology 58:537–541
Kim LG, Johnson TL, Marson AG, Chadwick DW, MRC MESS Study group (2006) Prediction of risk of seizure recurrence after a single seizure and early epilepsy: further results from the MESS trial. Lancet Neurol 5(4):317–322. https://doi.org/10.1016/S1474-4422(06)70383-0 (Erratum in: Lancet Neurol. 2006 May;5(5):383)
Malek N, Heath CA, Greene J (2017) A review of medication adherence in people with epilepsy. Acta Neurol Scandinavica 135:507–515
Angus-Leppan H, Liu RSN (2018) Weighing the risks of valproate in women who could become pregnant. BMJ 18(361):k1596. https://doi.org/10.1136/bmj.k1596
Keddie S, Angus-Leppan H, Parker T, Toescu S, Nash A, Adewunmi O, Liu R (2016) Discussing sudden unexpected death in epilepsy: are we empowering our patients? A questionnaire survey. JRSM Open 7(9):2054270416654358. https://doi.org/10.1177/2054270416654358
Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G et al (2021) The SANAD II study of the effectiveness and cost-effectiveness of levetiracetam, zonisamide, or lamotrigine for newly diagnosed focal epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomized controlled trial. Lancet 397:1363–1374
Marson A, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW et al (2007) The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomized controlled trial. Lancet 369:1000–1015
Chen Z, Brody MJ, Liew D, Kwan P (2018) Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs. JAMA Neurol 75:279–286
Patsalos PN, Spencer EP, Berry DJ (2018) Therapeutic drug monitoring of antiepileptic drugs in epilepsy: a 2018 update. Ther Drug Monit 40:526–548
Al-Roubaie Z, Guadagno E, Ramanakumar AV, Khan AQ, Myers KA (2020) Clinical utility of therapeutic drug monitoring of antiepileptic drugs. Neurol Clin Pract 10:344–355
Brinkmann BH, Karoly PJ, Nurse ES, Dumanis SB, Nasseri M, Viana PF, Schulze-Bonhage A, Freestone DR, Worrell G, Richardson MP, Cook MJ (2021) Seizure diaries and forecasting with wearables: epilepsy monitoring outside the clinic. Front Neurol 13(12):690404. https://doi.org/10.3389/fneur.2021.690404
Aicua-Rapun I, Andre P, Rossetti AO, Rynvlin P, Hottinger A, Decosterd LA, Buclin T, Novy J (2020) Therapeutic drug monitoring of newer antiepileptic drugs: a randomized trial for dosage adjustment. Ann Neurol 87:22–29
Battino D, Tomson T, Bonizzoni E, Craig J, Lindhout D, Sabers A, Perucca E, Vajda F, EURAP Study Group (2013) Seizure control and treatment changes in pregnancy: observations from the EURAP epilepsy pregnancy registry. Epilepsia 54:1621–1627
Thangaratinam S, Marlin N, Newton S, Weckesser A, Bagary M, Greenhill L, Rikunenko R, Khan KS et al (2018) Antiepileptic drug monitoring in pregnancy (EMPiRE): a double-blind randomised trial on effectiveness and acceptability of monitoring strategies. Health Technol Assess 22:1–152
Lamberink HJ, Otte WM, Geerts AT, Pavlovic M, Ramos-Lizana J, Marson AG, Overweg J, Sauma L, Specchio LM, Tennison M, Cardoso TMO, Shinnar S, Schmidt D, Geleijns K, Braun KPJ (2017) Individualised prediction model of seizure recurrence and long-term outcomes after withdrawal of antiepileptic drugs in seizure-free patients: a systematic review and individual participant data meta-analysis. Lancet Neurol 16(7):523–531
Schmidt D, Löscher W (2005) Uncontrolled epilepsy following discontinuation of antiepileptic drugs in seizure-free patients: a review of current clinical experience. Acta Neurol Scand 111(5):291–300
Josephson CB, Patten SB, Bulloch A, Williams JVA, Lavorato D, Fiest KM, Secco M, Jette N (2017) The impact of seizures on epilepsy outcomes: a national, community-based survey. Epilepsia 58(5):764–771
Man SL, Petersen I, Thompson M, Nazareth I (2012) Antiepileptic drugs during pregnancy in primary care: a UK population based study. PLoS One 7(12):e52339. https://doi.org/10.1371/journal.pone.0052339
Medical Research Council Antiepileptic Drug Withdrawal Study Group (1993) Prognostic index for recurrence of seizures after remission of epilepsy. BMJ 306(6889):1374–1378
Tombini M, Assenza G, Quintiliani L, Ricci L, Lanzone J, Ulivi M, Di Lazzaro V (2020) Depressive symptoms and difficulties in emotion regulation in adult patients with epilepsy: association with quality of life and stigma. Epilepsy Behav 107:107073. https://doi.org/10.1016/j.yebeh.2020.107073 (Epub 2020 Apr 19)
Boshuisen K, Arzimanoglou A, Cross JH, Uiterwaal CS, Polster T, van Nieuwenhuizen O, Braun KP, TimeToStop study group (2012) Timing of antiepileptic drug withdrawal and long-term seizure outcome after paediatric epilepsy surgery (TimeToStop): a retrospective observational study. Lancet Neurol 11(9):784–791
National Institute for Health and Care Excellence. Epilepsies: diagnosis and management. https://www.nice.org.uk/guidance/ng217. Last accessed Aug 18 2022.
Strozzi I, Nolan SJ, Sperling MR, Wingerchuk DM, Sirven J (2015) Early versus late antiepileptic drug withdrawal for people with epilepsy in remission. Cochrane Database Syst Rev 2015(2):CD001902. https://doi.org/10.1002/14651858.CD001902.pub2
Tang L, Xiao Z (2017) Can electroencephalograms provide guidance for the withdrawal of antiepileptic drugs: a meta-analysis. Clin Neurophysiol 128(2):297–302
Berg AT, Shinnar S (1994) Relapse following discontinuation of antiepileptic drugs: a meta-analysis. Neurology 44(4):601–608
Beghi E, Giussani G, Grosso S, Iudice A, La Neve A, Pisani F, Specchio LM, Verrotti A, Capovilla G, Michelucci R, Zaccara G (2013) Withdrawal of antiepileptic drugs: guidelines of the Italian League Against Epilepsy. Epilepsia 54(Suppl 7):2–12
Tellez-Zenteno JF, Hernandez-Ronquillo L, Moien-Afshari F (2012) Discontinuation of antiepileptic drugs after successful surgery: who and when? Epileptic Disord 14(4):363–370. https://doi.org/10.1684/epd.2012.0538
Téllez-Zenteno JF, Dhar R, Hernandez-Ronquillo L, Wiebe S (2007) Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain 130(Pt 2):334–345
Ferlazzo E, Giussani G, Gasparini S, Bianchi E, Cianci V, Belcastro V, Cantello R, Strigaro G, Lazzari M, Bianchi A, Guadagni M, Pradella S, La Neve A, Francavilla T, Pilolli N, Banfi P, Turco F, Piccioli M, Polidori L, Anna Cantisani T, Papetti R, Cecconi M, Pupillo E, Davide Arippol E, Enia G, Neri S, Aguglia U, Beghi E (2022) Rapid versus slow withdrawal of antiepileptic monotherapy in two-year seizure-free adults patients with epilepsy (RASLOW) study: a pragmatic multicentre, prospective, randomized, controlled study. Neurol Sci. https://doi.org/10.1007/s10072-022-06121-9
Ayuga Loro F, Gisbert Tijeras E, Brigo F (2020) Rapid versus slow withdrawal of antiepileptic drugs. Cochrane Database Syst Rev 1(1):CD005003. https://doi.org/10.1002/14651858
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Angus-Leppan, H., Sperling, M.R. & Villanueva, V. Antiseizure medications (antiepileptic drugs) in adults: starting, monitoring and stopping. J Neurol 270, 573–581 (2023). https://doi.org/10.1007/s00415-022-11378-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11378-3