Abstract
Objectives
To investigate the association between cognitive function at baseline and the progression of motor disability in Parkinson’s disease (PD).
Methods
We consecutively enrolled 257 drug-naïve patients with early-stage PD (follow-up > 2 years) who underwent a detailed neuropsychological test at initial assessment. Factor analysis was conducted to yield four cognitive function factors and composite scores thereof: Factor 1 (visual memory/visuospatial), Factor 2 (verbal memory), Factor 3 (frontal/executive), and Factor 4 (attention/working memory/language). The global cognitive composite score of each patient was calculated based on these factors. Subsequently, we assessed the effect of baseline cognitive function on long-term motor outcomes, namely levodopa-induced dyskinesia (LID), wearing-off, freezing of gait (FOG), and rate of longitudinal increases in levodopa-equivalent dose (LED).
Results
Cox regression analysis demonstrated that higher Factor 3 (frontal/executive) composite scores (i.e., better cognitive performance) were associated with early development of LID [hazard ratio (HR), 1.507; p = 0.003], whereas higher Factor 1 (visual memory/visuospatial) composite scores (i.e., better cognitive performance) were associated with a lower risk for FOG (HR 0.683; p = 0.017). We noted that higher global cognitive composite scores were associated with a lower risk for developing FOG (HR 0.455; p = 0.045). The linear mixed model demonstrated that higher global cognitive composite scores and better cognitive performance in visual memory/visuospatial function were associated with slower longitudinal increases in LED.
Conclusions
These findings suggest that baseline cognitive profiles have prognostic implications on several motor aspects in patients with PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment frequently occurs in the early stages of Parkinson’s disease (PD) [1]. The presence of early cognitive decline signals a high risk for the subsequent progression to dementia, which adversely affects the morbidity and mortality in patients with PD [2, 3]. Moreover, ample evidence suggests that cognitive impairment at baseline is a predictor of accelerated motor decline and increasing disability [4,5,6]. Conversely, the presence of axial motor features is associated with a faster rate of cognitive decline and an increased risk of incident dementia [7,8,9]. The association between cognitive dysfunction and motor deficits in PD may indicate common or parallel underlying pathological processes [8].
However, there is little evidence of the impact of individual cognitive domain on the progression of motor disability in PD. Cognitive impairment is remarkably heterogeneous among patients with PD, and the neural basis of cognitive decline varies for each of the cognitive function domains [10]. Some research has suggested that executive dysfunction is secondary to dopamine deficiency in frontostriatal circuits [11], while deficits in memory and visuospatial function domains are related to cholinergic deficits in the posterior cortical areas: this is known as the dual syndrome hypothesis [12,13,14]. Deficits in attention also appear to have overlapping cholinergic correlates with memory dysfunction in PD [15]. Moreover, the progression of motor deficits in PD results from two distinct processes [6], namely gradual degeneration of nigrostriatal dopaminergic neurons (i.e., the progression of levodopa-responsive symptoms) and widespread degeneration of the extra-nigrostriatal system (i.e., the progression of levodopa-nonresponsive symptoms). The longitudinal effects of cognitive dysfunction on motor outcomes could vary depending on the affected cognitive domain and the clinical parameters measured according to the underlying pathogenic mechanisms. Accordingly, in this study, we aimed to explore the cognitive profile specifically relevant to several parameters for disease progression related to motor aspects in PD [16], including levodopa-induced dyskinesia (LID), wearing-off, freezing of gait (FOG), and longitudinal requirement of dopaminergic medications.
Methods
Subjects
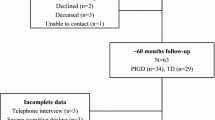
In the patient cohort enrolled in our previous study that assessed the effect of striatal dopamine depletion on cognition in patients with drug-naïve non-demented PD (n = 311) [17], we reviewed the follow-up data of patients treated with a PD medication for at least 2 years. PD was diagnosed according to the clinical diagnostic criteria proposed by the United Kingdom Parkinson’s Disease Society Brain Bank [18] and the presence of a PD drug response at follow-up. Patients (n = 9) who presented additional atypical features (e.g., ataxia, prominent autonomic dysfunction, vertical gaze limitation, early fall, cortical sensory loss, and early dementia) during follow-up were excluded. All patients showed decreased dopamine transporter (DAT) availability in the posterior putamen on [18F] N-(3-fluoropropyl)-2β-carbon ethoxy-3β-(4-iodophenyl) nortropane positron emission tomography (FP-CIT PET) scans. A detailed neuropsychological test [i.e., the Seoul Neuropsychological Screening Battery (SNSB) [19]] was administered to all patients as an initial diagnostic workup. Among all of the patients, 36 lost to follow-up within 2 years were excluded to reduce the likelihood of including patients with atypical Parkinsonism. Nine patients with illiteracy were also excluded from the study due to many restrictions on cognitive assessment. Finally, a total of 257 patients with drug-naïve non-demented PD (follow-up > 2 years) were enrolled in the present study. Parkinsonian motor deficit severity was assessed using the Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III). Depression was evaluated using the Beck Depression Inventory (BDI). The patients were classified into tremor-dominant, postural instability/gait difficulty (PIGD), and indeterminate motor subtypes based on UPDRS scores [20]. PD medication doses were calculated as levodopa-equivalent doses (LEDs) [21].
Following the diagnosis of PD, patients visited the outpatient clinic at 3-month intervals, and two movement disorders specialists (SYH and LPH) assessed the development of LID [22], wearing-off [23], or FOG [24] based on history-taking and clinical impression (see Supplementary Methods) and adjusted the dose of the PD medication for effective control of symptoms. This study was approved by the Yonsei University Severance Hospital Institutional Review Board. The need for informed consent was waived because of the retrospective nature of the study.
Neuropsychological assessment
All subjects underwent a comprehensive neuropsychological test battery (SNSB) widely used in Korea [19], which covers five cognitive domains: attention and working memory (forward and backward digit span task), language and related functions [the Korean version of the Boston Naming Test (K-BNT), calculation, and praxis], visuospatial function [the Rey Complex Figure Test (RCFT) copy and interlocking pentagon], verbal and visual memory [immediate recall/delayed recall/recognition test using the Seoul Verbal Learning Test (SVLT) for verbal memory; immediate recall/delayed recall/recognition test using the RCFT for visual memory], and frontal/executive function [contrasting program, go/no-go test, the Controlled Oral Word Association Test (COWAT), and the Stroop test]. Among scorable subtests of the SNSB, age and education-specific z-scores for the following 14 items were automatically calculated according to the established criteria when developing the SNSB: forward digit span task; backward digit span task; K-BNT; RCFT copy; the immediate recall, delayed recall, and recognition items using the SVLT for verbal memory; the immediate recall, delayed recall, and recognition items using the RCFT for visual memory; COWAT for animal names, COWAT for supermarket items, and COWAT for phonemic fluency; and the Stroop color reading test.
Assessment of dementia conversion during follow-up
After diagnosis of PD, patients or their caregivers were asked questions about daily functioning at every visit. Additionally, patients with PD underwent serial cognitive assessment using the Korean version of the Mini-Mental State Examination and Clock Drawing Test at an interval of 1 year (Level I tests) [25]. In cases of definite cognitive decline or evidence of impairments in daily life due to cognitive changes (Level I), a detailed neuropsychological battery (i.e., the SNSB [19]) was subsequently conducted to specify the pattern of cognitive deficits and diagnose PD with dementia (PDD) at Level II in most patients [25]. The diagnosis of PDD was made by achieving consensus between two neurologists and one neuropsychologist [26], according to the clinical diagnostic criteria proposed by the Movement Disorder Society Task Force [27, 28]. Patients who converted to PDD showed cognitive impairment in at least two of the core cognitive domains. All patients with PDD showed evidence of abnormalities in activities of daily living [29, 30].
Quantitative analyses of 18F-FP-CIT PET images
Image acquisition and processing were performed according to previously described methodology (Supplementary Methods) [17]. The volumes of interest (VOI) for the bilateral posterior putamen and one occipital VOI [i.e., calcarine fissure and surrounding cortex (V1) [31]] were drawn on the 18F-FP-CIT template using MRIcro version 1.37 (Chris Rorden, Columbia, SC). DAT availability in the posterior putamen was estimated using the specific/nonspecific binding ratio as a surrogate, which was defined as follows: (mean standardized uptake value of the posterior putamen VOIs—mean standardized uptake value of the occipital VOI)/(mean standardized uptake value of the occipital VOI).
Statistical analysis
Factor analysis was conducted based on the 14 tests derived from the SNSB [19] to reduce the redundancy of neuropsychological subtests and to determine the cognitive composite cores using the principal components method of factor extraction and the Varimax method of rotation [26]. The factor analysis yielded four cognitive function factors with eigenvalues > 1.0, which accounted for 63.8% of the variance in the cognitive performance of the subjects [32]. The component score coefficients were used to calculate the composite scores of the four cognitive function factors of each subject (Supplementary Table 1). Additionally, using the four cognitive function factors in an integrative formula, we calculated the global cognitive composite score of each patient with PD.
Subsequently, the effects of the composite scores of the four factors on the development of LID, wearing-off, and FOG were assessed using Cox regression analysis. Survival duration was defined as the time from treatment initiation to the date of the clinic visit when LID or wearing-off was first observed or reported (for patients with LID or wearing-off) or the time from parkinsonian symptom onset to the date of the clinic visit when FOG was first observed or reported (for patients with FOG) or the last clinic visit (for patients without these events). Cox regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for the development of LID or wearing-off according to neuropsychological profiles (i.e., composite scores of the four cognitive function factors) while adjusting for age, sex, DAT availability in the posterior putamen, BDI scores, and LED, which are known to be major risk factors for these events. The effect of the composite scores of the four cognitive function factors on the development of FOG was also assessed using Cox regression analysis while adjusting for age, sex, DAT availability in the posterior putamen, BDI scores, motor subtype (PIGD or not [33]), and LED. A log-minus-log plot and a time-dependent covariate analysis revealed that the assumption of proportionality was reasonable. There was no significant correlation among the composite scores of the cognitive function factors, and all these factors were included as predictor variables without concerns of multi-collinearity.
A linear mixed model was used to assess the longitudinal changes in LED over time, assuming that slopes were fixed and intercepts varied across subjects (i.e., a random intercepts model). Ten fixed effects were included in the model: nine were between-subject effects (age, sex, DAT availability in the posterior putamen, UPDRS-III scores, BDI scores, and composite scores of the four cognitive function factors) and one was a within-subject effect (time). The subject factor was considered a random effect. The effects of baseline cognitive function on the changes in LED over time were tested with (time × composite score of cognitive function factor) interaction terms. A significant interaction between time and cognitive composite scores indicates that the rate of longitudinal increases in LED would be affected by the baseline cognitive composite scores, while a negative value for the effect of interaction terms would imply that the higher the composite score, the slower the LED increases. Additionally, we evaluated the effects of global cognitive composite scores on the development of LID, wearing-off, or FOG and longitudinal increases in LED using the same statistical models. The statistical analyses were performed using SPSS software (version 23.0; IBM Corp., Armonk, NY, USA), and results with a two-tailed p < 0.05 were considered statistically significant.
Results
Demographic characteristics of the study participants
The baseline demographic characteristics of the patients with PD are listed in Table 1. The mean age at PD symptom onset was 65.94 ± 8.50 years, and the mean disease duration of PD (i.e., time from symptom onset to diagnosis) was 18.04 ± 15.98 months. The mean UPDRS-III score at baseline (i.e., drug-naïve status) was 22.50 ± 9.19. Approximately 40% of the patients had a PIGD clinical phenotype.
Factor analysis for cognitive composite scores
Table 2 shows the factor loadings of the 14 scorable cognitive subtests for each factor. The four cognitive function factors were consequently named according to the cognitive subtests that constituted each factor with a heavy factor loading: Factor 1 (visual memory/visuospatial), Factor 2 (verbal memory), Factor 3 (frontal/executive), and Factor 4 (attention/working memory/language).
Composite scores of the four cognitive function factors
The composite score of each cognitive function factor was calculated as the sum of the component score coefficient × standardized score for the neuropsychological subtest (see Supplementary Results). The global cognitive composite score was also calculated using the eigenvalues of the four cognitive function factors as follows: global cognitive composite score = (4.685 × Factor 1 + 1.637 × Factor 2 + 1.443 × Factor 3 + 1.167 × Factor 4)/14.
Clinical relevance of the cognitive composite scores in predicting the risk for dementia
To investigate the clinical relevance of cognitive composite scores for cognitive prognosis, Cox regression analysis was performed to assess the risk of dementia conversion according to the global cognitive composite scores after adjustment for age, sex, DAT availability in the posterior putamen, and years of education. During the follow-up period [mean ± standard deviation, 5.56 ± 1.82 years; median (minimum, maximum), 5.17 (2.19, 9.99) years], 45 (17.5%) patients developed PDD, and Cox regression analysis demonstrated that lower global cognitive composite scores were associated with a higher risk for PDD conversion [HR 0.151, 95% CI (0.063–0.359), p < 0.001; Supplementary Table 2].
Effect of cognitive function at baseline on long-term motor outcomes
Development of LID
During the follow-up period, LID developed in 72 (28.0%) of 257 patients with PD. Cox regression analysis demonstrated that higher Factor 3 composite scores (i.e., better cognitive performance on frontal/executive function) were associated with the early development of LID [HR 1.507, 95% CI (1.150–1.976), p = 0.003; Table 3], while composite scores for the other factors and global cognitive composite scores were not associated with the risk for developing LID (Tables 3 and 4).
Development of wearing-off
During the follow-up period, wearing-off developed in 46 (17.9%) of the patients with PD. Cox regression analysis demonstrated that composite scores for each cognitive function factor or global cognitive composite scores were not associated with the risk for developing wearing-off (Tables 3 and 4).
Development of FOG
During the follow-up period, FOG developed in 54 (21.0%) of the patients with PD. Cox regression analysis demonstrated that lower Factor 1 composite scores (i.e., poorer cognitive performance on visual memory/visuospatial function) were associated with a higher risk for FOG [HR 0.683, 95% CI (0.500–0.933), p = 0.017; Table 3]. Moreover, lower global cognitive composite scores were associated with a higher risk for FOG [HR 0.455, 95% CI (0.211–0.982), p = 0.045; Table 4].
Rate of longitudinal increases in doses of dopaminergic medications
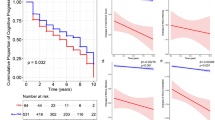
The linear mixed model demonstrated significant interactions between Factor 1 composite scores or global cognitive composite scores and time (p = 0.044 and 0.015, respectively), suggesting that higher Factor 1 composite scores and global cognitive composite scores were associated with slower longitudinal increases in LED (Table 5).
Discussion
The present study investigated the association between cognitive function at baseline and long-term motor outcomes in patients with early-stage PD. Factor analysis using the principal components method was conducted to determine cognitive composite scores. The main findings were as follows. (1) The factor analysis yielded four cognitive function factors (Factor 1, visual memory/visuospatial; Factor 2, verbal memory; Factor 3, frontal/executive; and Factor 4, attention/working memory/language) and global cognitive composite scores. In terms of cognitive prognosis, lower global cognitive composite scores were associated with a higher risk of conversion to dementia. (2) Higher Factor 3 composite scores were associated with the early development of LID, whereas the composite scores for each cognitive function factor or global cognitive composite scores were not associated with the risk for developing wearing-off. (3) Lower Factor 1 composite scores and global cognitive composite scores were associated with a higher risk for developing FOG. (4) Lower Factor 1 composite scores and global cognitive composite scores were associated with a more rapid rate of longitudinal increases in LED (see Fig. 1). These findings suggest that baseline cognitive profiles can predict prognosis in terms of motor aspects in patients with newly diagnosed PD.
There is increasing clinical evidence to support the potential associations between cognitive dysfunction and motor disability in patients with PD. Several prognostic models have consistently reported that the presence of cognitive decline is an important predictor of rapid motor decline [4,5,6]. Conversely, the motor subtypes of PD are also associated with a risk for incident dementia, and the PIGD phenotype is more relevant to rapid cognitive decline than the tremor-dominant phenotype [7,8,9]. Moreover, educational attainment, one of the most representative proxies of cognitive reserve, appears to be associated with baseline motor function and motor outcomes in patients with PD [34,35,36,37]. A possible explanation for this relationship is that several motor performance measures, especially gait, depend on the level of cognitive function [38]. Alternatively, a more reasonable explanation is that both cognitive and motor function are affected by shared or parallel pathological processes related to PD [8, 39, 40]. Anatomical and physiological evidence suggests that some cognitive and motor processes are facilitated by parallel loops that link the frontal cortex to the basal ganglia and thalamus [41]. In contrast, the close temporal relationship between motor and cognitive symptoms with a distinct neural basis (e.g., the transition from tremor-dominant to PIGD phenotype and accelerated cognitive decline [8]) suggests the coincidence of different neurodegenerative processes that develop in parallel.
The underlying pathophysiology of cognitive decline and motor disability in PD is highly variable [42]. Therefore, to better understand the association between cognitive and motor function, each of the cognitive domains and motor outcomes should be separately assessed. In this study, we explored the cognitive function factor predominantly associated with the following four clinical parameters that reflect the progression of motor disability in several aspects [16]. First, a higher level of cognitive performance in the frontal/executive function domain was associated with an increased risk for developing LID. A few studies have reported the contribution of frontal areas to the development of LID, showing the overactivation of the frontal motor areas [43, 44] and increased cortical thickness of the inferior frontal gyrus [45, 46] in patients with PD with LID. Notably, thickening in the prefrontal cortex may reflect neuroplastic changes induced by using the executive circuitry to suppress involuntary movements over several years [45,46,47]. Furthermore, in light of our findings, we suspect that increased prefrontal volume with better frontal/executive function may be a characteristic of patients with PD who are vulnerable to the development of LID. In other words, a thick prefrontal cortex would have profuse interconnections with the motor cortex and basal ganglia, which consequently provide a higher potential for neural plasticity, thereby leading to LID. We recently reported that patients with PD who experienced LID within 5 years of levodopa administration exhibited a more rapid progression of frontal/executive dysfunction than those without LID [48], suggesting the parallel development of aberrant corticostriatal plasticity in the motor and cognitive loops [41]. Altogether, the potential for plastic changes in the frontal areas would be closely associated with the development of LID and frontal/executive dysfunction.
Second, baseline cognitive function was not associated with the risk of developing wearing-off. Wearing-off is generally presumed to share the same pathogenic mechanisms as LID. However, several lines of evidence have suggested that the underlying mechanisms for wearing-off and LID may be different. The two motor complications may not necessarily occur in the same individual, although the onset of either LID or wearing-off commonly signals the risk of appearance of the other. Moreover, wearing-off may be viewed as more presynaptic than postsynaptic [49, 50], whereas LID appears to be a postsynaptic phenomenon considering that presynaptic dopamine depletion is the primary predisposing factor for LID induction but not a critical determinant [49, 51]. Therefore, no association between wearing-off and cognitive subsets may imply that the contribution of postsynaptic influence on the development of motor complications may differ between LID and wearing-off.
Third, poor cognitive performance in the visual memory/visuospatial function domain and global cognitive dysfunction were associated with a higher risk for developing FOG. Previous literature has highlighted some specific cognitive domains related to FOG in PD, namely frontal/executive [52,53,54], attention [55, 56], and visuospatial function [54, 57,58,59], in addition to global cognitive dysfunction [60]. In particular, patients with PD rely heavily on visual information, during the generation of motor plans [61] and the control of balance and locomotion [62]. Thus, baseline visual memory/visuospatial dysfunction may play a critical role in the development of FOG in this study. The lack of an association between baseline frontal/executive or attention function and the development of FOG, which appears to be inconsistent with previous studies [52,53,54,55,56], may be because of the differences in the study design, study population, and neuropsychological assessment.
Fourth, patients with lower composite scores in visual memory/visual function factor and lower global cognitive composite scores received higher doses of dopaminergic medications throughout the follow-up period. Usually, the required LED for effective symptom control increases as the disease progresses, and it appears to be indirectly associated with parkinsonian disability [63]. Our finding is in line with that of previous studies showing that visuospatial dysfunction significantly affects disability in patients with PD through its influence on cognition and locomotion [58, 64]. However, the result must be interpreted with caution as the longitudinal changes in LED may be affected by some confounding factors, including adverse effects of PD medications and the development of LID.
Our study had some limitations. First, the development of LID, wearing-off, and FOG was determined based on history-taking and neurological examination at every visit rather than quantitative assessment. Thus, the detection of mild motor features may be delayed in some cases. Additionally, direct measures of motor deficit, such as the UPDRS-III score, were not collected in a longitudinal manner. Second, factor analysis-derived cognitive composite scores can differ according to the study population, and additional analyses on an external dataset would be needed to replicate our findings. Moreover, all cognitive domains were not equally represented in the factor analysis. For example, six tests related to memory function domain were used for the factor analysis, whereas only one test related to language function was used in this study. However, none of the tests currently used for the factor analysis were included unnecessarily to provide accurate information about the nature of each cognitive domain. Third, because cognitive dysfunction and motor deficits seem to affect each other in PD [4,5,6,7,8,9], the impact of individual cognitive function factors on long-term motor outcomes may be altered in patients who experienced cognitive decline during the follow-up period. When we additionally estimated the risk for developing PDD in each cognitive domain using the Cox regression model, better performance in all cognitive function factors except Factor 4 was associated with a lower risk of PDD (Factor 1, HR = 0.641, p = 0.007; Factor 2, HR = 0.681, p = 0.014; Factor 3, HR = 0.413, p < 0.001). Therefore, the effects of visual memory/visuospatial dysfunction on the development of FOG and increases in LED would not be particularly mediated by the occurrence of PDD.
In conclusion, the present study suggests that baseline cognitive profiles may be used as a predictor of motor complications or motor disability in patients with PD. Given that coexisting cognitive dysfunction and motor impairment increase the risk of institutionalization [66], early identification of individuals at a high risk of rapid disease progression is necessary to implement appropriate therapeutic strategies.
References
Muslimovic D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65:1239–1245
Pedersen KF, Larsen JP, Tysnes OB, Alves G (2013) Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 70:580–586
Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G (2010) What predicts mortality in Parkinson disease? A prospective population-based long-term study. Neurology 75:1270–1276
Marras C, Rochon P, Lang AE (2002) Predicting motor decline and disability in Parkinson disease: a systematic review. Arch Neurol 59:1724–1728
Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP (2005) Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology 65:1436–1441
Velseboer DC, Broeders M, Post B, van Geloven N, Speelman JD, Schmand B, de Haan RJ, de Bie RM (2013) Prognostic factors of motor impairment, disability, and quality of life in newly diagnosed PD. Neurology 80:627–633
Burn DJ, Rowan EN, Allan LM, Molloy S, O’Brien JT, McKeith IG (2006) Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 77:585–589
Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D (2006) Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord 21:1123–1130
Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, Auinger P, Chou KL, Growdon JC (2009) Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology 73:1469–1477
Ray NJ, Strafella AP (2012) The neurobiology and neural circuitry of cognitive changes in Parkinson’s disease revealed by functional neuroimaging. Mov Disord 27:1484–1492
McKinlay A, Grace RC, Dalrymple-Alford JC, Roger D (2010) Characteristics of executive function impairment in Parkinson’s disease patients without dementia. J Int Neuropsychol Soc 16:268–277
Pillon B, Deweer B, Agid Y, Dubois B (1993) Explicit memory in Alzheimer’s, Huntington’s, and Parkinson’s diseases. Arch Neurol 50:374–379
Aarsland D, Litvan I, Salmon D, Galasko D, Wentzel-Larsen T, Larsen JP (2003) Performance on the dementia rating scale in Parkinson’s disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 74:1215–1220
Williams-Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA (2009) The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain 132:2958–2969
van der Zee S, Müller M, Kanel P, van Laar T, Bohnen NI (2020) Cholinergic denervation patterns across cognitive domains in Parkinson’s disease. Mov Disord 36:642–650
Coelho M, Ferreira JJ (2012) Late-stage Parkinson disease. Nat Rev Neurol 8:435–442
Chung SJ, Yoo HS, Oh JS, Kim JS, Ye BS, Sohn YH, Lee PH (2018) Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Parkinsonism Relat Disord 51:43–48
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Kang Y (2012) Seoul Neuropsychological Screening Battery (SNSB-II). Human Brain Research and Consulting Co., Seoul
Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 28:668–670
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Chung SJ, Yoo HS, Lee HS, Oh JS, Kim JS, Sohn YH, Lee PH (2018) The pattern of striatal dopamine depletion as a prognostic marker in de novo Parkinson disease. Clin Nucl Med 43:787–792
Chung SJ, Lee Y, Oh JS, Kim JS, Lee PH, Sohn YH (2018) Putaminal dopamine depletion in de novo Parkinson’s disease predicts future development of wearing-off. Parkinsonism Relat Disord 53:96–100
Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N (2003) Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol 10:391–398
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Chung SJ, Lee HS, Kim HR, Yoo HS, Lee YH, Jung JH, Baik K, Ye BS, Sohn YH, Lee PH (2020) Factor analysis-derived cognitive profile predicting early dementia conversion in PD. Neurology 95:e1650–e1659
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Yoo HS, Chung SJ, Lee PH, Sohn YH, Kang SY (2019) The influence of body mass index at diagnosis on cognitive decline in Parkinson’s disease. J Clin Neurol 15:517–526
Kang SJ (2002) The reliability and validity of the Korean instrumental activities of daily living (K-IADL). J Korean Neurol Assoc 20:8–14
Ku HM, Kim JH, Kwon EJ, Kim SH, Lee HS, Ko HJ, Jo S, Kim DK (2004) A study on the reliability and validity of seoul-instrumental activities of daily living (S-IADL). J Korean Neuropsychiatr Assoc 43:189–199
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Hair JF, Black WC, Babin BJ, Anderson RE (2013) Multivariate data analysis. Pearson Education Limited
Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G (2015) A 12-year population-based study of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 21:254–258
Sunwoo MK, Hong JY, Lee JJ, Lee PH, Sohn YH (2016) Does education modify motor compensation in Parkinson’s disease? J Neurol Sci 362:118–120
Kotagal V, Bohnen NI, Muller ML, Koeppe RA, Frey KA, Langa KM, Albin RL (2015) Educational attainment and motor burden in Parkinson’s disease. Mov Disord 30:1143–1147
Blume J, Rothenfusser E, Schlaier J, Bogdahn U, Lange M (2017) Educational attainment and motor burden in advanced Parkinson’s disease—the emerging role of education in motor reserve. J Neurol Sci 381:141–143
Lee PC, Artaud F, Cormier-Dequaire F, Rascol O, Durif F, Derkinderen P, Marques AR, Bourdain F, Brandel JP, Pico F, Lacomblez L, Bonnet C, Brefel-Courbon C, Ory-Magne F, Grabli D, Klebe S, Mangone G, You H, Mesnage V, Brice A, Vidailhet M, Corvol JC, Elbaz A (2019) Examining the reserve hypothesis in Parkinson’s disease: a longitudinal study. Mov Disord 34:1663–1671
Yogev-Seligmann G, Hausdorff JM, Giladi N (2008) The role of executive function and attention in gait. Mov Disord 23:329–342
Hausdorff JM, Buchman AS (2013) What links gait speed and MCI with dementia? A fresh look at the association between motor and cognitive function. J Gerontol A Biol Sci Med Sci 68:409–411
Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM (2012) Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 60:2127–2136
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Thenganatt MA, Jankovic J (2014) Parkinson disease subtypes. JAMA Neurol 71:499–504
Rascol O, Sabatini U, Brefel C, Fabre N, Rai S, Senard JM, Celsis P, Viallard G, Montastruc JL, Chollet F (1998) Cortical motor overactivation in parkinsonian patients with L-dopa-induced peak-dose dyskinesia. Brain 121(Pt 3):527–533
Cerasa A, Pugliese P, Messina D, Morelli M, Gioia MC, Salsone M, Novellino F, Nicoletti G, Arabia G, Quattrone A (2012) Prefrontal alterations in Parkinson’s disease with levodopa-induced dyskinesia during fMRI motor task. Mov Disord 27:364–371
Cerasa A, Messina D, Pugliese P, Morelli M, Lanza P, Salsone M, Novellino F, Nicoletti G, Arabia G, Quattrone A (2011) Increased prefrontal volume in PD with levodopa-induced dyskinesias: a voxel-based morphometry study. Mov Disord 26:807–812
Cerasa A, Morelli M, Augimeri A, Salsone M, Novellino F, Gioia MC, Arabia G, Quattrone A (2013) Prefrontal thickening in PD with levodopa-induced dyskinesias: new evidence from cortical thickness measurement. Parkinsonism Relat Disord 19:123–125
Aron AR, Obeso J (2012) Is executive control used to compensate for involuntary movements in levodopa-induced dyskinesia? Mov Disord 27:339–340
Yoo HS, Chung SJ, Lee YH, Lee HS, Ye BS, Sohn YH, Lee PH (2019) Levodopa-induced dyskinesia is closely linked to progression of frontal dysfunction in PD. Neurology 92:e1468–e1478
Jenner P (2013) Wearing off, dyskinesia, and the use of continuous drug delivery in Parkinson’s disease. Neurol Clin 31:S17-35
de la Fuente-Fernandez R, Schulzer M, Mak E, Calne DB, Stoessl AJ (2004) Presynaptic mechanisms of motor fluctuations in Parkinson’s disease: a probabilistic model. Brain 127:888–899
Boyce S, Rupniak NM, Steventon MJ, Iversen SD (1990) Nigrostriatal damage is required for induction of dyskinesias by L-DOPA in squirrel monkeys. Clin Neuropharmacol 13:448–458
Amboni M, Cozzolino A, Longo K, Picillo M, Barone P (2008) Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord 23:395–400
Teramoto H, Morita A, Ninomiya S, Shiota H, Kamei S (2014) Relation between freezing of gait and frontal function in Parkinson’s disease. Parkinsonism Relat Disord 20:1046–1049
Sunwoo MK, Cho KH, Hong JY, Lee JE, Sohn YH, Lee PH (2013) Thalamic volume and related visual recognition are associated with freezing of gait in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord 19:1106–1109
Tard C, Delval A, Duhamel A, Moreau C, Devos D, Dujardin K (2015) Specific attentional disorders and freezing of gait in Parkinson’s disease. J Parkinsons Dis 5:379–387
Maidan I, Jacob Y, Giladi N, Hausdorff JM, Mirelman A (2019) Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 63:77–82
Domellof ME, Elgh E, Forsgren L (2011) The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson’s disease. Mov Disord 26:2183–2189
Domellof ME, Forsgren L, Elgh E (2013) Persistence of associations between cognitive impairment and motor dysfunction in the early phase of Parkinson’s disease. J Neurol 260:2228–2236
Nantel J, McDonald JC, Tan S, Bronte-Stewart H (2012) Deficits in visuospatial processing contribute to quantitative measures of freezing of gait in Parkinson’s disease. Neuroscience 221:151–156
Vercruysse S, Devos H, Munks L, Spildooren J, Vandenbossche J, Vandenberghe W, Nieuwboer A, Heremans E (2012) Explaining freezing of gait in Parkinson’s disease: motor and cognitive determinants. Mov Disord 27:1644–1651
Helmich RC, de Lange FP, Bloem BR, Toni I (2007) Cerebral compensation during motor imagery in Parkinson’s disease. Neuropsychologia 45:2201–2215
Lewis GN, Byblow WD, Walt SE (2000) Stride length regulation in Parkinson’s disease: the use of extrinsic, visual cues. Brain 123(Pt 10):2077–2090
McColl CD, Reardon KA, Shiff M, Kempster PA (2002) Motor response to levodopa and the evolution of motor fluctuations in the first decade of treatment of Parkinson’s disease. Mov Disord 17:1227–1234
Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD (2005) Visual dysfunction in Parkinson disease without dementia. Neurology 65:1907–1913
Louis ED, Tang MX, Cote L, Alfaro B, Mejia H, Marder K (1999) Progression of parkinsonian signs in Parkinson disease. Arch Neurol 56:334–337
von Bonsdorff M, Rantanen T, Laukkanen P, Suutama T, Heikkinen E (2006) Mobility limitations and cognitive deficits as predictors of institutionalization among community-dwelling older people. Gerontology 52:359–365
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2019R1A2C2085462) and the Ministry of Education (NRF-2018R1D1A1B07048959).
Author information
Authors and Affiliations
Contributions
SJC and PHL conceived the study. SJC, HSY, HSL, YHL, KWB, and JHJ acquired and analyzed the data. SJC and PHL wrote the manuscript. HSY, HSL, YHL, KWB, JHJ, BSY, and YHS revised the manuscript for important intellectual content. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no financial or other conflicts of interest.
Ethical approval
This study was approved by the Institutional Review Board of Yonsei University Severance Hospital. The need for informed consent was waived because of the retrospective nature of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chung, S.J., Yoo, H.S., Lee, H.S. et al. Baseline cognitive profile is closely associated with long-term motor prognosis in newly diagnosed Parkinson’s disease. J Neurol 268, 4203–4212 (2021). https://doi.org/10.1007/s00415-021-10529-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-021-10529-2