Abstract
Objective
Despite high incidence of depression after stroke, few trials have investigated the therapeutic efficacy of repetitive transcranial magnetic stimulation (rTMS). Here, we aimed to evaluate clinical benefit of delivering a higher dose of rTMS compared to previous stroke trials. Secondary aims were to document adverse effects and investigate the role of functional connectivity as a potential mechanism of clinical response to rTMS treatment.
Methods
Eleven chronic stroke survivors were recruited to a double-blind, Sham-controlled, randomised trial to investigate 10 sessions of high-frequency rTMS for depression. Clinical assessments were obtained at baseline, after treatment and a 1-month follow-up. Adverse events were documented at completion of the treatment. Resting electroencephalography recordings were performed at baseline and after treatment to estimate functional connectivity.
Results
There were no differences in baseline characteristics between groups (all p ≥ 0.42). Beck Depression Inventory scores decreased for the Active rTMS group from baseline to 1-month follow-up (p = 0.04), but did not change for the Sham group at post-treatment or follow-up (p ≥ 0.17). Stronger theta frequency functional connectivity between the left frontal cortex and right parietal cortex was associated with lower baseline depression (r = − 0.71, p = 0.05). This network strength increased following Active rTMS, with change in connectivity associated with improvement in BDI scores (r = 0.98, p = 0.001). Adverse events were transient and minor and were not statistically different between groups (p ≥ 0.21).
Conclusions
Active rTMS significantly improved depression and was well tolerated. The mechanistic role of theta frequency functional connectivity appears worthy of further investigation.
The trial was registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12619001303134) on September 23, 2019.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a leading cause of adult disability and ~ 40% of stroke survivors subsequently experience depression [16, 38]. Depression may manifest from experiencing devastating impairment after stroke (reactive depression) or be underpinned by structural and/or functional abnormalities in frontal brain networks as a result of the lesion [15, 48]. Those with depression after stroke experience poor recovery, increased hospital stay, reduced activities of daily living, reduced self-efficacy and increased mortality [16, 33, 37, 45, 48]. Therapies to treat post-stroke depression could improve clinical outcomes, provide greater opportunity for recovery or reduce the burden of care for the whole health system.
Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive technique to induce plasticity within the human cortex and has shown promise as a treatment for depression. Low-frequency stimuli (< 1 Hz) decrease cortical excitability, while high frequencies (5–20 Hz) increase cortical excitability [8, 31, 35, 36]. Therapeutically, several studies have investigated the efficacy of high-frequency rTMS over the left DLPFC [13, 34], or low-frequency rTMS over the right DLPFC in depressed, but otherwise neurologically intact adults with promising results [24]. Of importance, rTMS is well tolerated compared to pharmacological therapy, where several side effects and poor treatment adherence have been reported [2, 9, 33, 41, 43]. rTMS could have therapeutic value in post-stroke depression.
However, to date, only a few randomized controlled trials with low treatment dose have investigated the efficacy of rTMS for post-stroke depression. Two trials both reported that 10 sessions of left DLPFC high-frequency (10 Hz) rTMS in chronic stroke survivors improved depressive symptoms [14, 23]. Of note, both trials employed similar rTMS protocols delivering 1000 pulses each session [14, 23]. Increasing pulse number provides greater treatment dose and could accelerate the trajectory of rTMS response [11]. Delivering 3000 pulses per session in depressed, but otherwise neurologically intact adults was not associated with elevated risk of serious adverse events and might produce more robust improvements in depression [32]. It may be that increasing pulse number can enhance rTMS improvements in depression and is worthy of investigation in people with stroke. However, stroke is associated with increased seizure risk [4], so increasing dosage could elevate potential for serious adverse events. Attempts to increase therapeutic response by increasing dose should be carefully monitored.
Furthermore, investigation of neurophysiological mechanism underpinning this treatment might help further inform and optimize rTMS treatment for post-stroke depression. Functional connectivity is one mechanism worth investigating as there is good evidence that a lesion can modify brain activity, even in the opposite hemisphere [18, 46, 47], and connectivity appears to influence neuromodulation. For example, in both healthy adults and people with stroke, greater functional connectivity of the stimulated network in alpha and beta bands was associated with a bigger increase in cortical excitability following excitatory brain stimulation [19, 20]. Understanding if and how functional connectivity mediates an improvement in depression with rTMS in people with post-stroke depression deserves investigation. Therefore, the purpose of this study was to investigate the efficacy of left DLPFC high-frequency (10 Hz) rTMS in people with post-stroke depression compared with a Sham control condition. Specifically, we were interested in delivering a higher dosage of rTMS compared to previous studies by increasing pulse number to 3000 per session, in line with the FDA approved protocol for depression. Secondary purposes were to document adverse effects and investigate the role of functional connectivity in mediating any improvements in depression with rTMS. It was hypothesized that rTMS would improve depressive symptoms to a greater extent for those receiving Active treatment compared to Sham treatment. Furthermore, we hypothesized that rTMS would be safe, well tolerated and improvements in depression would be associated with enhanced functional connectivity.
Materials and methods
Participants
Eleven chronic stroke survivors (9 male, aged 62.5 ± 11.1 years) participated in this study. All participants were aged > 18 years, had depression (Patient Health Questionnaire-9 (PHQ-9) > 5) with onset of symptoms occurring after stroke, had no change in antidepressant medication for 6 months prior to participation or during the trial, no contraindications to TMS such as metallic implants, pregnancy or a history of seizures [39] and no history of craniotomy or craniectomy as skull defects are known to affect electroencephalography (EEG) signal. The PHQ-9 was selected as the screening tool for depression given ease of administering via a telephone interview. Participants who were unable to communicate and provide informed consent were excluded. All participants provided written informed consent and ethical approval was provided by the University of South Australia Human Research Ethics Committee (200697). The trial was registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12619001303134).
Experimental protocol
This study was a double-blind randomized controlled trial with participants randomized to one of two parallel groups on enrolment (Active or Sham). Both participants and outcome assessors were blind to allocation. Participants were informed that there was an Active and Sham group and they would be unable to determine the difference between conditions. Randomization was performed by an assessor not involved in data collection, experimental procedures or analysis. Sequence generation was from a random number generator. Baseline assessments included demographics, clinical characteristics, depression (Beck Depression Inventory—II (BDI) and PHQ-9), self-efficacy (Stroke Self-Efficacy Questionnaire (SSEQ)) and neurophysiology (EEG). Following this, participants completed 10 daily sessions of Active or Sham rTMS (Monday to Friday over a 2-week period). Clinical assessments of depression and self-efficacy were repeated at the completion of the 10 rTMS sessions and a 1-month follow-up. Neurophysiology (EEG) was re-assessed at the completion of the 10 rTMS sessions.
Intervention
Electromyography
Surface electromyography (EMG) was used to record motor evoked potentials (MEPs) from the first dorsal interosseous (FDI) for determining resting motor threshold (RMT) of the non-lesioned hemisphere. Initially, the skin was prepared by cleaning with alcohol and lightly abrading with NuPrep paste. A ground strap was placed around the wrist. Signals were sampled at 5 kHz (CED 1401; Cambridge Electronic Design, UK), amplified (× 1000) (CED 1902; Cambridge Electronic Design) and filtered (20-1000 Hz).
Repetitive transcranial magnetic stimulation
At the first treatment session, RMT of the non-lesioned hemisphere was determined by applying single bi-phasic TMS pulses at 0.2 Hz ± 10%. The hotspot was identified by placing the coil over the non-lesioned motor cortex hand representation, with the coil held tangentially to the scalp and handle pointing 45° posterolaterally to induce a posterior–anterior current across the cortex. The coil position was systematically moved to identify the optimal position for evoking MEPs in the contralateral and relaxed FDI. RMT was defined as the minimum stimulus intensity for evoking MEPs with a peak-to-peak amplitude larger than 50 μV in at least 5 out of 10 trials.
Focal rTMS was administered using a Magstim Super Rapid (Magstim, UK) connected to either an active 70 mm figure-8 air-cooled coil (part number 3910-23-00), or placebo coil that was identical, but did not produced an electromagnetic field (part number 3950–23-00). For all participants, rTMS was applied at 110% RMT to the left DLPFC (F3 from the 10–20 EEG system). At each treatment session, 3000 pulses were applied at 10 Hz (4 s on and 26 s off; total duration 37.5 min) with a total of 10 rTMS sessions completed at a similar time of day over a 2-week period.
Outcome measures
Clinical assessment
The primary clinical outcome measure was the BDI. Secondary clinical assessments were the PHQ-9 and SSEQ. The BDI is a valid and reliable 21-item scale for assessing depression severity [10]. Total scores range from 0–63, with higher scores indicative of more severe depressive symptoms and a 17.5% reduction considered a minimal clinically important difference [3, 7]. The PHQ-9 is a valid and reliable 9-item scale for measuring depression [25, 26]. Total scores range from 0–27, with higher scores indicating greater depressive symptoms and a 5-point decrease considered a clinically meaningful improvement [28]. The SSEQ is a 13-item assessment of an individual’s confidence or belief in their ability to successfully achieve a task [21]. Self-efficacy was assessed as improved depression may lead to greater confidence in functional performance and aspects of self-management. Total scores range 0–130, with higher scores indicating greater self-efficacy.
EEG acquisition
Prior to and following the treatment phase, three minutes of EEG was acquired with an ASA-lab EEG system (ANT, Netherlands). Participants were fitted with a cap that had 64 sintered Ag–AgCl monopolar electrodes in standard 10–10 positions. During data collection, participants were seated in a comfortable chair in a quiet room and asked to relax while keeping their eyes open, maintaining their gaze toward a fixation point straight ahead, refraining from speaking or moving and not engaging in any cognitive or mental tasks. Signals were sampled at 2048 Hz, amplified 20x, filtered (high pass, DC; low pass 553 Hz) and online referenced CPz. Impedance was kept below 5 kΩ and recorded data were stored on a computer for offline analysis.
Adverse events and effectiveness of blinding
Adverse events following rTMS were documented as either absent, mild, moderate or severe (see supplementary materials for tool) [6]. To determine the effectiveness of blinding, participants were asked if they believed they had received the Active rTMS treatment (yes/no) and their confidence in this response (range; 0, not at all confident—10, completely confident).
Magnetic resonance imaging acquisition and processing
For a subset of participants (n = 9) magnetic resonance imaging (MRI) data were available and included T1- and T2-weighted sequences performed using a Siemens 3T MAGNETOM Skyra scanner (Siemens, Germany) with a 64-channel head coil. The scan protocol was as follows: T1-weighted image MPRAGE (voxel 1 mm x 1 mm x 1 mm, repetition time = 2300 ms, echo time = 2.98 ms, flip angle = 9°); T2-weighted fluid-attenuated inversion recovery (voxel 1 mm × 0.5 mm × 0.5 mm, repetition time = 5000 ms, echo time = 393 ms). Image processing was performed in FSL (FMRIB Software Library, Oxford, UK). T1-weighted images were linearly registered to T2-weighted images for lesion identification. Lesion masks were manually traced by an experienced investigator blind to group allocation and lesion volume determined.
EEG pre-processing and analysis
EEG data were exported to MATLAB 9.2.0 (MathWorks, Inc., Natick, MA) for pre-processing and analysis. Data were filtered 1–80 Hz (second order Butterworth) and band-stop filter 48–52 Hz. Visibly bad channels were removed, and data was segmented into 180 one-second epochs. An independent component analyses using the EEGLAB fast ICA function identified non-physiological artefactual components for removal (e.g., eye blinks and scalp muscle activity). Following artefact removal, missing channels were interpolated.
The debiased weighted phase lag index (dwPLI) was computed as a measure of functional connectivity (FieldTrip toolbox for MATLAB). The dwPLI is a conservative estimate of phase consistency that weights against zero phase lag relationships [44], limiting effects of volume conduction and common reference problems. Values range from 0 (negative values can incidentally occur due to limited sampling) to 1 (maximum phase coupling). The dwPLI was determined for delta (1–3 Hz), theta (4–7 Hz), alpha (8–13 Hz), low-beta (14–20 Hz), high-beta (21-30 Hz) and gamma (31–45 Hz) spectral frequencies.
To determine if baseline EEG functional connectivity was associated with response to rTMS, a partial least squares (PLS) regression analysis was performed using the N-way Toolbox for MATLAB. PLS identifies a model of connectivity between the seed electrode (F3, rTMS target) and all other electrodes that maximally accounted for baseline clinical scores for the primary (BDI) or secondary outcome measures (PHQ-9 or SSEQ). Similar to previous studies, the first component was used to generate all PLS models with a conservative threshold of 0.7 relative to the maximal correlation coefficient [19, 30]. Data were mean-centered and submitted to a direct orthogonal signal correction for increased efficiency. PLS models were cross-validated using leave-one-out and predict analysis. For the PLS model with greatest effect size, clusters of electrodes defined as 3 or more adjacent electrodes were identified and the mean dwPLI values of the clusters were then determined and correlated against the dependent variable.
Statistical analysis
Statistical analyses were performed using SPSS software (IBM Corp., V24, Armonk, NY, USA) with significance level set at p < 0.05. Normality of the data was checked, and where required, non-parametric statistics applied. Demographics and clinical characteristics were compared between Active and Sham groups with independent t-tests or Fisher’s Exact tests. Lesion volume was compared between groups with a Mann–Whitney U test. Adverse events were compared between groups with a Fisher’s Exact test. Clinical responses for the primary (BDI) and secondary (PHQ-9, SSEQ) outcomes following rTMS were analysed with separate 2 GROUP (Active, Sham) X 3 TIME (baseline, post-rTMS, follow-up) linear mixed models that were adjusted for time since stroke and lesion volume. Main effects or interactions were evaluated with Bonferroni corrected t-tests. PLS analysis to identify EEG connectivity models were further investigated by correlating dwPLI and baseline clinical measures for both the primary (BDI) and secondary (PHQ-9, SSEQ) outcomes. For significant correlations between dwPLI and baseline clinical measures, data were further investigated by comparing the rTMS-induced change in dwPLI (normalized to baseline) between Active and Sham groups (independent t-test) and correlating the change in dwPLI with change in clinical measure (baseline to post-rTMS). Finally, lesion volume was correlated with both baseline and change scores for both the primary (BDI) and secondary (PHQ-9, SSEQ) outcomes. Where appropriate, Cook’s distance identified influential data points in correlational analyses. A threshold of 4/n was used to classify an observation as a statistical outlier [5].
Results
Participant characteristics, missing data and adverse events
Six participants were randomized to Active and five to Sham rTMS. Demographics and baseline characteristics are reported in Table 1 and individual MRI scans for the subset of participants with MRI data (n = 9) are shown in Fig. 1. There were no group differences in age, sex, time since stroke, lesion characteristics, RMT or baseline BDI, PHQ-9 and SSEQ (all p ≥ 0.42). All participants completed all 10 rTMS sessions and all clinical and neurophysiological assessments. A detailed summary of individual participant demographics, clinical characteristics and MRI outcomes is provided in the supplementary materials.
Lesion imaging for a subset of participants in the trial. The slice with greatest cross-sectional area of the lesion is shown. The lesion tracing is highlighted in red. The top row shows four participants in the Active rTMS group and the bottom row shows five participants from the Sham rTMS group. L = left; R = right; rTMS = repetitive transcranial magnetic stimulation. Note, MRI data were only available for four of the six participants in the Active rTMS group
One participant randomized to the Sham condition experienced strong dental pain that appeared to be linked to the delivery of Sham rTMS but likely underpinned by other mitigating circumstances. This has been detailed in a separate report [17]. Participation in the study was delayed by 3 months and upon re-enrolment this participant experienced very minor, but tolerable, discomfort and completed all experimental procedures. At completion of treatment, four participants (n = 2 Active, n = 2 Sham) reported mild headaches; two participants from the Active condition reported mild neck pain and two from the Sham condition reported moderate neck pain; two participants (n = 1 Active, n = 1 Sham) reported mild sleep disturbances; and five participants (n = 4 Active, n = 1 Sham) reported mild mood changes (noted as an improved mood) at the completion of treatment. No other adverse effects were noted and there were no statistical differences in reported adverse events between groups (all p ≥ 0.21).
Participant blinding was effective. All participants in the Sham rTMS group believed they had received the Active treatment with a moderate level of confidence (mean 5.6 ± 2.9, range 2–10). Of interest, only 50% of participants in the Active group believed they had received the Active treatment (high level of confidence; mean 8.7 ± 2.9, range 7–10), while the remaining participants believed they had received the Sham treatment (moderate level of confidence; mean 5.3 ± 2.3, range 4–8).
Treatment responses
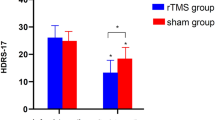
Participants that received Active rTMS had a reduction in depression scores measured with the primary clinical outcome (BDI) from baseline to follow-up (Fig. 2). There was no main effect of GROUP (F(1,7) = 1.82, β = − 0.75, p = 0.22), but there was a main effect of TIME (F(2,14) = 8.28, β = − 4.60, p = 0.004) and a GROUP X TIME interaction (F(2,14) = 4.55, β = − 11.55, p = 0.05). Post-hoc analysis found BDI scores reduced in the Active rTMS group from baseline to post-rTMS, but became insignificant following correction for multiple comparisons (t(5) = 2.71, p = 0.08, Bonferroni corrected). There was a significant decrease in BDI from baseline to follow-up in the Active group (t(5) = 3.42, p = 0.04, Bonferroni corrected). For Sham, there were no statistical differences between baseline BDI and either post-rTMS BDI (t(4) = 2.30, p = 0.17, Bonferroni corrected) or follow-up BDI (t(4) = 1.55, p = 0.39, Bonferroni corrected).
Clinical response to repetitive transcranial magnetic stimulation from baseline to post-treatment and a 1-month follow-up. Data are presented as mean ± SEM for (a) Beck Depression Inventory, (b) Patient Health Questionnaire 9 Item scale, and (c) Stroke self-efficacy questionnaire. There was a significant improvement in Beck Depression Inventory scores at follow-up for participants in the Active rTMS group, but not the Sham rTMS group. *indicates a statistically significant difference
For the secondary clinical outcome measures, there was a main effect of TIME for the PHQ-9 (F(2,14) = 7.56, β = − 6.20, p = 0.04), with post-hoc analysis finding PHQ-9 scores decreased from baseline to post-rTMS (t(10) = 3.95, p = 0.006, Bonferroni corrected), and follow-up (t(10) = 3.80, p = 0.006, Bonferroni corrected). However, there was no effect of GROUP (F(1,7) = 2.70, β = − 0.80, p = 0.14) or GROUP X TIME interaction (F(2,14) = 0.72, β = − 3.80, p = 0.50; Fig. 2). These findings suggest that PHQ-9 scores improved from baseline to post-rTMS and follow-up; however, there was no difference between groups. For SSEQ there was a main effect of TIME (F(2,14) = 16.85, β = 19.4, p = 0.02), with post-hoc analysis finding SSEQ scores increased from baseline to post-rTMS (t(10) = − 3.25, p = 0.02, Bonferroni corrected), and follow-up (t(10) = − 3.36, p = 0.01, Bonferroni corrected). However, there was no effect of GROUP (F(1,7) = 1.12, β = 4.40, p = 0.33) or GROUP X TIME interaction (F(2,14) = 2.11, β = 13.30, p = 0.16; Fig. 2). These findings suggest SSEQ scores improved from baseline to post-rTMS and follow-up; however, there was no difference between groups.
Electroencephalography and baseline clinical outcomes
For the baseline primary clinical outcome (BDI), a theta frequency PLS model identified a cluster of electrodes approximately overlying the right parietal cortex (fitted R2 = 0.46, cross-validated R2 = 0.41; Fig. 3). Greater theta band dwPLI for this model was associated with lower BDI scores (r = − 0.71, Bonferroni corrected p = 0.05, Fig. 3). Electrode clusters for PLS models in alpha, low-beta and gamma frequencies were also identified but were not significantly correlated with baseline BDI scores (all Bonferroni corrected p > 0.20). PLS models for delta and high-beta frequencies did not identify any electrode clusters.
PLS models identified electrode clusters where dwPLI functional connectivity between F3 and the identified electrodes maximally accounted for variance in baseline clinical outcomes. For the EEG topoplots, the white circle represents F3, where rTMS was applied to the left DLPFC and was the seed electrode. Stars represent electrodes within the identified PLS models. Black filled stars represent electrodes in a cluster. Electrodes that were in the PLS model, but not in a cluster are show as grey stars. BDI, Beck Depression Inventory—II; dwPLI, debiased weighted phase lag index; PHQ-9, Patient Health Questionnaire-9
For the baseline secondary clinical outcome (PHQ-9), a low-beta frequency PLS model identified a cluster of electrodes approximately overlying the left parietal cortex (fitted R2 = 0.38, cross-validated R2 = 0.27; Fig. 3). Greater low-beta dwPLI for this model was associated with higher PHQ-9 scores (r = 0.74, Bonferroni corrected p = 0.03, Fig. 3). Electrode clusters for PLS models in alpha and gamma frequencies were also identified but were not significantly correlated with baseline PHQ-9 scores (all Bonferroni corrected p > 0.45). PLS models for delta, theta and high-beta frequencies did not identify any electrode clusters.
For the baseline secondary clinical outcome (SSEQ), electrode clusters for PLS models in delta, theta, high-beta and gamma frequencies were identified but did not reach significance when correlated with baseline SSEQ scores (all Bonferroni corrected p > 0.35). PLS models for alpha and low-beta frequencies did not identify any electrode clusters.
A detailed summary for all PLS analyses for each frequency and clinical measure is provided in the supplementary materials.
rTMS-induced changes in neurophysiology
For theta connectivity between F3 and the cluster approximating the right parietal cortex, there was a non-significant trend indicating a greater increase in dwPLI for the Active group compared to Sham (t(9) = 1.6, p = 0.08). However, there was no significant difference between groups for change in low-beta dwPLI between F3 and the cluster approximately overlying the left parietal cluster (t(9) = 0.24, p = 0.41; Fig. 4).
Change in dwPLI following rTMS treatment and correlation with improvement in depression. Top left, change in dwPLI from baseline to post-rTMS treatment for PLS model (F3—Right parietal) in the theta frequency (mean ± SEM). An increase in dwPLI was observed for the active rTMS group and a slight decrease for the Sham rTMS group. Top right, change in dwPLI from baseline to post-rTMS treatment for PLS model (F3—Left parietal) in the low-beta frequency (mean ± SEM). Both Active and Sham rTMS groups exhibited a slight decrease in dwPLI after treatment. Bottom, a significant positive correlation between the improvement in depression (change beck depression inventory) following Active rTMS and the change in dwPLI for PLS model (F3—Right parietal) in the theta frequency. This finding suggests an increase in dwPLI in the theta frequency between F3—Right parietal region was associated with improvement in depression. Inset bottom right, the theta dwPLI PLS model of connectivity between F3 (seed electrode, white circle) and a cluster approximating the right parietal region (black stars)
For the active group, there was a correlation between change in theta band dwPLI from F3 to the electrode cluster approximately overlying the right parietal cortex and change in BDI scores after treatment (r = 0.98, p = 0.001; Fig. 4). Cook’s distance found that no datapoints had significant influence over the correlation and even a conservative approach of re-analysing the data after removal of the upper right data point demonstrated that this correlation remained strong (r = 0.86, p = 0.06). These findings suggest that an increase in theta dwPLI after treatment was associated with improved depression measured by BDI. For the Sham group, the correlation between change in theta band dwPLI and BDI was not significant (p = 0.29). Similarly, for both groups, the change in low-beta dwPLI for the PHQ-9 PLS model and change in PHQ-9 scores were not significant (all p > 0.48).
Lesion volume and clinical outcomes
Lesion volume did not correlate with baseline clinical measures (all p > 0.28) or improvements in clinical outcomes (all p > 0.16).
Discussion
This preliminary investigation demonstrated that left DLPFC high-frequency rTMS in chronic stroke survivors with moderate post-stroke depression reduced symptoms of depression to a greater extent in those receiving Active compared to Sham treatment. This finding was evident for the primary clinical outcome measure (BDI). Both secondary clinical outcomes (PHQ-9 and SSEQ) reduced following treatment but were not different between groups. Stronger theta frequency resting state functional connectivity between F3 and a cluster of electrodes overlying the right parietal cortex was associated with lower BDI scores at baseline. For those in the Active treatment group, rTMS appeared to increase this model of theta connectivity, with the magnitude of increase in connectivity strongly associated with improvements in the BDI scores after treatment. Finally, the rTMS protocol employed in this study appears safe with only mild and temporary side effects that are common in rTMS literature noted [29]. Together, these findings suggest rTMS is a promising treatment for post-stroke depression. Clinical improvements in post-stroke depression following rTMS might be related to theta band connectivity between an approximate left DLPFC and right parietal cortex network.
Previous studies have provided good evidence that left DLPFC high-frequency rTMS is an effective treatment for depression, but little work has been performed in post-stroke depression. Here, in a small sample, we demonstrate that rTMS produced statistically significant and clinically meaningful improvement in depressive symptoms. For the primary clinical outcome measure (BDI), the magnitude of improvement after treatment (14 points) and at follow-up (11.8 points) exceeded the minimal clinically important difference of a 17.5% reduction, equating to 4 points in this study [7]. Similarly, for the secondary clinical outcome measure (PHQ-9), improvements of 8.5 points after treatment and 6.7 points at follow-up exceeded minimal clinically important differences (5 points), although this was not statistically significant compared to Sham [28]. Our findings, therefore, support the few available trials investigating rTMS in post-stroke depression that have reported significant improvements in BDI and the Hamilton Depression Rating Scale [14, 23]. Of note, this study did safely deliver a greater dosage of rTMS compared to previous stroke studies (3000 vs 1000 pulses per session). This may be one factor that led to greater BDI improvement in this study (14 point improvement) compared to previous work (7.3 point improvement) [14]. Further extending previous literature, this study also investigated the effect of rTMS on self-efficacy as a secondary clinical outcome. Although no group level differences were observed between Active and Sham treatment, it is noteworthy that self-efficacy, a measure of internal sense of control, improved across assessment time points irrespective of group allocation. One explanation for both groups showing improved self-efficacy may be that involvement in the trial provided a sense of increased confidence in their ability to participate within society. Although not directly assessed in this study, participation aspects such as leaving home to attend treatment sessions, transportation and social interactions may have contributed to greater self-efficacy. Given the importance of self-efficacy in stroke recovery [22], we suggest future studies continue to investigate the effects of rTMS on the confidence of people with stroke to perform functional tasks and aspects of self-management.
A novel finding was that enhanced theta frequency functional connectivity between an electrode approximating the left DLPFC and a cluster of electrodes approximating the right parietal cortex appeared to underpin improvement in BDI following rTMS. Previous studies with depressed, but otherwise neurologically intact, people have indicated that rTMS treatment efficacy might rely on functional connectivity with the DLPFC [1, 12, 27]. In particular, there is some indication that connectivity within a frontoparietal central executive network is abnormally diminished, while connectivity of a prefrontal–parietal default mode network is abnormally increased in people with depression [27]. High-frequency DLPFC rTMS was found to normalize default mode network connectivity, but did not alter frontoparietal central executive network connectivity [27]. Although spatial resolution of EEG recordings limits interpretation of anatomical brain regions generating the recorded signal, it is possible our findings provide some support for a role of the default mode and central executive networks in rTMS treatment for depression after stroke. That theta connectivity is thought to reflect cognitive processes underpinned by these networks provides further support for our finding that theta interhemispheric frontoparietal connectivity is an important mechanism of response to rTMS in post-stroke depression [40]. Although further investigation is required, theta resting state functional connectivity may be a neurophysiological mechanism that enables rTMS to improve clinical outcomes in post-stroke depression.
Along similar lines, we observed that reduced low-beta connectivity between F3 and a cluster of electrodes approximating the left parietal cortex was associated with lower levels of depression on the PHQ-9 at baseline. However, our findings do not support a relationship between connectivity of the left frontoparietal network and improved depression with rTMS. This is because rTMS did not appear to modify low-beta connectivity and there was no evidence that a change in connectivity was associated with clinical improvement. It appears unlikely low-beta connectivity has a relationship with depression and response to rTMS.
There are several limitations to this study. First, the sample size was small, limiting our ability to conclusively demonstrate rTMS is an effective and generalizable treatment for post-stroke depression. However, it is important to note that this well-designed, registered, double-blind randomized controlled trial adds to the available evidence supporting the efficacy of left DLPFC high-frequency rTMS for post-stroke depression. Second, we note that, on average, participants had a moderate level of depression. It is, therefore, not clear whether similar improvements in depression would be evident for those with more severe symptoms. Third, we did not have a measure of stroke severity. Although MRI data were available and there is some indication of a strong association between lesion volume and stroke severity (National Institutes of Health Stroke Scale; NIHSS) [42], we suggest future studies should assess NIHSS to gain further insight to the relationship between depression and rTMS treatment. Fourth, EEG suffers from poor spatial resolution and neural signal can be affected by volume conduction, making it difficult to directly infer the neural generator. To mitigate this limitation we have; (1) used a conservative measure of functional connectivity that biases against phase differences of 0 or 180 degrees; and (2) cautiously referred to our findings by referring to electrode clusters that approximate cortical regions as we do not know where the recorded EEG signals were generated. Finally, we acknowledge that imaging data were not available for all participants in this study. Although not required to address our research questions, this did partially limit ability to accurately describe lesion characteristics for all study participants and determine if lesion metrics correlated with clinical measures.
In conclusion, post-stroke depression is a serious consequence of stroke that requires new treatment options. This preliminary study investigated rTMS of the left DLPFC and found that those receiving the Active treatment had greater improvements in depression compared to Sham. Delivering an increased dosage of rTMS compared to previous stroke studies appears to enhance clinical benefit and was well tolerated, with only mild and temporary side effects noted. Therefore, our findings support the possible use of rTMS as a treatment for post-stroke depression. The mechanistic role of theta frequency functional connectivity appears worthy of further investigation.
Abbreviations
- BDI:
-
Beck depression inventory—II
- DLPFC:
-
Dorsolateral prefrontal cortex
- dwPLI:
-
Debiased weighted phase lag index
- FDI:
-
First dorsal interosseous
- MEP:
-
Motor evoked potential
- PHQ-9:
-
Patient health questionnaire-9
- PLS:
-
Partial least squares
- rTMS:
-
Repetitive transcranial magnetic stimulation
- RMT:
-
Repetitive transcranial magnetic stimulation
- SSEQ:
-
Stroke self-efficacy questionnaire
References
Bailey NW, Hoy KE, Rogasch NC, Thomson RH, McQueen S, Elliot D, Sullivan CM, Fulcher BD, Daskalakis ZJ, Fitzgerald PB (2018) Responders to rTMS for depression show increased fronto-midline theta and theta connectivity compared to non-responders. Brain Stimul 11:190–203
Bakker N, Shahab S, Giacobbe P, Blumberger DM, Daskalakis ZJ, Kennedy SH, Downar J (2015) rTMS of the dorsomedial prefrontal cortex for major depression: safety, tolerability, effectiveness, and outcome predictors for 10 Hz versus intermittent theta-burst stimulation. Brain Stimul 8:208–215
Beck AT, Steer RA, Brown G (1996) Beck depression inventory–II. Psychol Assess
Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Coté R, Lebrun L, Pirisi A, Norris JW (2000) Seizures after stroke: a prospective multicenter study. Arch Neurol 57:1617–1622
Bollen KA, Jackman RW (1990) Regression diagnostics: An expository treatment of outliers and influential cases. Modern methods of data analysis:257–291
Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F (2011) A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol 14:1133–1145
Button KS, Kounali D, Thomas L, Wiles NJ, Peters TJ, Welton NJ, Ades AE, Lewis G (2015) Minimal clinically important difference on the Beck Depression Inventory—II according to the patient’s perspective. Psychol Med 45:3269–3279
Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48:1398–1403
Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J (2011) Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ 343
Dozois DJ, Dobson KS, Ahnberg JL (1998) A psychometric evaluation of the beck depression inventory–II. Psychol Assess 10:83
Fitzgerald PB, Daskalakis ZJ (2011) The effects of repetitive transcranial magnetic stimulation in the treatment of depression. Expert Rev Med Devices 8:85–95
Fox MD, Liu H, Pascual-Leone A (2013) Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 66:151–160
George MS, Wassermann EM, Kimbrell TA, Little JT, Williams WE, Danielson AL, Greenberg BD, Hallett M, Post RM (1997) Mood improvement following daily left prefrontal repetitive transcranial magnetic stimulation in patients with depression: a placebo-controlled crossover trial. AJ Psychiatry 154:1752–1756
Gu SY, Chang MC (2017) The effects of 10-Hz repetitive transcranial magnetic stimulation on depression in chronic stroke patients. Brain Stimul 10:270–274
Hackett ML, Pickles K (2014) Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke 9:1017–1025
Hackett ML, Yapa C, Parag V, Anderson CS (2005) Frequency of depression after stroke: a systematic review of observational studies. Stroke 36:1330–1340
Hordacre B, Comacchio K, Moseley GL (2020) The unusual case of dental pain with sham repetitive transcranial magnetic stimulation: A benign idiosyncrasy or diagnostic opportunity? Brain Stimul 13:422–423
Hordacre B, Goldsworthy MR, Welsby E, Graetz L, Ballinger S, Hillier S (2020) Resting state functional connectivity is associated with motor pathway integrity and upper-limb behavior in chronic stroke. Neurorehabil Neural Repair 34:547–557
Hordacre B, Moezzi B, Goldsworthy MR, Rogasch NC, Graetz LJ, Ridding MC (2017) Resting state functional connectivity measures correlate with the response to anodal transcranial direct current stimulation. Eur J Neurosci 45:837–845
Hordacre B, Moezzi B, Ridding MC (2018) Neuroplasticity and network connectivity of the motor cortex following stroke: a transcranial direct current stimulation study. Hum Brain Mapp 39:3326–3339
Jones F, Partridge C, Reid F (2008) The stroke self-efficacy questionnaire: measuring individual confidence in functional performance after stroke. J Clin Nurs 17:244–252
Jones F, Riazi A (2011) Self-efficacy and self-management after stroke: a systematic review. Disabil Rehabil 33:797–810
Jorge RE, Robinson RG, Tateno A, Narushima K, Acion L, Moser D, Arndt S, Chemerinski E (2004) Repetitive transcranial magnetic stimulation as treatment of poststroke depression: a preliminary study. Biol Psychiatry 55:398–405
Klein E, Kreinin I, Chistyakov A, Koren D, Mecz L, Marmur S, Ben-Shachar D, Feinsod M (1999) Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry 56:315–320
Kroenke K, Spitzer RL (2002) The PHQ-9: a new depression diagnostic and severity measure. Psychiatric annals 32:509–515
Kroenke K, Spitzer RL, Williams JB (2001) The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 16:606–613
Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ (2014) Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 76:517–526
Löwe B, Unützer J, Callahan CM, Perkins AJ, Kroenke K (2004) Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care:1194–1201
Machii K, Cohen D, Ramos-Estebanez C, Pascual-Leone A (2006) Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clin Neurophysiol 117:455–471
Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, del Campo N, Sahakian BJ, Robbins TW, Bullmore E (2007) Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain 130:3223–3236
Muellbacher W, Ziemann U, Boroojerdi B, Hallett M (2000) Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol 111:1002–1007
O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA (2007) Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 62:1208–1216
Paolucci S (2008) Epidemiology and treatment of post-stroke depression. Neuropsychiatr Dis Treat 4:145–154
Pascual-Leone A, Rubio B, Pallardó F, Catalá MD (1996) Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. The Lancet 348:233–237
Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M (1994) Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117:847–858
Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant’Angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P (2005) Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res 161:114-124
Robinson RG, Spalletta G (2010) Poststroke depression: a review. Can J Psychiatry 55:341–349
Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS (2012) Heart disease and stroke statistics—2012 update a report from the American heart association. Circulation 125:e2–e220
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2011) Screening questionnaire before TMS: an update. Clin Neurophysiol 122:1686
Sauseng P, Klimesch W (2008) What does phase information of oscillatory brain activity tell us about cognitive processes? Neurosci Biobehav Rev 32:1001–1013
Sawada N, Uchida H, Suzuki T, Watanabe K, Kikuchi T, Handa T, Kashima H (2009) Persistence and compliance to antidepressant treatment in patients with depression: a chart review. BMC Psychiatry 9:38
Schiemanck SK, Post MW, Witkamp TD, Kappelle LJ, Prevo AJ (2005) Relationship between ischemic lesion volume and functional status in the 2nd week after middle cerebral artery stroke. Neurorehabil Neural Repair 19:133–138
Turner-Stokes L, Hassan N (2002) Depression after stroke: a review of the evidence base to inform the development of an integrated care pathway. Part 2: treatment alternatives. Clin Rehabil 16:248–260
Vinck M, Oostenveld R, Van Wingerden M, Battaglia F, Pennartz CMA (2011) An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 55:1548–1565
Volz M, Möbus J, Letsch C, Werheid K (2016) The influence of early depressive symptoms, social support and decreasing self-efficacy on depression 6 months post-stroke. J Affect Disord 206:252–255
Ward NS, Brown MM, Thompson AJ, Frackowiak RS (2003a) Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126:2476–2496
Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ (2003b) Neural correlates of outcome after stroke: A cross-sectional fMRI study. Brain 126:1430–1448
Whyte EM, Mulsant BH (2002) Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry 52:253–264
Acknowledgements
The authors would like to acknowledge Dr Tobias Loetscher for assistance during study design.
Funding
This research was funded by a Research Themes Investment Scheme—University of South Australia grant. BH was supported by National Health and Medical Research Council (NHMRC) fellowship (1125054).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None of the authors have potential conflicts of interest to be disclosed.
Ethical standard
This study was approved by the University of South Australia Human Research Ethics Committee (ID number 200697). The study conformed to the standards defined in the latest revision of the Declaration of Helsinki. All patients and controls signed a written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hordacre, B., Comacchio, K., Williams, L. et al. Repetitive transcranial magnetic stimulation for post-stroke depression: a randomised trial with neurophysiological insight. J Neurol 268, 1474–1484 (2021). https://doi.org/10.1007/s00415-020-10315-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10315-6