Abstract
Objective
Determine toxicity and efficacy of autologous hematopoietic stem cell transplantation (HSCT) for patients with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) who are dependent on intravenous immunoglobulins or plasmapheresis.
Methods
Unselected peripheral blood stem cells were re-infused on day 0 after conditioning with cyclophosphamide 200 mg/kg/intravenously (IV), rATG (thymoglobulin) 5.5 mg/kg/IV, and rituximab 1000 mg/IV.
Results
Sixty-six patients underwent HSCT for CIDP. Data on sixty patients with a mean follow-up of 4.5 years (range 2–5 years) were available for analysis. There were no treatment-related deaths, and overall survival was 97%. Post-transplant immune medication-free remission was 80%, 78%, 76% 78%, and 83% at 1, 2, 3, 4, and 5 years. Ambulation without assistance improved from 33% pre-HSCT to 82% 82%, 81%, 86%, and 83% at 1, 2, 3, 4, and 5 years, respectively. Mean right/left hand grip strength (kg) improved significantly (all p values < 0.01) from 18.1/16.5 pre-HSCT to 26.3/25.4, 29.2/28.2, 28.8/28.6, 30.3/25.5, and 30.8/29.1 at 1, 2, 3, 4, and 5 years, respectively. Average nerve conduction velocity (NCV) (m/s) improved significantly (all p values ≤ 0.001) from a mean of 27.2 pre-HSCT to 33.5, 33.8, 37.7, 38.2, and 38.3 at 1, 2, 3, 4, and 5 years, respectively. Average compound motor action potential (CMAP) (mv) improved significantly (p values ≤ 0.001) from a mean of 3.6 pre-HSCT to 4.6, 4.6, 5.0, 5.1, and 4.1 at 1, 2, 3, 4, and 5 years, respectively.
Conclusion
A randomized trial is indicated to verify these results and confirm that HSCT reverses disability and offers long-term immune therapy independence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an immune-mediated demyelinating disease of the peripheral nervous system that may present acutely or insidiously with a progressive, stepwise, or relapsing clinical course [1]. “Typical” CIDP, as defined by the European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS), affects proximal and distal motor and sensory function, results in diminished deep tendon reflexes, and evolves over at least 2 months [2, 3]. “Atypical” CIDP variants have heterogeneous phenotypic patterns that may be multifocal (Multifocal Acquired Demyelinating Sensory and Motor (MADSAM), i.e. also known as Lewis–Sumner syndrome), distally accentuated (distal acquired demyelinating symmetric, i.e. DADS), pure motor, or pure sensory [2, 3]. The pathogenesis of CIDP and atypical variants has yet to be well characterized but is likely to involve multiple pathways that contribute to chronic neuronal dysfunction [4, 5].
CIDP remains one of the few peripheral neuropathies for which effective therapy exists [6, 7]. Corticosteroids [8, 9], intravenous immunoglobulin (IVIG) [10, 11], and plasmapheresis (PLEX) [12] have each been shown to be effective in randomized clinical trials. Retrospective studies have shown that when more than one of these conventional therapies are utilized, approximately 80% of patients respond [13, 14]. However, some individuals do not respond to first-line treatments [15] and many become dependent on conventional treatments [16]. Furthermore, side-effect profiles, quality of life restrictions, financial burdens, and/or losing insurance benefits can limit the use of corticosteroids, IVIG, and PLEX [17,18,19]. These limitations are especially relevant when treatment is chronic, as is often the case in patients with CIDP. Herein, we report the results of non-myeloablative autologous hematopoietic stem cell transplantation (HSCT) for patients with CIDP dependent on conventional treatment options of IVIG and/or PLEX.
Methods
Patients
Patients enrolled in this open-label prospective study were treated at Northwestern Memorial Hospital (Chicago, IL, USA) on an IRB and FDA approved protocol (www.clinicaltrials.gov NCT00278629). The study was an exploratory phase I/II trial designed to capture immune-based peripheral neuropathies with the intention of analyzing the results of CIDP separately. Of the 80 patients enrolled, 66 were identified as CIDP and are reported herein. Fourteen were non-CIDP immune-mediated peripheral neuropathies (predominately multifocal motor neuropathy or anti-myelin-associated glycoprotein (anti-MAG) neuropathy) that responded differently and will be reported separately. Before 2010, study eligibility was CIDP based on National Institutes of Neurologic Disease and Stroke (NINDS) CIDP criteria. Thereafter, study eligibility was modified to definite CIDP based on the European Federation Neurologic Societies/Peripheral Nerve Society (EFNS/PNS) criteria [2, 3]. Eligibility required dependence on or failure of at least two of three first-line treatments (corticosteroids, IVIG, or PLEX). Failure was defined as inability to tolerate treatment. Dependency was defined as unsatisfactory partial clinical response (persistent 3/5 weakness in one muscle or 4/5 in multiple muscles). An immunotherapy dependency test prior to enrollment was not mandated.
All patients underwent a radiographic skeletal survey, serum protein electrophoresis (SPEP), and immunoelectrophoresis (IEP), and those with monoclonal gammopathy or skeletal lesions underwent a bone marrow biopsy and aspirate to rule out multiple myeloma versus monoclonal gammopathy of undetermined significance (MGUS). General health exclusion criteria included age < 18 or > 65 years old, left ventricular ejection fraction < 40%, diffusing capacity for carbon monoxide (DLCO) < 40%, creatinine > 2 mg/dl, or hepatic transaminases or bilirubin greater than twice the upper limit of normal. After initiation, the protocol was amended to exclude patients with a history of cancer (except localized basal or squamous cell skin cancer), poorly controlled hypertension, hypertension-related end organ dysfunction, or sclerotic bone lesions.
Stem cell collection and transplant regimen
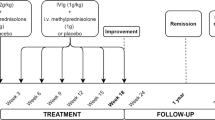
Peripheral blood stem cells (PBSC) were harvested and cryopreserved without manipulation 10 days after intravenous (IV) cyclophosphamide (2 g/m2) and 5 days after subcutaneous filgrastim (5–10 mcg/kg/day). The conditioning regimen was cyclophosphamide IV (50 mg/kg) on day − 5 to day − 2 and rATG (thymoglobulin) IV dosed at 0.5 mg/kg on day − 5, 1 mg/kg on days − 4 and − 3, and 1.5 mg/kg on days − 2 and − 1. After the second enrollee, all subsequent patients also received rituximab 500 mg/IV on days − 6 and + 1. PBSC were infused on day 0.
Supportive care guidelines
Blood products were irradiated, cytomegalovirus (CMV) safe, and leukocyte depleted. Filgrastim (5–10 mcg/kg/day) was started on day + 4 and continued until engraftment. Either intravenous cefepime or piperacillin–tazobactam was started prophylactically on day 0. Oral acyclovir was started upon admission and continued for 12 months after transplant. Patients received daily oral fluconazole and oral trimethoprim–sulfamethoxazole three times a week or monthly aerosolized pentamidine for six months. Hydration (125 ml/h 0.9% normal saline) and diuretics were continued for 24 h after each dose of cyclophosphamide. CMV was monitored post-transplant for 90 days and pre-emptively treated by switching from acyclovir to oral valganciclovir (900 mg twice daily) until negative.
Study end points
Patients were evaluated in Chicago by the study team at baseline, 6 months, 12 months, and then yearly thereafter for up to 5 years. Between visits, patients were in phone and email contact with the study team and also continued follow-up with their local neurologist. The primary outcome was survival. We initially listed normalization of strength in the absence of conduction blocks as a secondary outcome for remission. However, because randomized IVIG trials never reported nerve conduction study (NCS) findings as an endpoint, because improvement in strength and NCS may occur without complete normalization of either, and because slow gradual improvements in NCS parameters lag behind drug-free duration of remission, we define treatment-free remission as free from IVIG, PLEX, and all immunosuppressive medications with either a normal neurological examination or abnormal examination that was stable or improving [20]. Immediately after transplantation, immunomodulating medications were usually discontinued. In a minority of cases requiring multiple weekly dosing of IVIG, a more conservative ad hoc tapering strategy was employed. Other outcomes included changes in: Medical Research Council (MRC) sum score for muscle strength of six bilaterally evaluated muscle groups [21], nerve conduction velocity (NCV), compound motor action potential (CMAP), and quality of life by SF-36. After enrolling the first 20 patients, the Overall Neuropathy Limitation Scale (ONLS) [22], Rasch-built Overall Disability Scale (RODS) [23], Inflammatory Neuropathy Cause and Treatment (INCAT) Scale [24], and grip strength tests [25] were added.
Standard nerve conduction studies (NCS) included median, ulnar, and peroneal motor responses. The first 11 enrolled patients had unilateral recordings at the pre-transplant evaluation. The remaining patients underwent bilateral limb NCS. Averaged CMAP amplitude was calculated for each subject by summing all amplitude measurements recorded and dividing by the total number of nerves sampled. For NCV, the tibial nerves that are prone to compression and nerves that could not be obtained were excluded.
Statistical analysis
The study began as a phase I trial that was subsequently extended in order to capture phase II efficacy data on a larger group of patients with a presumed immune-mediated polyneuropathy that was not limited to CIDP. Statistical analysis was performed by Wilcoxon signed-rank test for all outcome measures except for quality of life SF-36 that was analyzed by paired 2 tailed student T test (Microsoft excel 2017). Wilcox signed-ranked test and the paired 2 tail T test were always performed on paired samples with the same number of subjects being compared between time points. A repeated-measures ANCOVA model was also fit to evaluate effect of age on change over time.
Results
Eligibility (Fig. 1)
Over a 10-year interval, 216 consecutive patients were reviewed. Upon evaluation, 96 patients were excluded for a neurology diagnosis other than definitive CIDP, and 40 patients were excluded for a non-neurologic diagnosis or medical contraindication. Fourteen patients were enrolled for non-CIDP peripheral neuropathies presumed to be immune-mediated: multifocal motor neuropathy (MMN) 4, anti-MAG neuropathy 4, chronic immune sensory polyradiculopathy 2, and 1 each for chronic ataxic ophthalmoplegia IgM paraprotein cold agglutinins disialosyl antibodies (CANOMAD), autoimmune autonomic ganglionopathy (AAG), paraneoplastic peripheral neuropathies, and myopathy with undefined peripheral neuropathy. These patients will be reported separately due to variability in response, e.g. MMN and paraneoplastic neuropathy related to a previously irradiated plasmacytoma had no response. Sixty-six patients were transplanted, 12 before 2010 based on the NINDS criteria and 54 on or after 2010 based on the EFNS/PNS criteria.
Of the 66 patients transplanted for CIDP, six patients never reached the first 6-month evaluation and without follow-up documentation were subsequently excluded from analysis. Of these, two patients who had pre-transplant sclerotic bone lesions but normal bone marrow biopsies developed POEMS disease within months of HSCT and after referral for a melphalan-based myeloma transplant regimen did not return for follow-up but remain alive. Two patients did not return for follow-up, one relapsed within 6 months and restarted IVIG but remains alive, and one patient moved and was lost to follow-up. Two patients died from non-transplant related conditions that were present before transplant. Both deaths occurred more than 100 days after hospital discharge when routine laboratories are no longer drawn or followed. One with hypertension-related cardiac diastolic dysfunction, hypertension-related renal proteinuria, a history of medication non-compliance, and poorly controlled hypertension was discovered unresponsive at home. The death certificate listed the cause of death as cardiovascular. The other patient who was treated with mantle irradiation and chemotherapy for Hodgkin’s lymphoma ten years before HSCT, died eight months post-transplant from signet ring gastric cancer occurring within the prior radiation port. Before HSCT, the patient reported epigastric discomfort and endoscopy revealed giant stomach folds but a biopsy was not performed. In retrospect, the patient had pre-transplant linitis plastica from adenocarcinoma due to prior mantle field irradiation for Hodgkin’s lymphoma. Both deaths were formally reported to the FDA and were accepted as being non-treatment related.
After two patients died from pre-HSCT coexisting diseases (hypertension, prior Hodgkin’s radiation induced signet ring carcinoma) present before transplant, and two patients with isolated sclerotic bone lesions developed POEMS, the protocol was amended to exclude poorly controlled hypertension, prior malignancy or plasmacytoma, or isolated sclerotic skeletal lesions.
One patient, who did not respond to HSCT and whose NCV and CMAP continued to gradually decline at 6 months and at 1 and 2 years after transplant, was diagnosed at 3 years with Charcot Marie Tooth (CMT) 1A hereditary polyneuropathy due to PMP22 gene duplication [26]. Pre-transplant the patient met the EFNS/PNS criteria for definite CIDP, had elevated CSF protein, and a history of a good response to IVIG. Because the patient returned for post-HSCT follow-up, the data were not censored but included in an intention-to-treat analysis despite, in retrospect, having the wrong diagnosis, i.e. CMT1A.
Baseline demographic and diagnostic data (Table 1).
The patients had a mean and median age of 43 years (range 20–63 years old), sex ratio of 23 females to 37 males, and a mixed ethnic origin (52 Caucasian, 4 Hispanic, 2 Asian, and 2 African American). The mean/median duration of symptoms before undergoing HSCT was 80/57 months (range 9–348 months). Fifty-four patients had typical and six atypical CIDP [MADSAM four, distal acquired demyelinating symmetrical (DADS) one, motor dominant one]. Disease onset was insidious in 40, acute in 16, and acute relapsing in 4. Before HSCT, all patients had been treated with IVIG and 47% (28/60) with PLEX. Numerous other immune-based medications (azathioprine, mycophenolate mofetil, rituximab, cyclophosphamide, methotrexate, and cyclosporine) had also been tried (Table 1).
Safety/toxicity (Table 2)
There were no treatment-related deaths in the 66 patients who underwent HSCT for definitive CIDP. Three patients experienced grade 4 toxicities: one patient required total parenteral nutrition for vomiting, one required potassium replacement for asymptomatic hypokalemia, and one required bilevel positive airway pressure for transient dyspnea. Grade 3 electrolyte toxicities were: hypophosphatemia 29/66 (44%), hypokalemia 19/66 (29%), and 5/66 (7%) for hypocalcemia, hyponatremia, and hyperglycemia. Infections during hospitalization were four clostridium difficile, one respiratory syncytial virus, one rhinovirus, one sinusitis, and one subcutaneous abscess. There were no positive blood cultures except one case of coagulase negative staphylococcus thought to be a skin contaminant. Other grade 3 toxicities include dyspnea in eight patients, hypotension and hypertension in three patients each, elevated transaminases in two patients, and one patient each had atrial fibrillation, syncope, deep vein thrombosis (DVT), or sinus tachycardia requiring intravenous fluids, and one allergic drug reaction treated with diphenhydramine. The mean day of discharge was day + 11 after stem cell infusion. There were no early or late post-transplant fungal, Pneumocystis jirovecii, cytomegalovirus, Epstein Barr virus, or John Cunningham (JC) virus infections.
In the 60 patients with a mean follow-up of 4.5 years and in whom all except one had three years of follow-up, post-hospital discharge infectious events (number of patients) were: oral antibiotics for upper respiratory tract infections or sinusitis [19], oral acyclovir for cutaneous varicella zoster [12], pneumonia (11—of whom 5 were treated with intravenous antibiotics), influenza treated with oral oseltamivir [8], urinary tract infection [5], Clostridium difficile diarrhea [2], cutaneous skin abscess [1], Streptococcus bacteremia [1], Helicobacter pylori gastritis [1]. Two patients underwent post-transplant cholecystectomies, two developed hypothyroidism and two hyperthyroidism, and one patient developed Parkinson’s disease. Three patients had post-transplant immunoglobulin deficiency and restarted either subcutaneous immunoglobulin or IVIG to prevent infections. Two of the three were immunodeficient before transplant with hypogammaglobulinemia and low peripheral blood B cells, and in one case prior rituximab therapy.
Immune medication-free remission
Remission defined as free of all immune modulating or suppressive drugs with stable or improving neurological exam was 80% (48/60) at 6 months, 80% (48/60) at 1 year, 78% at 2 years (47/60), 76% (45/59) at 3 years, 78% (39/50) at 4 years and 83% (35/42) at 5 years (Fig. 2a). Of interest, prior history of response to immune-based therapy did not predict outcome since four of six patients who did not respond to immune-based therapy before HSCT improved after HSCT and have not relapsed since transplant. Although all patients had previously received immunoglobulin, forty-seven patients were receiving IVIG regularly before HSCT. Forty patients discontinued IVIG during transplant admission, two discontinued IVIG by 6 months, two by 1-year, two by 18 months, and one patient remained IVIG dependent. Seven patients receiving PLEX regularly before HSCT discontinued this treatment the day of transplant admission and have remained PLEX-free. Twenty-six patients were on corticosteroids regularly before HSCT and in order to avoid adrenal insufficiency were gradually tapered off it with three patients remaining on adrenal maintenance dosage (prednisone 5–7.5 mg/day) at 6 months. Thirteen patients on mycophenolate mofetil, two patients on rituximab, one on cyclosporine, two on azathioprine, one on cyclophosphamide, and two on methotrexate had those medications discontinued the day of transplant admission. During the 5 years of follow-up, eleven of the sixty patients (18%) restarted some form of immune-based therapy for CIDP such as IVIG, subcutaneous immunoglobulin, PLEX, or rituximab.
a Percentage of patients free of all immune therapies after HSCT. b Percent and type of ambulatory assistance requiring to ambulate before and after HSCT. a @Remission is defined as free of immune modulating or immune suppressive drugs with stable or improving neurological exam. Before HSCT, no patients were in remission. b AFO ankle–foot orthosis, HSCT hematopoietic stem cell transplantation
Ambulatory assistance
Despite discontinuation of medications, patients who required no assistance to ambulate improved from 32% (19/60) pre-transplantation to 68% (41/60) at 6 months, 82% (49/60) at 1 year, 82% (49/60) at 2 years, 81% (48/59) at 3 years, 86% (43/50) at 4 years, and 83% (35/42) at 5 years (Fig. 2a). The percent of patients requiring a wheelchair decreased from 22% pre-HSCT to 0%, 0%, 0%, 2%, 0%, and 0% at 6 months and 1, 2, 3, 4, and 5 years, respectively (Fig. 2b).
Requirement of a cane decreased from 28% pre-HSCT to 13%, 7%, 5%, 3%, 0%, and 2% at 6 months, 1, 2, 3, 4, and 5 years, respectively. As patients were liberated from using wheelchairs, walkers, and canes, the requirement for AFOs increased from 7% pre-HSCT to between 8 and 12% from 6 months to 5 years after HSCT, respectively (Fig. 2b).
Disability and strength functional scales
The MRC sum score for muscle strength improved significantly (p < 0.001) at all time points from a mean (median) of 51.8 (53.5) pre-HSCT to 54.8 (58) at 6 months, and 57 (median between 59 and 60) at 1, 2, 3, 4, and 5 years (interquartile ranges are shown in Fig. 3a). The sum INCAT score [24] improved significantly (p < 0.001) for all time intervals from a pre-HSCT mean (median) of 4.4 (4) to 3.1 (3) at 6 months, 2.1 (2) at 1 year, 2.1 (2) at 2 years, 2.1 (2) at 3 years, 2.0 (1) at 4 years, and 1.8 (1) at 5 years (interquartile ranges are shown in Fig. 3b). The total ONLS and R-ODS centile metric score are not shown due to redundant and similar significant improvements to the INCAT.
Pre- and post-transplant physical assessment and disability scores. Box whisker plots: 1st quartile is lower box line, median is middle line within the box, 3rd quartile is top line of box, X = mean, whiskers are minimum and maximum, a small circle represents an outlier. CMAP compound motor action potential, INCAT inflammatory neuropathy cause and treatment disability score, kg kilogram, m months, MRC Medical Research Council., m/s meters per second, pre before hematopoietic stem cell transplantation, NCV nerve conduction velocity, y year
Mean (median) right hand grip strength (kg) improved significantly from 18 (18) pre-transplant to 22.7 (22) at 6 months (p = 0.009), 26.3 (25.5) at 1 year (p = 0.003), 29.2 (27) at 2 years (p < 0.0001), 28.8 (31) at 3 years (p < 0.0001), 30.4 (30) at 4 years (p < 0.0001), and 30.8 (31) at 5 years (p = 0.002) (interquartile ranges are shown Fig. 3c). Mean (median) left hand grip strength (kg) improved from 16.6 (15) pre-transplant to 21.8 (20.4) at 6 months (p = 0.02), 25.4 (25.6) at 1 year (p = 0.001), 28.2 (29) at 2 years (p < 0.0001), 28.6 (29) at 3 years (p < 0.0001), 25.5 (27) at 4 years (p < 0.0001), and 29.1 (29) at 5 years (p = 0.0002) (interquartile ranges are shown Fig. 3d).
Electrophysiology
The mean (median) NCV (m/s) for all nerves improved significantly (p < 0.0001) at all time points from 27.2 (23.7) pre-transplant to 30.5 (29.8) at 6 months, 33.5 (35.8) at 1 year, 33.8 (34) at 2 years, 37.7 (38.5) at 3 years, 38.1 (38.1) at 4 years, and 38.3 (37.1) at 5 years post-transplant (interquartile ranges are shown Fig. 3e). CMAP amplitude (millivolts) for all nerves improved from a mean (median) of 3.55 (2.95) pre-HSCT to 3.69 (3.4) at 6 months (p = 0.39), 4.63 (4.6) at 1 year (p = 0.0002), 4.58 (4.9) at 2 years (p < 0.0001), 5.04 (4.8) at 3 years (p < 0.0001), 5.07 (5.2) at 4 years (p < 0.0001), and 4.12 (4.07) (p = 0.002) at 5 years post-transplant (interquartile ranges are shown Fig. 3f).
Effect of age correction and multiple comparisons on outcome
A repeated-measures ANCOVA model revealed that age did not affect outcome. For example, type III p values were obtained from a repeated-measures ANCOVA, involving 463 observations, assuming a compound symmetry within person correlation, and involving groups CMAP and NCV. Values included all time points from baseline to 60 months. This model was adjusted for age, but age was not significant (p = 0.92). For multiple comparisons, Bonferroni correction and repeated-measures ANCOVA led to the same conclusions regarding significant differences over time.
Quality of life (Table 3)
Patients’ quality of life as measured by the SF-36 physical, mental, and total score improved significantly (p < 0.001) at all post-transplant evaluations. Total quality of life improved (all p values < 0.001) from pre-HSCT mean (median) of 39.20 (39.31) to 64.01 (64.63), 63.89 (63.53), 69.72 (76.54), 65.89 (70.47), and 72.71 (82.5) at 1, 2, 3, 4, and 5 years, respectively.
Discussion
When using the word “transplantation” in the terminology hematopoietic stem cell transplantation (HSCT), the rationale of HSC infusion should not be confused with mesenchymal stem cell (MSC) or neural stem cell (NSC) transplantation that are performed without a conditioning regimen (i.e. no chemotherapy or biologics) as MSC and NSC trials are instigated for an independent NSC neuro-regenerative effect or a stand-alone MSC immune suppressive effect. In comparison, HSCs, by themselves, have no beneficial or clinical stand-alone neuro-regenerative or immune suppressive effects. HSCs are infused for their normal homologous purpose, i.e. blood transfusion support to hasten recovery of hematopoiesis after chemotherapy and biologics (conditioning regimen). Toxicity and efficacy of autologous HSCT arises from the immune suppressive conditioning regimen and patient selection, not the autologous HSC per se. For diseases that are associated with pathologic autoantibodies such as neuromyelitis optica or disease suspected to be associated with such autoantibodies, we found, when using an unmanipulated graft, that the addition of rituximab to cyclophosphamide and ATG induced remission in most patients [27].
Prior publications of autologous HSCT for CIDP, although generally encouraging, were small case reports [28,29,30,31], included heterogeneous neuropathies such as POEMS [32], were reporting different conditioning regimens, or evaluated retrospectively to fulfil CIDP criteria [33]. Using patients referred for this study with an outside diagnosis of “CIDP,” one of our neurologists previously reported that CIDP is frequently misdiagnosed [34]. In our prospective study, after 2010, minimization of diagnostic errors was accomplished by strict adherence to EFNS/PNS CIDP diagnostic criteria and by exclusion of patients with any diagnostic uncertainty or those with a potential alternative neuropathic etiology [35]. Despite the rigor of the EFNS/PNS exclusion criteria, two patients with pre-HSCT sclerotic bone lesions and a normal bone marrow biopsy were found to have a polyneuropathy due to a plasma cell dyscrasia (POEMS), and the protocol was subsequently amended to exclude patients with sclerotic bone lesions. In addition, despite rigorously screening for definite CIDP by EFNS/PNS criteria, NCS of one patient, who is included in this analysis, continued to gradually decline after treatment following which genetic analysis confirmed the diagnosis of CMT1A. Formal screening for hereditary neuropathies was not performed in this trial or to our knowledge in the IVIG or PLEX trials for CIDP in part because of the expense and potential failure to exclude other yet unknown genetic abnormalities. However, this study suggests that failure to respond to the intense immune suppressive effect of autologous HSCT should raise the question not of therapy failure for CIDP, but rather of a misdiagnosis.
We found no statistically significant differences in response based on patient age, gender, illness duration, MGUS status, baseline nerve conduction studies, or typical or atypical CIDP. Due to the limited number of patients with atypical CIDP: (4 MADSAM, and one each with, motor predominate and sensory predominate), it is difficult to draw conclusions on outcome for atypical CIDP. However, all four MADSAM variants responded.
In the landmark IVIG CIDP Efficacy (ICE) trial [11], 59 patients were randomized to IVIG and 58 to placebo. In the prednisolone versus dexamethasone in chronic inflammatory demyelinating polyradiculopathy trial (PREDICT) [36], 24 patients were randomized to dexamethasone and 16 to prednisolone. In the Polyneuropathy And Treatment with Hizentra® (PATH) trial, patients were randomized to either low (57 patients) or high (58 patients) dose weekly subcutaneous immunoglobulin (SCIg) [37]. In both the ICE and PREDICT trials, the majority of patients had not been exposed to prior IVIG. In the PATH trial, the majority of patients had previously received at least four doses of IVIG over a period of 9 months [37]. In comparison with ICE, PREDICT, and PATH trials, our study enrolled a similar number of patients, but unlike the other trials cohorts, our patients that underwent HSCT had persistent neurological deficits despite being maintained on chronic IVIG or PLEX, and the majority of our patients had exposure to multiple other immune suppressive drugs (azathioprine, mycophenolate mofetil, rituximab, cyclophosphamide, methotrexate, and cyclosporine) and needed assistance (wheelchair, walker, cane, or ankle–foot orthosis) to ambulate.
The goal of HSCT, unlike other immune-based therapies, is to become and remain free from all immune modulating or suppressive drugs after a one-time treatment. To this end, the primary end point after HSCT is the percentage of patients remaining immune therapy free after HSCT which in this study was 76–83% over a 5-year interval. The outcome of the clinical examination was based on formal assessments during the follow up visits and a relapse was defined as any clinical worsening, based on the assessment of either the local neurologist or the study neurologist, necessitating restarting any immune therapy. Therefore, the outcome measure used in this study is more stringent than those used in other CIDP studies which have depended on detecting change to the INCAT score while continuing therapies. During 5 years of follow-up, eleven out of the sixty patients (18%) relapsed and restarted some form of immune-based therapy. In comparison, the primary outcome of the PATH immunoglobulin trial [37] was relapse defined as an increase in the INCAT by 1 or more points while staying on subcutaneous immunoglobulin and although the overall INCAT and MRC sum scores remained unchanged, grip strength declined while receiving low or high dose subcutaneous immunoglobulin [37]. Similarly, the main outcome in the ICE study was a 1 point change in INCAT [11]. In contrast, in our study besides the primary outcome of the percentage of patients remaining immune drug free, all secondary outcomes including INCAT, MRC sum score, grip strength, nerve conduction velocities, and compound motor action potentials all improved. Neither ICE [11] nor PATH [37] studies reported the outcome of the electrophysiological assessments.
After completing this trial an extended period of time (mean 4.5 years) passed in order to gather longer-term outcomes and to confirm the durability of results before publication. From current IVIG trials (11, 36,37,38), retrospective series of IVIG [39] or heterogeneously treated CIDP patients, it appears that two-thirds of patients who are treated for CIDP relapse or remain dependent on long-term IVIG. In this trial, approximately 80% of patients were free from any immune-based therapy for up to 5 years.
The most significant unexpected long-term toxicity after HSCT using this regimen was hypogammaglobulinemia in three of 60 patients; however, two of the patients were already B cell deficient before transplant. Hypogammaglobulinemia has been documented to persist for up to 2 years after exposure to rituximab [40] and was probably associated with addition of rituximab in the conditioning regimen.
The limitations of this open label study include possible unblinded investigator bias, inconsistency related to the fact that the university neurology team evaluating the patients varied over the duration of the trial, lack of a mandated immunotherapy dependency test before entry into the trial, and the absence of a control arm. Unlike the ICE trial, the PATH trial performed an immune dependency test and reported that 28 of 245 (10%) patients were not IVIG dependent, i.e. did not decline off IVIG, and as such were excluded from the trial because they would be unlikely to respond. Our results demonstrated marked improvements in the study cohort after HSCT despite stopping IVIG or PLEX after transplantation. While no immunotherapy dependency test was performed, should our results have simply been a consequence of stopping unnecessary immunotherapy in patients with inactive disease, we would not have expected subsequent improvements in strength (MRC sum score, grip strength), electrophysiology (NCV, CMAP), and disability (INCAT). Had we enrolled patients with inactive disease and permanent deficits who would have been stable without IVIG, this would have biased our results towards a negative outcome, i.e. no improvement after HSCT.
We reported NCS findings in terms of overall mean changes in NCV and CMAP. In comparison, the landmark ICE [11] and PATH [37] immunoglobulin studies did not report any NCS changes. Furthermore, following HSCT, the majority of patients who required assistance to ambulate regained their ambulation independence (Fig. 2b). The slight increase in assisted ambulation using AFOs reflected gait improvement, i.e. becoming independent of a wheelchairs, walkers, or canes. Post-HSCT medication-free remission was approximately 80% , and this is unlikely to have been secondary to different study neurologists or to clinician bias because the threshold for re-initiation of immunotherapy after HSCT, i.e. namely CIDP relapse, was low and patients could have immunotherapy restarted at the discretion of the study team or their independent local neurologist’s judgment. These results are supported not only by qualitative physician clinical assessments but also by objective grip strength and quantitative electrophysiologic (NCV and CMAP) measurements.
Similar to our previous work in multiple sclerosis in which we reported the results of phase I [41, 42] and II [43] trials before initiating and subsequently reporting on a randomized phase III trial in relapsing–remitting multiple sclerosis [44], we report herein the safety and efficacy of a phase I/II trial in CIDP as rationale, justification, and foundation for a randomized trial.
Finally, the economic burden from CIDP in the USA for each patient on IVIG was $108,016 per year in 2014 [45] and $165,000 per year in 2017 [46]. The mean HSCT charges for CIDP using the regimen utilized herein are approximately $125,000 (manuscript in development). Since HSCT is a one-time treatment in which approximately 76–83% remain free of all immune drugs including IVIG for 5 years, this therapy may be cost effective especially when analyzed over long-term treatment costs. While this therapy requires committed expertise within the fields of hematologic stem cell transplantation and neurology, most university have the necessary components for this treatment [47].
Summary
When used as a salvage therapy in patients with CIDP dependent on IVIG or PLEX, non-myeloablative HSCT can improve ambulation, hand grip strength, NCV and CMAP amplitude, and quality of life, as well as provide long-term independence from conventional immune-based therapies. Further investigation is warranted.
Availability of data and material
All data and materials support the authors’ published claims and comply with field standards.
References
Vallat JM, Sommer C, Magy L (2010) Chronic inflammatory demyelinating polyradiculoneuropathy: diagnostic and therapeutic challenges for a treatable condition. Lancet Neurol 9(4):402–412
Hughes RA, Bouche P, Cornblath DR, Evers E, Hadden RD, Hahn A, Illa I, Koski CL, Léger JM, Nobile-Orazio E, Pollard J, Sommer C, Van den Bergh P, van Doorn PA, van Schaik IN (2006) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 13(4):326–332
Van den Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, Koski CL, Léger JM, Nobile-Orazio E, Pollard J, Sommer C, van Doorn PA, van Schaik IN, European Federation of Neurological Societies; Peripheral Nerve Society (2010) European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—first revision. Eur J Neurol. 17(3):356–363
Peltier AC, Donofrio PD (2012) Chronic inflammatory demyelinating polyradiculoneuropathy: from bench to bedside. Semin Neurol 32:187–195
Hughes RA, Allen D, Makowska A, Gregson NA (2006) Pathogenesis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst 11(1):30–46
Dalakas MC (2011) Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol 7(9):507–517
Köller H, Kieseier BC, Jander S, Hartung HP (2005) Chronic inflammatory demyelinating polyneuropathy. NEJM 352(13):1343–1356
Nobile-Orazio E, Cocito D, Jann S, Uncini A, Beghi E, Messina P, Antonini G, Fazio R, Gallia F, Schenone A, Francia A, Pareyson D, Santoro L, Tamburin S, Macchia R, Cavaletti G, Giannini F, Sabatelli M, IMC Trial Group (2012) Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurol. 11(6):493–502
Dyck PJ, O'Brien PC, Oviatt KF, Dinapoli RP, Daube JR, Bartleson JD, Mokri B, Swift T, Low PA, Windebank AJ (1982) Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann Neurol 11(2):136–141
Mendell JR, Barohn RJ, Freimer ML, Kissel JT, King W, Nagaraja HN, Rice R, Campbell WW, Donofrio PD, Jackson CE, Lewis RA, Shy M, Simpson DM, Parry GJ, Rivner MH, Thornton CA, Bromberg MB, Tandan R, Harati Y, Giuliani MJ, Working Group on Peripheral Neuropathy (2001) Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 56(4):445–449
Hughes RA, Donofrio P, Bril V, Dalakas MC, Deng C, Hanna K, Hartung HP, Latov N, Merkies IS, van Doorn PA, ICE Study Group (2008) Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): a randomised placebo-controlled trial. Lancet Neurol. 7(2):136–144
Dyck PJ, Daube J, O'Brien P, Pineda A, Low PA, Windebank AJ, Swanson C (1986) Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N Engl J Med 314(8):461–465
Cocito D, Paolasso I, Antonini G, Benedetti L, Briani C, Comi C, Fazio R, Jann S, Matà S, Mazzeo A, Sabatelli M, Nobile-Orazio E, Italian Network for CIDP Register (2010) A nationwide retrospective analysis on the effect of immune therapies in patients with chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 17(2):289–294
Viala K, Maisonobe T, Stojkovic T, Koutlidis R, Ayrignac X, Musset L, Fournier E, Léger JM, Buche P (2010) A current view of the diagnosis, clinical variants, response to treatment and prognosis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 15(1):50–56
Gorson KC, Allam G, Ropper AH (1997) Chronic inflammatory demyelinating polyneuropathy: clinical features and response to treatment in 67 consecutive patients with and without monoclonal gammopathy. Neurology 48:321–328
Kuitwaard K, van Doorn PA (2009) Newer therapeutic options for chronic inflammatory demyelinating polyradiculoneuropathy. Drugs 29(69):987–1001
Mahdi-Rogers M, McCrone P, Hughes RA (2014) Economic costs and quality of life in chronic inflammatory neuropathies in southeast England. Eur J Neurol 21(1):34–39
Robertson EE, Donofrio PD (2010) Treatment of chronic inflammatory demyelinating polyneuropathy. Curr Treat Options Neurol 12(2):84–94
Blackhouse G, Gaebel K, Xie F, Campbell K, Assasi N, Tarride JE, O'Reilly D, Chalk C, Levine M, Goeree R (2010) Cost-utility of intravenous immunoglobulin (IVIG) compared with corticosteroids for the treatment of chronic inflammatory demyelinating polyneuropathy (CIDP) in Canada. Cost Eff Resour Alloc 8:14
Gorson KC, van Schaik IN, Merkies IS, Lewis RA, Barohn RJ, Koski CL, Cornblath DR, Hughes RA, Hahn AF, Baumgarten M, Goldstein J, Katz J, Graves M, Parry G, van Doorn PA (2010) Chronic inflammatory demyelinating polyneuropathy disease activity status: recommendations for clinical research standards and use in clinical practice. J Peripher Nerv Syst 15(4):326–333
Kleyweg RP, van der Meché FG, Schmitz PI (1991) Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain–Barré syndrome. Muscle Nerve 14(11):1103–1109
Graham RC, Hughes RAC (2006) A modified peripheral neuropathy scale: the overall neuropathy limitations scale. J Neurol Neurosurg Psychiatry 77:973–976
van Nes SI, Vanhoutte EK, van Doorn PA, Hermans M, Bakkers M, Kuitwaard K et al (2011) Rasch-built overall disability scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology 76:337–345
Hughes R, Bensa S, Willison H, Van den Bergh P, Comi G, Illa I, Nobile-Orazio E, van Doorn P, Dalakas M, Bojar M, Swan A (2001) Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculopathy. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Ann Neurol. 50(2):195–201
Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, Casaer MP, Wouters P, Gosselink R, Van Den Berghe G (2012) Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve 45(1):18–25
van Paassen BW, van der Kooi AJ, van Spaendonck-Zwarts KY, Verhamme C, Baas F, de Visser M (2014) PMP22 related neuropathies: charcot-marie-tooth disease type 1A and hereditary neuropathy with liability to pressure palsies. Orphanet J Rare Dis. 19(9):38
Burt RK, Balabanov R, Han X, Burns C, Gastala J, Jovanovic B, Helenowski I, Jitprapaikulsan J, Fryer JP, Pittock SJ (2019) Autologous nonmyeloablative hematopoietic stem cell transplantation for neuromyelitis optica. Neurology. 93(18):e1732
Oyama Y, Sufit R, Loh Y, Statkute L, Yaung K, Quigley K, Gonda E, Spahovic D, Bronesky D, Burt RK (2007) Nonmyeloablative autologous hematopoietic stem cell transplantation for refractory CIDP. Neurology. 69(18):1802–1803
Vermeulen M, Van Oers MH (2002) Successful autologous stem cell transplantation in a patient with chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry 72(1):127–128
Axelson HW, Oberg G, Askmark H (2009) Successful repeated treatment with high dose cyclophosphamide and autologous blood stem cell transplantation in CIDP. BMJ Case Rep. https://doi.org/10.1136/bcr.09.2008.0927
Scheibe F, Alexander T, Prüss H, Wengert O, Harms L, Angstwurm K, Hiepe F, Arnold R, Meisel A (2016) Devastating humoral CIDP variant remitted by autologous stem cell transplantation. Eur J Neurol 23(3):e12–e14. https://doi.org/10.1111/ene.12896
Mahdi-Rogers M, Kazmi M, Ferner R, Hughes RA, Renaud S, Steck AJ, Fuhr P, Halter J, Gratwohl A, Tyndall A (2009) Autologous peripheral blood stem cell transplantation for chronic acquired demyelinating neuropathy. J Peripher Nerv Syst 14(2):118–124
Press R, Askmark H, Svenningsson A, Andersen O, Axelson HW, Strömberg U, Wahlin A, Isaksson C, Johansson JE, Hägglund H (2014) Autologous haematopoietic stem cell transplantation: a viable treatment option for CIDP. J Neurol Neurosurg Psychiatry 85(6):618–624
Allen JA, Lewis RA (2015) CIDP diagnostic pitfalls and perception of treatment benefit. Neurology 85(6):498–504
Allen JA, Ney J, Lewis RA (2018) Electrodiagnostic errors contribute to chronic inflammatory demyelinating polyneuropathy misdiagnosis. Muscle Nerve 57(4):542–549. https://doi.org/10.1002/mus.25997
van Schaik IN, Eftimov F, van Doorn PA, Brusse E, van den Berg LH, van der Pol WL, Faber CG, van Oostrom JC, Vogels OJ, Hadden RD, Kleine BU, van Norden AG, Verschuuren JJ, Dijkgraaf MG, Vermeulen M (2010) Pulsed high-dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double-blind, randomised, controlled trial. Lancet Neurol 9(3):245–253
Van Schaik IN, Bril V, van Geloven N, Hartung HP, Lewis RA, Sobue G, Lawo JP, Praus M, Mielke O, Durn BL, Cornblath DR, Merkies ISJ, PATH Study Group (2018) Subcutaneous immunoglobulin for maintenance treatment in chronic inflammatory demyelinating polyneuropathy (PATH): a randomized, double blinded, placebo controlled, phase 3 trial. Lancet Neurol 17:35–46
Eftimov F, Vermeulen M, van Doorn PA, Brusse E, van Schaik IN (2012) PREDICT Long-term remission of CIDP after pulsed dexamethasone or short-term prednisolone treatment. Neurology 78(14):1079–1084
Querol L, Rojas-Garcia R, Casasnovas C, Sedano MJ, Muñoz-Blanco JL, Alberti MA, Paradas C, Sevilla T, Pardo J, Capablo JL, Sivera R, Guerrero A, Gutierrez-Rivas E, Illa I (2013) Long-term outcome in chronic inflammatory demyelinating polyneuropathy patients treated with intravenous immunoglobulin: a retrospective study. Muscle Nerve 48(6):870–876
Chiou FK, Beath SV, Patel M, Gupte GL (2019) Hypogammaglobulinemia and bacterial infections following pediatric post-transplant lymphoproliferative disorder in the rituximab era. Pediatr Transplant. 17:e13519. https://doi.org/10.1111/petr.13519(Epub ahead of print)
Burt RK, Cohen BA, Russell E, Spero K, Joshi A, Oyama Y, Karpus WJ, Luo K, Jovanovic B, Traynor A, Karlin K, Stefoski D, Burns WH (2003) Hematopoietic stem cell transplantation for progressive multiple sclerosis: failure of a total body irradiation-based conditioning regimen to prevent disease progression in patients with high disability scores. Blood 102(7):2373–2378
Burt RK, Loh Y, Cohen B, Stefoski D, Stefosky D, Balabanov R, Katsamakis G, Oyama Y, Russell EJ, Stern J, Muraro P, Rose J, Testori A, Bucha J, Jovanovic B, Milanetti F, Storek J, Voltarelli JC, Burns WH (2009) Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol 8(3):244–253
Burt RK, Balabanov R, Han X, Sharrack B, Morgan A, Quigley K, Yaung K, Helenowski IB, Jovanovic B, Spahovic D, Arnautovic I, Lee DC, Benefield BC, Futterer S, Oliveira MC, Burman J (2015) Association of nonmyeloablative hematopoietic stem cell transplantation with neurological disability in patients with relapsing-remitting multiple sclerosis. JAMA 313(3):275–284
Burt RK, Balabanov R, Burman J, Sharrack B, Snowden JA, Oliveira MC, Fagius J, Rose J, Nelson F, Barreira AA, Carlson K, Han X, Moraes D, Morgan A, Quigley K, Yaung K, Buckley R, Alldredge C, Clendenan A, Calvario MA, Henry J, Jovanovic B, Helenowski IB (2019) Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: a randomized clinical trial. JAMA 321(2):165–174
Guptill JT, Bromberg MB, Zhu L, Sharma BK, Thompson AR, Krueger A, Sanders DB (2014) Patient demographics and health plan paid costs in chronic inflammatory demyelinating polyneuropathy. Muscle Nerve 50(1):47–51
Divino V, Mallick R, DeKoven M, Krishnarajah G (2018) The Economic Burden of CIDP in the United States: a case-control study. PLoS ONE 13(10):e0206205 (Epub only)
Sharrack B, Saccardi R, Alexander T, Badoglio M, Burman J, Farge D, Greco R, Jessop H, Kazmi M, Kirgizov K, Labopin M, Mancardi G, Martin R, Moore J, Muraro PA, Rovira M, Sormani MP, Snowden JA European Society for Blood, and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP), and the Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT), and EBMT (JACIE) (2020) Autologous haematopoietic stem cell transplantation and other cellular therapy in multiple sclerosis and immune-mediated neurological diseases: updated guidelines and recommendations from the EBMT Autoimmune Diseases Working Party (ADWP) and the Joint Accreditation Committee of EBMT and ISCT (JACIE). Bone Marrow Transplant 55(2):283–306
Acknowledgements
We wish to thank Professor Richard A. C. Hughes MD, Cochrane Neuromuscular Disease Review Group, MRC Centre of Neuromuscular Diseases, institute of Neurology, University Colleague London, London, UK, for his thoughtful discussions and comments. This study was made possible by financial support from the Danhakl family, the McNamara Purcell Foundation, and Morgan Stanley and Company. The principal investigator (RKB), statisticians (IBH, BJ), and fellow (XH) had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by RKB, XH, KQ, IA, and IH. The first draft of the manuscript was written by RKB, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest except the following author. Rouman Balabanov—Scientific Advisory Boards-Biogen, Sanofi-Genzyme, Genentech, Alexion Speakers' Bureaus–Biogen Idec, Sanofi-Genzyme.
Ethics approval
Patients enrolled in this open-label prospective study were treated at Northwestern Memorial Hospital (Chicago, Illinois, USA) on an IRB and FDA approved protocol (www.clinicaltrials.gov NCT00278629) and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
All persons gave their informed consent prior to their inclusion in the study.
Rights and permissions
About this article
Cite this article
Burt, R.K., Balabanov, R., Tavee, J. et al. Hematopoietic stem cell transplantation for chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol 267, 3378–3391 (2020). https://doi.org/10.1007/s00415-020-10010-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-10010-6