Abstract
While the epidemic of Coronavirus disease 2019 (COVID-19) continues to spread globally, more and more evidences are collected about the presence of neurological manifestations and symptoms associated with it. A systematic review has been performed of papers published until 5 April 2020. 29 papers related to neurological manifestations associated with COVID-19 were examined. The results show presence of central and peripheral nervous system manifestations related to coronavirus. Neurological manifestations, or NeuroCOVID, are part of the COVID-19 clinical picture, but questions remain regarding the frequency and severity of CNS symptoms, the mechanism of action underlying neurological symptoms, and the relationship of symptoms with the course and severity of COVID-19. Further clinical, epidemiological, and basic science research is urgently needed to understand and address neurological sequalae of COVID-19. Concomitant risk factors or determinants (e.g. demographic factors, comorbidities, or available biomarkers) that may predispose a person with COVID-19 to neurological manifestations also need to be identified. The review shows that although more and more papers are reporting neurological manifestations associated with COVID-19; however, many items remain unclear and this uncertainty calls for a global action that requires close coordination and open-data sharing between hospitals, academic institutions and the fast establishment of harmonised research priorities and research consortia to face the NeuroCOVID-19 complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reports are emerging from China and Italy and increasingly from several countries of neurological symptoms associated with SARS-CoV-2, which may be worsening clinical pictures, respiratory outcomes and mortality rates in patients with COVID-19. While most coronaviruses cause mild respiratory illness, it is well known that many beta-coronaviruses have nervous system involvement [1]. Sharing similar genetic traits with MERS and SARS [2, 3] as well as a common host cell entry receptor with SARS [4], SARS-CoV-2 may also demonstrate neurotropism via possible invasion through the cribriform plate, olfactory nerve, thalamus and brainstem resulting in suppression of central cardiorespiratory drive [5]. Reports from China describe neurological symptoms in COVID-19 patients with one retrospective case series from Wuhan, China showing 78 of 214 patients (36%) with neurological manifestations [6,7,8]. Observations from Italy have confirmed Chinese data noting a high number of patients with hyposmia, anosmia and varying patterns of possibly centrally mediated symptoms including respiratory manifestations. In the evolving pandemic, healthcare professionals, therefore, need to recognize and address neurological consequences. We summarized the available knowledge to guide further research, clinical surveillance and management protocols.
Methods
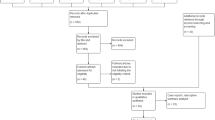
We searched papers published in English by 5 April 2020 using PubMed. Search terms relating to COVID-19 (including COVID*, novel coronavirus, nCoV*, *CoV-2, or *CoV2) in titles and abstracts were crossed with terms relating to neurological symptoms or neurotropism including (neurolog*, nervous, dizz*, delirium, encephal*, cereb*, headache, hyposmia, *geusia, hypopsia, myalgia, neurotrop*, or neuroinv*) in full texts. The search resulted in 198 papers, with 154 unique results retrieved, of which 122 were excluded on title/abstract screen and four were excluded on full text screen. Exclusion was based on topic, outcomes covered, and full text unavailability. 29 papers related to neurological manifestations associated with COVID-19 were examined.
Results
Evidence of neurological symptoms of COVID-19
Respiratory distress is the most distinctive symptom (55%) reported in COVID-19 patients [7]. Reported neurological findings fall into three categories: central (headache [6, 7], dizziness [6], impaired consciousness, acute cerebrovascular disease, ataxia and seizures), peripheral (hypogeusia, hyposmia) and musculoskeletal [8]. Mao et al. reported that 6 of 214 patients had either ischemic or haemorrhagic strokes, although it was not reported whether the strokes occurred before or after SARS-CoV-2 infection. Approximately 5% reported hypogeusia (5.6%) and/or hyposmia (5.1%) [8]. Additionally, a clinical picture reminiscent of a central hypoventilation syndrome (“Ondine’s curse”) was reported from a COVID-19 survivor in Wuhan [5]. There have been increasing reports of delirium in COVID-19 patients, and delirium may also be associated with more severe disease. A recent retrospective case series from China found that 22% of people who died from COVID-19 experienced delirium compared with 1% of people who recovered [9]. There has also been a case report of altered mental status in a COVID-19 patient with encephalopathy on EEG [10] and acute haemorrhagic necrotizing encephalopathy, the latter of which was thought to be due to cytokine storm [11]. Myalgia and muscle injury were reported in 10.7% of the cases in Wuhan [8] and rhabdomyolysis has been reported in another case from Wuhan [12].
Neurological manifestations have also been observed first-hand in Italy’s Brescia province which documented 9340 COVID-19 patients as of April 5, 2020 (personal observations of authors AP ML).Many patients experienced hyposmia or anosmia, dysgeusia, dysarthria and either allodynia or acroparesthesias. An atypical onset has been observed in few patients characterized by a delirious presentation that occurs prior to the onset of any respiratory syndromes. Within a cohort of 2660 hospitalized COVID-19 patients, 6 patients presented with encephalitis as the first and only symptom, 2 of whom subsequently died. Four patients presented with new onset seizures with no fever. These symptoms were then followed by the respiratory syndrome. There is evidence that many patients displayed varying patterns of respiratory manifestations. One such presentation consists of a sudden onset of respiratory failure in contrast to severe acute pulmonary failure. There also seems to be increase in number of both ischemic stroke and haemorrhagic stroke compared to historical series. Finally, more recently a case report has been published about the onset of Guillaine Barrè syndrome (GBS) during SARS-CoV-2, suggesting a pattern of a parainfectious profile, instead of the classic postinfectious profile [13].
Discussion
Neurological manifestations or NeuroCOVID are part of the COVID-19 clinical picture, but questions remain regarding the frequency and severity of CNS symptoms, the mechanism of action underlying neurological symptoms, and the relationship of symptoms with the course and severity of COVID-19. Further clinical, epidemiological, and basic science research is urgently needed to understand and address neurological sequalae of COVID-19. We identified three key priority areas for NeuroCOVID:
First, are patients with COVID-19 in other countries experiencing neurologic symptoms and do these symptoms affect the severity and course of the illness, specifically are they associated with increased respiratory failure or death? Documentation of neuropsychiatric comorbidities and drug treatment regimens is essential to aid ongoing discussions of drug–drug interactions and pharmacodynamic effects. Vaccine research discoveries need to be implemented while keeping in mind possible adverse events as shown by our experiences with the swine flu [14].
Second, what is the mechanism of action causing the neurological symptoms seen in patients with COVID-19? It is possible that SARS-CoV-2 causes neurological sequelae via inflammation, as elevated inflammatory biomarkers in patients with COVID-19 have been noted. Pro-inflammatory cytokine release is known to cause severe pulmonary damage in COVID-19, termed “cytokine storm”, and likely affects the CNS as well. Indirect CNS damage, through cytokine storm, can cause high mortality rates, encephalopathy, and posterior reversible encephalopathy (PRES). In acute infections, cytokine release can also result in strokes, a number of which have been reported in SARS post infection [17]. MR imaging might provide further information to elucidate the role of brainstem respiratory centres in COVID-19 patients.
Third, is SARS-CoV-2 a neurotropic virus? While we know that other coronaviruses demonstrate neurotropism, it is unknown how much of this knowledge is relevant for SARS-CoV-2. Li et al. postulate that SARS-CoV-2 neuroinvasion via the olfactory nerves is partially responsible for respiratory failure [5]. Is hyposmia or anosmia part of a prodrome of symptoms in COVID-19? Further research is needed in this area to further understand why steroids may be counterproductive in management of COVID-19 [18]. This signifies the importance of carrying out research into therapeutic options systematically with shared protocols and critical comparison of results.
Many patients present dysgeusia, dysphagia, dysarthria (personal observation AP) indicating a possible vagal involvement which could also indicate the possibility for diaphragmatic paresis and tachycardia thus potential bulbar infections. We know that SARS-CoV-1 enters cells through the ACE2 receptor mainly in renal, cardiovascular and gastrointestinal systems [19,20,21,22]. In mice, infected hippocampal cells were isolated in the CNS several days post-infection indicating that SARS-CoV-1 has the capability to spread to the CNS after clearance by the lungs [23]. In humans, SARS-CoV-1 has been found in the CSF [24] and in neurons on autopsy [25, 26]. Another coronavirus (swine hemagglutinating encephalomyelitis virus) travels via peripheral nerves to the brainstem via trigeminal and vagal sensory nuclei [27,28,29]. HCoV OC43 (a coronavirus responsible for the common cold) infects human microglia leading to persistent infection and has been found in the CSF and brain tissue of patients [30,31,32]. In mice, it travels to the CNS from the olfactory bulbs and, importantly, is blocked by destroying olfactory sensory neurons [33]. This potential mechanism becomes crucially important clinically when considering that wearing masks could likely be the most effective prevention against viral entry into the CNS.
Finally, prospective cohort studies are needed to understand the long-term impacts of COVID-19 on neurological functions. It is currently unknown if people who have recovered from severe COVID-19 suffer any lasting neurological sequalae. It is certainly true and well known that several patients discharged from ICU, thus also several COVID 19 patients, suffer of chronic illness myopathy or neuropathy or both which might worsen the clinical outcomes. Concomitant risk factors or determinants (e.g. demographic factors, comorbidities, or available biomarkers) that may predispose a person with COVID-19 to neurological manifestations should be identified.
We are aware that this review has limitations including that it was limited to articles published in English and that there is a tremendous growth in the volume of published literature on COVID-19, so that findings and recommendations are constantly evolving as new evidence arises and thus other relevant information and data could be lacking. Answers to all the above questions, however, require close coordination and open-data sharing between hospitals, academic institutions and fast establishment of harmonised research priorities and research consortia to face the NeuroCOVID-19 complications.
References
Arbour N, Day R, Newcombe J, Talbot PJ (2000) Neuroinvasion by human respiratory coronaviruses. J Virol 74(19):8913–8921
Yu F, Du L, Ojcius DM, Pan C, Jiang S (2020) Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect 22(2):74–79
Lu R, Zhao X, Li J et al (2020) Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224):565–574
Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94(7):e00127–e220. https://doi.org/10.1128/JVI.00127-20
Li Y-C, Bai W-Z, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92:552–555. https://doi.org/10.1002/jmv.25728
Wang D, Hu B, Hu C et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323(11):1061–1069. https://doi.org/10.1001/jama.2020.1585
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.1127
Chen T, Wu D, Chen H et al (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091
Filatov A, Sharma P, Hindi F et al (2020) Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 12(3):e7352. https://doi.org/10.7759/cureus.7352
Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B (2020) COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020:201187. https://doi.org/10.1148/radiol.2020201187[Epub ahead of print]
Jin M, Tong Q (2020) Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. https://doi.org/10.3201/eid2607.200445
Zhao H, Shen D, Zhou H, Liu J, Chen S (2020) Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 19:383–384. https://doi.org/10.1016/S1474-4422(20)30109-5
Krause R (2006) The swine flu episode and the fog of epidemics. Emerg Infect Dis 12(1):40–43
Lippi G, Plebani M (2020) Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. https://doi.org/10.1515/cclm-2020-0198[Epub ahead of print]
Qin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa248
Umapathi T, Kor AC, Venketasubramanian N et al (2004) Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol 251(10):1227–1231
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet 395(10223):473–475
Harmer D, Gilbert M, Borman R, Clark KL (2002) Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett 532(1–2):107–110
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637
Donoghue M, Hsieh F, Baronas E et al (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 87(5):E1–9
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. https://doi.org/10.1016/j.cell.2020.02.052[Epub ahead of print]
Glass WG, Subbarao K, Murphy B, Murphy PM (2004) Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J Immunol 173(6):4030–4039
Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY (2004) Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 10(2):342–344
Gu J, Gong E, Zhang B et al (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202(3):415–424
Xu J, Zhong S, Liu J et al (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 41(8):1089–1096
Andries K, Pensaert MB (1980) Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am J Vet Res 41(9):1372–1378
Yagami K, Hirai K, Hirano N (1986) Pathogenesis of haemagglutinating encephalomyelitis virus (HEV) in mice experimentally infected by different routes. J Comp Pathol 96(6):645–657
Li YC, Bai WZ, Hirano N, Hayashida T, Hashikawa T (2012) Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res 163(2):628–635
Morfopoulou S, Brown JR, Davies EG et al (2016) (2016) Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med 375(5):497–498
Arbour N, Cote G, Lachance C, Tardieu M, Cashman NR, Talbot PJ (1999) Acute and persistent infection of human neural cell lines by human coronavirus OC43. J Virol 73(4):3338–3350
Bonavia A, Arbour N, Yong VW, Talbot PJ (1997) Infection of primary cultures of human neural cells by human coronaviruses 229E and OC43. J Virol 71(1):800–806
Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ (2018) Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 92(17):e00404. https://doi.org/10.1128/JVI.00404-18
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Leonardi, M., Padovani, A. & McArthur, J.C. Neurological manifestations associated with COVID-19: a review and a call for action. J Neurol 267, 1573–1576 (2020). https://doi.org/10.1007/s00415-020-09896-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09896-z