Abstract
Objective
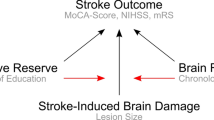
Although post-stroke cognitive deficit can significantly limit patient independence and social re-integration, clinical routine predictors for this condition are lacking. ‘Cognitive reserve’ limits the detrimental effects of slowly developing neurodegeneration. We aimed to determine whether comparable effects also exist in acute stroke. Using 'years of education' as a proxy, we investigated whether cognitive reserve beneficially influences cognitive performance and disability after stroke, whilst controlling for age and lesion size as measure of stroke pathology.
Methods
Within the first week of ischemic right hemisphere stroke, 36 patients were assessed for alertness, working memory, executive functions, spatial neglect, global cognition and motor deficit at 4.9 ± 2.1 days post-stroke, in addition to routine clinical tests (NIH Stroke Scale, modified Rankin Scale on admission < 24 h post-stroke and at discharge 9.5 ± 4.7 days post-stroke). The impact of education was assessed using partial correlation analysis adjusted for lesion size, age, and the time interval between stroke and assessment. To validate our results, we compared groups with similar age and lesion load, but different education levels.
Results
In the acute stroke phase, years of education predicted both severity of education independent (alertness) and education dependent (working memory, executive functions, global cognition) cognitive deficits and disability (modified Rankin Scale). Spatial neglect seemed to be independent.
Interpretation
Proxies of cognitive reserve should be considered in stroke research as early as in the acute stroke phase. Cognitive reserve contributes to inter-individual variability in the initial severity of cognitive deficits and disability in acute stroke, and may suggest individualised rehabilitation strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke-induced dependency is frequently associated with severe motor impairment and deficits of higher cognitive functions such as aphasia, apraxia or neglect but also memory and attention deficits and disturbances of executive functions. Prediction of stroke outcome in both cognitive and motor domains remains to be challenging due to the inter-individual variability [1, 2].
Research on normal ageing as well as neurodegeneration has revealed that the variation in individual responses to brain pathology can be explained by the concepts of ‘brain reserve’ and ‘cognitive reserve’ [3]. These concepts, which address different factors protecting against cognitive decline, might also be applicable to stroke [4], though their terminology—introduced for degenerative disorders—might not be ideally fitting for stroke.
In neurodegeneration, ‘brain reserve’ is defined by quantitative brain parameters, such as the maximal lifetime brain volume typically estimated by intracranial volume [3]. In stroke, age may be applicable as proxy for ‘brain reserve’, since it correlates with brain volumetric characteristics [5] and influences stroke outcome [6, 7] including post-stroke cognition [8, 9]. In addition, ageing is also associated with cortical atrophy and leukoaraiosis, which have each been shown to impact on both functional outcome [10, 11] and stroke-induced cognitive impairment [12]. This interrelation makes it difficult to dissociate the effects of age itself and of age-related pathology on stroke outcome. Overall, however, age is the most commonly used proxy of brain reserve in stroke research, which measures the inter-individual variability of stroke outcome.
‘Cognitive reserve’ is defined as the function of a lifetime intellectual activities, which serves to shape network efficiency, processing capacity and flexibility [3]. Proxies such as level of education, crystallised intelligence, socioeconomic status, etc. are used to estimate cognitive reserve, and have even been suggested to influence stroke recovery [13, 14]. At the same time, such factors as socio-economic status, participation in social networks or depression might considerably change with ageing. Proxies of cognitive reserve have barely been considered in stroke research. Indeed, low education level has been shown to predict post-stroke dementia [12]. However, stroke-induced dementia is much rarer than mild cognitive deficits, which considerably limit rehabilitation options and worsen stroke outcome [15]. There is also evidence for the impact of education level on post-stroke global cognition [8, 16] and recovery of language functions and visual memory in the chronic stroke phase [17]. However, whether ‘cognitive reserve’ has an independent impact on severity of stroke-induced deficits in other cognitive domains is yet to be determined. Furthermore, whether or not a possible impact of ‘cognitive reserve’ is also present in the acute stroke phase remains to be investigated. Moreover, we recently suggested that ‘cognitive reserve’ might affect non-cognitive domains [4]. Clarifying these issues is essential for the improvement of individual prognoses and individualised rehabilitation approaches.
The present study, therefore, evaluated the impact of cognitive reserve on clinical parameters and cognitive performance in the acute phase of stroke whilst adjusting for age and lesion load. As proxy for ‘cognitive reserve’, we used years of education [3]. To avoid any confounds being potentially introduced in behavioural tests by comprehension or other disturbances of the language system, we exclusively looked at patients after right hemisphere stroke.
Materials and methods
Subjects
As part of a large study (Freiburg Large Scale Project), patients were recruited from the stroke unit of the Department of Neurology, University Medical Centre Freiburg, Germany, in the period from September 2014 to December 2015. An inclusion criterion was a first-ever territorial ischemic right middle cerebral artery stroke. Exclusion criteria were (i) conditions compromising study participation and examination (e.g. low consciousness or arousal level, general MRI contraindications); (ii) pre-stroke neurological or psychiatric conditions compromising data interpretation (pre-stroke cognitive impairment, previous stroke, structural brain lesions besides stroke, etc.); and (iii) left-handedness [18]. Thirty-six patients aged 66.4 ± 14.6 years (mean ± SD) were included (Table 1). All patients underwent standard treatment in line with the stroke guidelines of the German Neurological Society.
Neuropsychological and clinical testing
Clinical scoring using the NIH Stroke Scale (NIHSS) and modified Rankin Scale (mRS) was performed routinely by physicians upon patient admission in the acute clinic (< 24 h post-stroke) and at discharge (9.5 ± 4.8 days post-stroke). Neuropsychological testing was performed at 4.9 ± 2.1 days post-stroke and consisted of the following variables: (i) global cognition, which was assessed using the Montreal Cognitive Assessment (MoCA, max 30, norm ≥ 26 points) [19]. Besides total MoCA score, we also included the MoCA after subtraction of visuospatial items to avoid double consideration of spatial neglect (max 25); (ii) tonic alertness, which was based on the reaction time (ms) to single targets (circles with a diameter of ~ 3° of visual angle) presented centrally on a screen for 300 ms, with inter-trial intervals between 2250 and 3800 ms. In patients with neglect, the screen was adjusted accordingly by moving it towards the right so as to ensure target perception. (iii) Working memory, which was assessed by digit span (forwards and backwards) measured in percentile rank; normative data were stratified by age [20]. Normative data that are stratified according to education years are not available. (iv) Executive functions, assessed using a word fluency test with phonemic (‘M’- and alternating ‘G–R’-letters) and semantic (‘food’ and alternating ‘sport-fruits’) tasks, measured in percentile rank; normative data were stratified by age [21]. Although normative data stratified by both age and education years are available, there is poor representation of subjects > 65 years. By including in the study only patients with right hemisphere stroke, we avoided confounds potentially introduced by stroke-induced language disorder. (v) Severity of spatial neglect was assessed by calculating the mean Center of Cancellation (CoC) score [22] for Bells test [23], Ota task [24], line cancellation, star cancellation, letter cancellation and reading tests [25], as well as an adapted score for a drawing test [26]. For the reading test, CoC was calculated as the center of mass for the words read. (vi) Crystallised intelligence was evaluated as an additional representation of cognitive reserve using the IQ score of the Multiple-Choice Vocabulary Test—Version A (Mehrfachwahl-Wortschatz-Test—Version A) [27]. Despite this measure being possibly confounded by stroke deficits, it appeared to be applicable and valid, since we neither included aphasic left hemisphere patients, nor set a time limit for patients. (vii) Motor deficits were assessed using the Fugl–Meyer test for the upper limb [28]. Since the testing took place in the acute stroke phase, some tests (i–vi above) were not feasible for all patients due to a number of factors including fatigue and limited compliance. In case of missing data, the remaining number of patients is provided additionally.
MRI examination and lesion mapping
All patients underwent an MRI examination within the first week of admission that included diffusion-weighted imaging (DWI) for lesion mapping and anatomical T1 imaging (sagittal magnetization-prepared rapid-acquisition gradient echo sequence [MPRAGE]), with previously described parameters [18]. Lesion mapping was performed semi-automatically by roughly delineating the lesion on the DWI images using a customised region-of-interest toolbox implemented in SPM8 (https://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Individual intensity thresholds were then applied to find the best match between the binary lesion map and the diffusion-restricted area. The resulting lesion map was subsequently inspected with MRIcron (https://www.cabiatl.com/mrico/mricon/stats.html) and manually adjusted where necessary [18]. T1 images were segmented using the VBM8 Toolbox, after which deformation field parameters for nonlinear normalization were calculated with DARTEL (diffeomorphic anatomical registration through exponentiated lie algebra [29]), and applied to T1 images and co-registered lesion maps for normalization in the standard Montreal Neurological Institute (MNI) space.
Statistical analysis
Since all behavioural and clinical variables were approximately normally distributed according to the Kolmogorov–Smirnov test, we, therefore, employed parametric statistical analyses. According to our main working hypothesis that cognitive reserve impacts stroke deficit severity besides premorbid brain reserve and stroke damage [4], we evaluated the effect of their proxies in a single analysis. The measure 'years of education' was used as a proxy for cognitive reserve. We controlled for age, also as it represents the approximated proxy of brain reserve in stroke, because it correlates with brain volumetric characteristics [5] and influences stroke outcome [6, 7]. We did not apply brain volume characteristics directly (e.g. whole brain or grey matter volume) but used age instead, since we aimed to find proxies that are easily available in routine clinical praxis, and as age has an impact on functional besides structural network characteristics [30]. As proxy for the severity of stroke damage, we used relative lesion volume, which was calculated as ratio between lesion volume and total brain volume [absolute lesion size/total brain volume], both derived from native non-normalised images to avoid lesion size underestimation in patients with age-related brain atrophy. To evaluate the impact of education level on clinical and behavioural variables, we applied a partial correlation analysis that was adjusted for age and relative lesion size. Considering that the data were acquired during the acute stroke phase, the time interval between stroke onset and examination could have had a significant influence on the results; therefore, it was also introduced into the model. We applied the false discovery rate (FDR) correction for multiple comparisons with q = 0.05 (false discovery detection rate of 5%).
As a validation, we carried out additional control analyses by forming two groups of patients who were matched by relative lesion size and age but differed in education level, by applying a multi-dimensional matching method [31]. To this end, the sample was divided along its median of 13 years of education into two subsamples comprising patients with a higher (> 13 years; n = 18) and a lower (≤ 13 years; n = 18) education level. From these two subgroups, patient pairs showing the least differences in age and relative lesion volume in the multi-dimensional space were created [32]. The analysis of pairwise distance showed a step after 13 pairs, which were used for further groups’ comparisons using two-sample t test. For the group comparisons of NIHSS and mRS scores at admission and discharge, we applied two-way repeated measure ANOVA. An alpha level of 0.05 was applied in all behavioural analyses. One-tailed testing was chosen, if specifically justified by the a priori hypothesis that higher education is associated with better abilities [4]. Statistical analyses were performed using SPSS 21.
Lesion anatomy was analyzed with MRIcron software using the voxel-based lesion-behaviour mapping (VLBM) tool [33]. To exclude any association between lesion anatomy and variables of interest, we performed Brunner–Munzel test where age, education years and lesion volume served as regressors [1000 permutations, family-wise error (FWE) correction, p < 0.05]. Comparison of lesion anatomy between the two subgroups matched for age and relative lesion size but with disparate education levels was performed using the Liebermeister test [false discovery rate (FDR) correction, p < 0.05].
Results
Table 1 summarises the clinical characteristics of the study cohort, whilst Fig. 1 shows the distribution of lesion anatomy. Years of education correlated strongly with crystalised intelligence (Pearson correlation, MWT-IQ, r = 0.820, p < 0.001, n = 19). There was no correlation between years of education and age (r = − 0.238, p = 0.181), between age and relative lesion volume (r = − 0.072, p = 0.682); and between relative lesion volume and education level (r = − 0.032, p = 0.858). Neither age nor years of education nor relative lesion size was associated with damage to specific brain regions, as the VLBM analysis did not yield any voxels significantly associated with them (Brunner–Munzel test, p < 0.05).
Partial correlation analyses
To investigate the impact of cognitive reserve in the acute stroke phase, we applied a partial correlation analysis, in which years of education were correlated with neuropsychological parameters and clinical routine scores at admission and discharge, whilst controlling for the variables age, relative lesion size and the time interval between stroke onset and examination. Years of education had an effect both on severity of disability and cognitive deficits (Table 2). Years of education negatively correlated with disability scores, as assessed by the mRS at admission and discharge (p = 0.026 and p = 0.019 correspondingly, significant after FDR correction; here and further one-tailed), but no significant correlation was found for NIHSS (at admission p = 0.056, at discharge p = 0.145).
Years of education correlated positively each with post-stroke alertness (p = 0.013), global cognition (MoCA-score, p = 0.004), working memory (assessed as forwards and backwards digit span, p = 0.005 and p = 0.001), and executive functions (assessed by word fluency ‘M’, ‘Sport/Fruit’, ‘G–R’, p = 0.001, p = 0.004 and p = 0.007 correspondingly, all significant after FDR correction), explaining at least 20% of their variance after adjusting for lesion size and age. In contrast, education years did not play any significant role in the severity of either upper limb paresis or spatial neglect (p = 0.342 and p = 0.171). Thus, years of education served as a predictor for cognitive deficit severity and disability in the acute stroke phase, independent of age and relative lesion size.
In the additional control analysis, we used crystallised intelligence as proxy of cognitive reserve and applied the same statistical model. The result remained the same as in the analysis above. However, in addition, NIHSS both at admission and discharge, severity of neglect and motor function of paretic arm significantly correlated with cognitive reserve assessed as IQ scores based on vocabulary (Table 3).
Contrast of patients with distinct cognitive reserve
To confirm the beneficial effects of cognitive reserve on post-stroke performance, we compared stroke deficit severity amongst 13 pairs of patients who had different levels of education (p < 0.001, two-tailed) but were matched for age and relative lesion volumes (p = 0.293 and p = 0.795 correspondingly). The groups also did not differ in relation to lesion anatomy (VLBM analysis using the Liebermeister test, FDR corr, p < 0.05, also with reduced statistical threshold p < 0.001 uncorr; Fig. 2a).
Comparison of age- and lesion-volume-matched patients with distinct education levels. a Simple lesion overlap for each subgroup; voxel-based lesion symptom mapping analysis did not reveal any differences in lesion anatomy between the two subgroups (Liebermeister test, FDR corr, p < 0.05). Colour bar indicates the number of overlapping lesions. b Group comparisons of proxies for cognitive reserve (years of education at school), stroke damage (absolute lesion size) and brain reserve (total brain volume). c Group differences in stroke severity and disability, and cognitive deficits in the acute stroke phase (mean ± SEM, *p < 0.05, **p < 0.01)
According to the group analysis, beneficial effect of cognitive reserve was observed for level of disability (mRS) and severity of stroke impairment (NIHSS) in acute stroke (Fig. 2). In detail, patients with higher education level had overall lower mRS than those with lower education (two-way repeated measure ANOVA, main group effect p = 0.038, two-tailed), whereas mRS improved from admission to discharge in both groups (main effect of time, p = 0.040); groups did not differ in their course of disability improvement (interaction effect group × time, p = 0.539) There was a comparable group difference in stroke impairment: higher cognitive reserve was associated with lower NIHSS (two-way repeated measure ANOVA, main group effect, p = 0.041, two-tailed) by overall NIHSS improvement from admission to discharge (main effect of time, p = 0.002), and by insignificant interaction effect (p = 0.891).
Patient groups also differed in cognitive deficit severity, where alertness, global cognition, working memory (digit span forwards and backwards), and executive functions (word fluency) were better in subjects with higher cognitive reserve (all p < 0.039, one-tailed, Table 1, Fig. 2). Moreover, arm paresis in patients with higher cognitive reserve was less severe than in the group with lower cognitive reserve (p = 0.012), whereas patient groups did not differ significantly in spatial neglect severity (p = 0.098, one-tailed).
Discussion
The present study shows the impact of ‘cognitive reserve’, as approximated by years of education on (i) initial severity of cognitive deficits (alertness, working memory, executive functions and global cognition) as early as in the acute stroke phase and (ii) the level of disability in the acute stroke phase, independently from age and lesion load.
There are only few studies on the impact of education level on functional stroke outcome. Patients with longer educational history had a lower NIHSS score in acute stroke [16], but the results were not adjusted for age. Low level of education was also associated with a higher mRS around 5 years after stroke, independent from age [34]. However, these studies did not take lesion load into account. Moreover, it is unclear whether patients with low level of education had poor outcome in the chronic stroke phase due to lower cognitive reserve itself, or rather due to low socio-economic status, which is often associated with lower education level and potentially results in restricted treatment and rehabilitation programmes [35]. The present findings are, therefore, the first to show the beneficial impact of cognitive reserve on disability in acute stroke, independent from lesion load and age. The absent effect of cognitive reserve on recovery of disability within the acute stroke phase might be confounded by (i) the short observation time (9.5 ± 4.7 days), (ii) by considering mRS at admission before thrombolysis, making this score dependent on multiple factors (e.g. hypoperfusion, blood pressure, etc.) and making the recovery rate being dependent on success of thrombolysis, (iii) insufficient sample size of patients. Therefore, the negative result might be false negative. Further studies are needed to investigate the impact of cognitive reserve on recovery of disability with larger patient sample, longer observation time and with clinical scores acquired after thrombolysis as the baseline.
Previous work exploring the impact of cognitive reserve on stroke-induced cognitive impairment is limited. Low education level was shown to be associated with worse global cognition as assessed by MoCA in the acute stroke phase [36] and the Mini-Mental State Examination in the chronic stroke phase [8, 16]. Bilingualism was also associated with a better cognitive outcome after stroke [37]. The present study revealed that as early as 1 week post-stroke, lower education level is associated with poorer performance in different cognitive domains including education-independent alertness [38], besides education-dependent working memory, executive functions and MoCA. The impact of educational history was reported for memory impairment, aphasia, visuospatial and constructive deficits at 3 months post-stroke, independent of age and stroke severity [16]. However, the latter study did not control for lesion volume and anatomy. The influence of cognitive reserve in acute stroke appears to affect functions differently. Whilst alertness, working memory, executive functions and global cognition were better in subjects with higher cognitive reserve, we did not observe significant effects of cognitive reserve on motor deficits or on spatial neglect. This differential impact might depend on how particular networks are distributed in human brain: the more a functional network is represented in restricted brain regions, the more important the lesion anatomy [39] and the less critical the brain reserve and cognitive reserves. In fact, though spatial attention is provided by a large-scale network [40], the neural correlate of spatial neglect appears to be restricted to a perisylvian network [41], strongly lateralised in the right hemisphere. Therefore, the effect of education on neglect severity may either not be present or may be weaker than in other cognitive domains studied, as this protective effect has been reported previously by AE Hills and Tippett (2014). Interestingly, in the control analyses, both motor function of the paretic arm and NIHSS were influenced by educational attainment. Further studies are required to investigate these results.
The impact of cognitive reserve on disability and cognitive deficits might comprise several mechanisms [4]. First, patients with higher education years might have better pre-stroke cognition, and therefore show better post-stroke cognitive performance than patients with lower education despite of comparable stroke damage. In other words, in case of higher reserve, brain can simply tolerate more pathology before it reaches a critical threshold and the patient presents a cognitive deficit [42]. This might be partly true for MoCA scores and the word fluency tests, although it has been shown that the semantic word fluency tasks applied here are more dependent on age, for which we adjusted, than on education [43]. However, a simple additive effect of education appears to be insufficient to explain the present results, as it does not account for its impact on mRS-assessed disability level or education-independent cognitive functioning (e.g. alertness), meaning that other beneficial mechanisms exist. Second, higher cognitive reserve is associated with better network efficiency or capacity [3]. For example, higher education level is related to increased dendritic measures [44] or plasticity [45] in humans, whilst enriched environment fosters neurogenesis and synaptogenesis according to animal studies [46]. Third, individuals with higher cognitive reserve may be more capable of recruiting alternative neural networks to maintain function [47]. In other words, higher cognitive reserve might provide easier and faster compensation for stroke damage, e.g. by unmasking latent connections and recruiting (new) functional centers. Finally, subjects with higher education levels usually have a healthier life style and better compliance with vascular risk factor management, and cognitive reserve might interact with brain reserve [48].
The most critical limitation of the study is the impossibility to differentiate whether or not the better post-stroke performance in higher educated patients might just mirror their better pre-stroke cognitive functioning, as the cognitive deficits were assessed only cross sectionally. On the other side, this limitation is relevant only for education-dependent cognitive domains (e.g. working memory, executive functions); and it concerns all studies of stroke-induced education-dependent cognitive deficits, as reliable normative data stratified by both age and education are not available for patients > 65 years old. The existence of the strong impact of education on education-independent alertness and stroke disability ensures, however, the post-stroke mechanism of this protective effect. Also, the presence of this protective impact of cognitive reserve as early as one week post-stroke points to a strong effect, despite of diaschisis [49] and disruption through an acute lesion [39]. It is clear that future research needs to investigate the impact of cognitive reserve on stroke recovery. Nevertheless, the present data strongly highlight its protective influence on initial stroke-induced cognitive deficit and stroke disability. Thus, cognitive reserve has to be considered in stroke research as early as in the acute stroke phase, independent from its underlying pre-stroke or “post-stroke only” mechanisms.
There are several other limitations. Since cognitive testing was only performed in patients in whom testing was feasible within the first week post-stroke, the patient sample is relatively small and biased towards the exclusion of patients with severe stroke deficits, i.e. patients with larger lesions. This represents a concern for wider generalisability and limited the optimal group matching. Thus, additional studies on a larger and more balanced patient cohort are required to further elucidate the impact of cognitive reserve on patients’ abilities in acute stroke. Moreover, the proxy used in the present study for cognitive reserve (= years of education) is only a rough measure of lifetime intellectual activities and, hence, might not sufficiently cover the whole concept of cognitive reserve. However, the advantage of this particular proxy is that it is extremely easy to assess in the clinical setting. Furthermore, years of education correlated strongly with crystallised intelligence, underscoring its reliability as a proxy for cognitive reserve.
To summarise, the present data demonstrate that cognitive reserve measured as years of education can serve as an independent predictor of cognitive performance and disability in the acute phase of stroke, and should, therefore, be considered alongside age and lesion load in therapeutic trials as well as in individual therapeutic approaches. The findings arise new discussions on impact of life experience on stroke outcome.
References
Amunts K, Schleicher A, Burgel U et al (1999) Broca’s region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341
Ward NS, Brown MM, Thompson AJ, Frackowiak RS (2003) Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126:2476–2496. https://doi.org/10.1093/brain/awg245
Barulli D, Stern Y (2013) Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 17:502–509. https://doi.org/10.1016/j.tics.2013.08.012
Umarova RM (2017) Adapting the concepts of brain and cognitive reserve to post-stroke cognitive deficits: Implications for understanding neglect. Cortex 97:327–338. https://doi.org/10.1016/j.cortex.2016.12.006
Franke K, Ziegler G, Klöppel S et al (2010) Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. NeuroImage 50:883–892. https://doi.org/10.1016/j.neuroimage.2010.01.005
Knoflach M, Matosevic B, Rücker M et al (2012) Functional recovery after ischemic stroke—a matter of age: data from the Austrian Stroke Unit Registry. Neurology 78:279–285. https://doi.org/10.1212/WNL.0b013e31824367ab
Beumer D, Rozeman AD, Lycklama À, Nijeholt GJ et al (2016) The effect of age on outcome after intra-arterial treatment in acute ischemic stroke: a MR CLEAN pretrial study. BMC Neurol 16:68. https://doi.org/10.1186/s12883-016-0592-5
Jaillard A, Grand S, Le Bas JF, Hommel M (2010) Predicting cognitive dysfunctioning in nondemented patients early after stroke. Cerebrovasc Dis Basel Switz 29:415–423. https://doi.org/10.1159/000289344
Nys GM, Van Zandvoort MJ, De Kort PL et al (2005) Domain-specific cognitive recovery after first-ever stroke: a follow-up study of 111 cases. J Int Neuropsychol Soc 11:795–806
IST-3 collaborative group (2015) Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol 14:485–496. https://doi.org/10.1016/S1474-4422(15)00012-5
Ryu W-S, Woo S-H, Schellingerhout D et al (2017) Stroke outcomes are worse with larger leukoaraiosis volumes. Brain 140:158–170. https://doi.org/10.1093/brain/aww259
Pendlebury ST, Rothwell PM (2009) Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 8:1006–1018. https://doi.org/10.1016/S1474-4422(09)70236-4
Cramer SC (2008) Repairing the human brain after stroke: I Mechanisms of spontaneous recovery. Ann Neurol 63:272–287. https://doi.org/10.1002/ana.21393
Hillis AE (2014) Tippett DC (2014) Stroke recovery: surprising influences and residual consequences. Adv Med. https://doi.org/10.1155/2014/378263
Hachinski V, Iadecola C, Petersen RC et al (2006) National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke 37:2220–2241. https://doi.org/10.1161/01.STR.0000237236.88823.47
Ojala-Oksala J, Jokinen H, Kopsi V et al (2012) Educational history is an independent predictor of cognitive deficits and long-term survival in postacute patients with mild to moderate ischemic stroke. Stroke J Cereb Circ 43:2931–2935. https://doi.org/10.1161/STROKEAHA.112.667618
Ramsey LE, Siegel JS, Lang CE et al (2017) Behavioural clusters and predictors of performance during recovery from stroke. Nat Hum Behav 1:0038. https://doi.org/10.1038/s41562-016-0038
Beume L-A, Martin M, Kaller CP et al (2017) Visual neglect after left-hemispheric lesions: a voxel-based lesion-symptom mapping study in 121 acute stroke patients. Exp Brain Res 235:83–95. https://doi.org/10.1007/s00221-016-4771-9
Nasreddine ZS, Phillips NA, Bédirian V et al (2005) The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699. https://doi.org/10.1111/j.1532-5415.2005.53221.x
Wechsler D (2000) Wechsler-Gedächtnistest—Revidierte Fassung: WMS-R; Testmanual; deutsche Adaptation der revidierten Fassung der Wechsler Memory Scale von David Wechler, 1st edn. Hans Huber, Bern
Aschenbrenner A, Tucha O, Lange K (2000) RWT Regensburger Wortflüssigkeits-Test. Hogrefe Verlag, Göttingen
Rorden C, Karnath HO (2010) A simple measure of neglect severity. Neuropsychologia 48:2758–2763. https://doi.org/10.1016/j.neuropsychologia.2010.04.018
Gauthier L, Dehaut F, Joanette Y (1989) The Bells test: a quantitative and qualitative test for visual neglect. Int J Clin Neuropsychol 11(2):49–54
Ota H, Fujii T, Suzuki K et al (2001) Dissociation of body-centered and stimulus-centered representations in unilateral neglect. Neurology 57:2064–2069
Wilson B, Cockburn J, Haligan PW (1987) Behavioural inattention test. Thames Valley Company, Titchfield
Umarova RM, Reisert M, Beier T-U et al (2014) Attention-network specific alterations of structural connectivity in the undamaged white matter in acute neglect. Hum Brain Mapp 35:4678–4692. https://doi.org/10.1002/hbm.22503
Lehrl S (1991) Manual zum MWT. Perimed, Balingen
Fugl-Meyer AR, Jääskö L, Leyman I et al (1975) The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 7:13–31
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007
Geerligs L, Renken RJ, Saliasi E et al (2015) A brain-wide study of age-related changes in functional connectivity. Cereb Cortex 25:1987–1999. https://doi.org/10.1093/cercor/bhu012
Kaller CP, Loosli SV, Rahm B et al (2014) Working memory in schizophrenia: behavioral and neural evidence for reduced susceptibility to item-specific proactive interference. Biol Psychiatry 76:486–494. https://doi.org/10.1016/j.biopsych.2014.03.012
Mahalanobis PC (1936) On the generalized distance in statistics. National Institute of Science of India, vol 2, pp 49–55
Rorden C, Karnath H-O, Bonilha L (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19:1081–1088. https://doi.org/10.1162/jocn.2007.19.7.1081
Yang Y, Shi Y-Z, Zhang N et al (2016) The disability rate of 5-year post-stroke and its correlation factors: a National Survey in China. PLoS ONE 11:e0165341. https://doi.org/10.1371/journal.pone.0165341
Grimaud O, Roussel P, Schnitzler A et al (2016) Do socioeconomic disparities in stroke and its consequences decrease in older age? Eur J Public Health 26:799–804. https://doi.org/10.1093/eurpub/ckw058
Pasi M, Salvadori E, Poggesi A et al (2013) Factors predicting the Montreal cognitive assessment (MoCA) applicability and performances in a stroke unit. J Neurol 260:1518–1526. https://doi.org/10.1007/s00415-012-6819-5
Alladi S, Bak TH, Mekala S et al (2016) Impact of bilingualism on cognitive outcome after stroke. Stroke 47:258–261. https://doi.org/10.1161/STROKEAHA.115.010418
Bodenburg S, Popp B, Kawski S (2001) Ergänzende Normdaten zu dem Untertest Alertness aus der Testbatterie zur Aufmerksamkeitsprüfung (TAP) in der Altersgruppe 60+. Z Für Neuropsychol 12:125–130. https://doi.org/10.1024//1016-264X.12.2.125
Corbetta M, Ramsey L, Callejas A et al (2015) Common behavioral clusters and subcortical anatomy in stroke. Neuron 85:927–941. https://doi.org/10.1016/j.neuron.2015.02.027
Corbetta M, Shulman GL (2002) Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3:201–215
Karnath H-O, Rorden C (2012) The anatomy of spatial neglect. Neuropsychologia 50:1010–1017. https://doi.org/10.1016/j.neuropsychologia.2011.06.027
Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11:1006–1012. https://doi.org/10.1016/S1474-4422(12)70191-6
Tombaugh TN, Kozak J, Rees L (1999) Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 14:167–177. https://doi.org/10.1016/S0887-6177(97)00095-4
Jacobs B, Schall M, Scheibel AB (1993) A quantitative dendritic analysis of Wernicke’s area in humans. II. Gender, hemispheric, and environmental factors. J Comp Neurol 327:97–111. https://doi.org/10.1002/cne.903270108
Brosnan MB, Demaria G, Petersen A et al (2017) Plasticity of the right-lateralized cognitive reserve network in ageing. Cereb Cortex N Y N 1991:1–11. https://doi.org/10.1093/cercor/bhx085
Mandolesi L, Gelfo F, Serra L et al (2017) Environmental factors promoting neural plasticity: insights from animal and human studies. Neural Plast 2017:7219461. https://doi.org/10.1155/2017/7219461
Steffener J, Reuben A, Rakitin BC, Stern Y (2011) Supporting performance in the face of age-related neural changes: testing mechanistic roles of cognitive reserve. Brain Imaging Behav 5:212–221. https://doi.org/10.1007/s11682-011-9125-4
EClipSE Collaborative Members, Brayne C, Ince PG et al (2010) Education, the brain and dementia: neuroprotection or compensation? Brain J Neurol 133:2210–2216. https://doi.org/10.1093/brain/awq185
Monakow CV (1906) Aphasie und diaschisis. Neurol Cent 25:1026–1038
Acknowledgements
The authors thank Sebastian Kuebel for his help in the neuropsychological testing. This work was supported by funds of the Department of Neurology, Freiburg, the Brain-Links Brain-Tools Cluster of Excellence (Grant number EXC 1086) as well as by Grant KA1258/23-1 funded by the Deutsche Forschungsgemeinschaft (DFG). Christoph Sperber was supported by the Friedrich Naumann Foundation.
Author information
Authors and Affiliations
Contributions
RMU and SK contributed to conception; RMU, CW and CPK contributed to the study design; RMU, HU, CSMS and CW contributed to data acquisition, RMU, CS, CPK, SK and H-OK contributed to data analysis; RMU, CS and H-OK wrote the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Nothing to report.
Ethical standards
The Ethics Committee of the University Medical Centre Freiburg approved the study (10/2013), which was conducted according to the principles of the Declaration of Helsinki.
Informed consent
Written informed consent was obtained from each subject.
Rights and permissions
About this article
Cite this article
Umarova, R.M., Sperber, C., Kaller, C.P. et al. Cognitive reserve impacts on disability and cognitive deficits in acute stroke. J Neurol 266, 2495–2504 (2019). https://doi.org/10.1007/s00415-019-09442-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09442-6