Abstract
The aim of this study was to evaluate the effect of cognitive reserve (CR), in progression from subjective cognitive decline (SCD) to mild cognitive impairment (MCI) and Alzheimer’s disease (AD). For this purpose, we followed up 263 patients (154 SCD; 109 MCI) for a mean time of 7 years. CR was assessed by the Test di Intelligenza Breve (TIB), functionally equivalent to the National Adult Reading Test. High CR resulted as a protective factor for progression from SCD to MCI. Age at conversion to MCI was delayed 9 years on average in SCD with high CR with respect to SCD with low CR. On the contrary, high CR resulted as a risk factor for progression from MCI to AD dementia only in APOE ε4 carriers. Conversion time from MCI to AD dementia was 3 years shorter in ε4 carriers with high CR than subjects with low CR and ε4 non-carriers with high CR. Consistent with the CR hypothesis, our results showed that higher levels of CR protect against the earliest clinical manifestations of AD. In line with the previous researches, we found an interaction between CR and APOE in progression from MCI to AD dementia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The identification of protection and risk factor is a crucial issue in the management of a chronic, widely prevalent disorder such as Alzheimer’s disease (AD). Several studies have reported that lifetime experiences that are associated with cognitive stimulation (e.g., years of education [1], occupational attainment [2], and engagement in mentally stimulating leisure activities [3]) may protect against dementia. These findings have been interpreted by means of the cognitive reserve (CR) hypothesis, which assume that highly intelligent or educated individuals appear to be able to cope better with the presence of a neurodegenerative pathology, maintaining a normal functional level for a longer time than less educated people [4]. This model, first proposed by Stern, is supported by neuropathology and functional neuroimaging studies showing that there is much variability in the relationship between the amount of cerebral damage and the degree of cognitive deficit [5,6,7]. Another prediction of Stern’s CR model is that higher functioning individuals will show a more rapid deterioration over time. Indeed, if greater cognitive reserve allows the brain to cope with a greater amount of damage, when this reserve is overcome, a faster decline is evident [2, 4]. As CR is a hypothetical construct, direct measurements of reserve are not available [8]. Therefore, surrogate or proxy measurements are used to approach CR. Education [9] and engagement in leisure and cognitive activities [10] are considered as standard proxies of CR. Premorbid intelligence quotient investigated by performance-based measurements (such as vocabulary or reading tests), which show little change with age and remain relatively preserved in the early stages of dementia, has been used as proxy of CR [11, 12].
Subjective cognitive decline (SCD) was defined as a self-experienced persistent decline in cognitive capacity in comparison with the subject’s previously normal status, during which the subject had normal age-, gender-, and education-adjusted performance on standardized cognitive tests [13]. Subjective cognitive complaints constitute an important criterion for diagnosis of mild cognitive impairment (MCI) [14, 15] and individuals with SCD are more likely to develop dementia than those without [16,17,18]. Taking into account the protective effect of CR, knowing how CR influences cognitive performance and the risk of progression to objective cognitive impairment in subjects experiencing SCD may be a promising research field. Many cross-sectional studies focused on SCD and CR showing that high CR is associated with high level of cognitive performance [19,20,21]. More recently, longitudinal studies have been conducted in cognitively healthy subjects to explore the effect of CR on cognitive function over time and on progression from normal cognition to full-blown cognitive decline [3, 22,23,24]. Soldan et al. [23] found that higher CR scores were associated with better cognitive performance, but did not modify the rate of change in cognition in a cognitively healthy subjects sample. A study by Lojo-Seoane et al. [24] first showed that CR had a positive influence on cognitive performance at follow-up in a in a sample of older adults with SCD. Bessi et al. [3] demonstrated that having carried out intellectual activities in the decades before the onset of SCD could reduce the risk of progression to MCI up to 30%. Finally, proof to support CR hypothesis in SCD comes from a recent work by Aghjayan and colleagues [25] which showed that correlation between SCD measures and cortical Aβ burden was stronger in higher educated subjects.
The effect of APOE ε4 allele as risk factor for memory decline and progression from normal cognition to objective cognitive decline (MCI or AD) over time has been widely demonstrated by the previous study [26,27,28,29,30]. A number of studies showed that the effect of APOE ε4 on cognitive impairment or dementia risk appears to be diminished by higher CR [31,32,33,34,35]. For instance, Carlson et al. [33] showed that higher CR was associated with lower risk of dementia in cognitively healthy subjects, particularly among ε4 carriers, with a 30% risk reduction. Pettigrew and colleagues [36] then found that ε2 allele was protective in individuals with high but not low CR. Ferrari et al. [37] recently found a positive effect of CR on progression of AD dementia only in APOE ε4 carriers. Furthermore, interaction between CR and APOE was supported also by neuroimaging investigations [38, 39]. Nevertheless, at the best of our knowledge, there are no longitudinal studies investigating the interaction between APOE and CR neither on progression to MCI in people with carefully phenotyped SCD nor on rate of progression from MCI to AD. Taking into account these evidences, in the present work, we aimed to investigated if CR could interact with APOE and influence the onset of symptoms in SCD and in MCI. Furthermore, we wanted to evaluate the effect of CR and its interaction with APOE on risk of progression from SCD to MCI and from MCI to AD. Finally, according to CR model, in subjects with higher CR, we expect to find a slower rate of conversion from SCD to MCI and a faster rate of progression from MCI to AD.
Materials and methods
Participants and clinical assessment
As part of a longitudinal, clinical–neuropsychological–genetic survey on SCD and MCI, we included 284 consecutive patients who self-referred to the Centre for Alzheimer’s Disease and Adult Cognitive Disorders of Careggi Hospital in Florence between March 1990 and March 2017.
All participants underwent a comprehensive family and clinical history, general and neurological examination, extensive neuropsychological investigation, assessment of depression by means of the 22-item Hamilton Depression Rating Scale (HDRS) [52], and estimation of premorbid intelligence as a proxy of CR at baseline. A positive family history was defined as one or more first-degree relatives with documented cognitive decline. Cognitive complaints were explored during the neurological interview at baseline using a survey based on the Memory Assessment Clinics Questionnaire [40]. 191 out of 284 participants had APOE genotype analysis. At the baseline evaluation, a diagnosis was made by a team of neurologists with expertise in neurodegenerative disorders based on clinical, neuropsychological, and instrumental examination according to international diagnostic criteria.
For this study, inclusion criteria were: (1) complaining of cognitive decline with a duration of ≥ 6 months; (2) normal functioning on the Activities of Daily Living and the Instrumental Activities of Daily Living scales [41]; (3) not satisfied criteria for dementia at baseline [42, 43]; (4) attainment of the clinical endpoint, i.e., conversion to MCI according to the National Institute on Aging-Alzheimer’s Association (NIA-AA) criteria [15] or conversion to AD according to the NIA-AA criteria [43] during follow-up, regardless of the follow-up duration; (5) a follow-up time more than 2 years from the baseline visit for those patients who did not develop MCI or AD. Exclusion criteria were: (1) history of head injury, current neurological and/or systemic disease, symptoms of psychosis, major depression, alcoholism or other substance abuse; (2) the complete data loss of patients’ follow-up; and (3) progression to dementia other than AD.
From the initial sample, we excluded 13 subjects who did not convert to MCI or to AD dementia with a follow-up shorter than 2 years. We excluded 1 subject who developed a brain tumor, 7 subjects who were diagnosed with other forms of dementia (6 Vascular Dementia, according to NINDS–AIREN criteria [44] and 1 fronto-temporal dementia, according to Neary criteria [45]). Therefore, we in the end included 263 subjects. We divided this sample in two groups: 154 subjects classified as SCD, according to the terminology proposed by the Subjective Cognitive Decline Initiative (SCD-I) Working Group [13] (i.e., presence of a self-experienced persistent decline in cognitive capacities with normal performance on standardized cognitive tests); 109 subjects classified as MCI according to (NIA-AA) criteria for the diagnosis of MCI [15] (i.e., evidence of lower performance in one or more cognitive domains with preserved independence of function in daily life).
All the patients underwent clinical and neuropsychological follow-up every 6 or 12 months. Baseline evaluation and annual clinical and cognitive assessments have been described in detail elsewhere [3].
On the basis of progression from SCD to MCI during the follow-up, SCD subjects were classified, respectively, as SCD-non-converters (SCD-nc) and SCD converters (SCD-c). In the same way, MCI subjects were classified as MCI-non-converters (MCI-nc) and MCI converters (MCI-c) according to the progression to AD dementia.
Estimated premorbid intelligence
To estimate the premorbid intelligence, all cases were assessed at baseline by the Test di Intelligenza Breve (TIB, i.e., Brief Intelligence Test) [46], an Italian version of the National Adult Reading Test (NART) [47]. The NART is a single word, oral reading test consisting of 50 items. All the words are irregular, that is, they violate grapheme–phoneme correspondence rules. Since Italian is a transparent language, the reading task could not be based on the irregularity in the grapheme-to-phoneme conversion as for the NART but rather on the irregularity of words with less frequent stress patterns.
Apolipoprotein E genotyping
DNA was extracted from peripheral blood samples from all subjects by use of the phenol–chloroform procedure, and the APOE gene was amplified in the polymorphic region [48]. The frequencies of the ε2, ε3, and ε4 alleles were estimated by gene counting. The APOE genotype was coded as APOE ε4 − (no APOE ε4 alleles) and APOE ε4 + (presence of one or two APOE ε4 alleles).
Statistical analysis
Patient groups were characterized using means and standard deviations (SD). We tested for the normality distribution of the data using the Kolmogorov–Smirnov test. Depending on the distribution of our data, we used t test or non-parametric Mann–Whitney U tests for between groups’ comparisons and Pearson’s correlation coefficient or non-parametric Spearman’s ρ (rho) to evaluate correlations between groups’ numeric measures. We used Chi-square test to compare categorical data. We run multiple linear regression models to assess relationship between variables which showed statistically significant correlations. We constructed Cox regression models to ascertain the effect of demographic and clinical variables which showed differences between groups on risk of conversion from SCD to MCI and from MCI to AD. Kaplan–Meier analysis was used to compare conversion time (as years from baseline visit to conversion) in different groups of SCD and MCI and pairwise log rank comparisons were conducted to determine if there were differences in conversion time among groups. All statistical analyses were performed with SPSS software v.13 (SPSS Inc., Chicago, USA). The significance level was set at p < 0.05.
Results
Demographic and clinical features
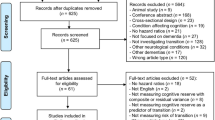
At follow-up, 44 out of 154 SCD subjects (29%) converted to MCI (SCD-c). Mean conversion time from SCD to MCI was 9.53 (± 4.04) years. A total of 110 subjects (71%) remained stable (SCD-nc) and their mean follow-up time (from baseline to last evaluation) was 6.40 (± 3.53) years (range 2.00–18.48 years, IQR 4.11 years). Including 44 SCD-c and 109 subjects who referred as MCI at baseline, our total MCI subjects consisted of 153 subjects. Of 153 MCI subjects, 51 (33%) developed AD dementia (MCI-c) and 100 (66%) remained stable (MCI-nc). 12 out of 51 MCI-c subjects were SCD-c. Two MCI subjects (1%) regressed to SCD and were included in the MCI-nc group, as we considered them as non-converters. Mean follow-up time of MCI-nc was 7.51 (± 4.78) years (range 2.00–27.20 years, IQR 5.21 years). A summary flowchart of the groups is reported in Fig. 1.
44 Out of 154 SCD subjects (29%) converted to MCI (SCD-c) and 110 subjects (71%) remained stable (SCD-nc). Including 44 SCD-c and 109 subjects who referred as MCI at baseline, our total MCI subjects consisted of 153 subjects. Of 153 MCI subjects, 51 (33%) developed AD dementia (MCI-c). 102 Subjects were considered as MCI-nc, including 100 (66%) MCI subjects who remained stable and two (1%) MCI subjects who regressed to SCD. Follow-up time and conversion time are expressed in years
No significant differences were found neither between SCD-nc and SCD-c nor between MCI-nc and MCI-c in sex, familiarity, disease duration (time from onset of symptoms and baseline evaluation), HDRS, and TIB. Age at baseline evaluation and age at onset of symptoms were lower in SCD-nc compared to SCD-c and in MCI-nc compared to MCI-c. SCD-nc had higher education and MMSE at baseline compared to SCD-c. MCI-nc had higher MMSE at baseline compared to MCI-c. In the SCD group, 29 subjects were APOE ε4 carriers (one ε2/ε4, 25 ε3/ε4 and three ε4/ε4) and 81 subjects were APOE ε4 non-carriers (14 ε2/ε3, 67 ε3/ε3). There was no statistically significant difference in genotype distribution of APOE ε4 between SCD-nc and SCD-c (p = 0.088). In the MCI group, 20 subjects were APOE ε4 carriers (two ε2/ε4, 17 ε3/ε4 and one ε4/ε4) and 39 subjects were APOE ε4 non-carriers (five ε2/ε3, 34 ε3/ε3). APOE ε4 was more frequent in MCI-c subjects than MCI-nc subjects (p < 0.001). Follow-up time in MCI-nc was significantly longer than conversion time in MCI-c (p < 0.001) (Table 1).
No significant differences were found between APOE ε4 + (46 subjects: 28 SCD, 18 MCI) and APOE ε4− (121 subjects: 82 SCD, 39 MCI) groups with regards to sex, familiarity, disease duration, age at onset, age at baseline, years of education, MMSE, HDRS and TIB neither in the SCD nor in the MCI groups. No subjects had depression according to HDRS scores.
Influence of cognitive reserve in SCD
First of all, we aimed to evaluate if there were any correlations between CR and age at baseline of SCD. In the SCD group, age at baseline was positively correlated with TIB (χ2 = 0.237, p = 0.004) and age at onset (χ2 = 0.911, p < 0.001), but inversely correlated with years of education (χ2 = − 0.171, p < 0.035). Positive correlations between TIB and MMSE (χ2 = 0.249, p = 0.002) and between TIB and years of education (χ2 = 0.722, p < 0.001) were also found. To evaluate if the interaction between ages at baseline and TIB was independent from other variables, a multiple regression analysis on the SCD group was run. We included age at baseline as dependent variable and age at onset, years of education, MMSE, and TIB as covariate. The regression model statistically significantly predicted age at baseline (adj. R2 = 0.825, p < 0.001) including TIB (β = 0.107, p = 0.036, I.C. 95%= 0.009:0.243) and age at onset (β = 0.898, p < 0.001, I.C. 95% = 0.785:0.928) in the final model.
Second, to the evaluate the effect of TIB on the risk of progression from SCD to MCI and to ascertain that this effect was independent from other confounding factors, a backward Cox regression analysis was performed on SCD group considering age at conversion as time and including age at baseline, age at onset, TIB, years of education, MMSE, and APOE as covariates. The Cox regression model was statistically significant (χ2 = 28.39, p < 0.001) and TIB (HR = 0.925, p = 0.020, I.C. 95% = 0.927:0.993), age at onset (HR = 0.760, p < 0.001, I.C. 95% = 0.795:1.011) and APOE (HR = 2.532, p < 0.045, I.C. 95% = 1.088:5.582) were included in the final model.
We assessed if CR might influence time of progression from SCD to MCI. In the SCD-c group, age at conversion positively correlated with TIB (χ2 = 0. 409, p = 0.007), age at onset (χ2 = 0.844, p > 0.001) and age at baseline (χ2 = 0.891, p > 0.001), but not with years of education (p = 0.102) nor MMSE (p = 0.222). No correlation was found between conversion time and TIB (p = 0.799). There were no differences between ε4 − and ε4 + subjects neither in age at conversion (p = 0.518) nor in conversion time (p = 0.819). To ascertain that the effect of TIB on age at conversion was independent from confounding factors, a multiple regression analysis on the SCD-c group was run, including age at conversion as dependent variable and age at onset, age at baseline and TIB as covariate. The regression model statistically significantly predicted age at conversion (adj. R2 = 0.775, p < 0.001) including TIB (β = 0.160, p = 0.041, I.C. 95% = 0.006:0.278) and age at onset (β = 0.844, p < 0.001, I.C. 95% = 0.727:1.047) in the final model.
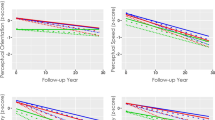
Finally, SCD-c subjects were ranked into three percentiles according to TIB score (low, n = 14; intermediate, n = 17; high, n = 13). Kaplan–Meier survival analysis was conducted to compare age at conversion in the three different groups of SCD-c (low, intermediate, and high TIB). Subjects in the high TIB group had a median age at conversion of 77.52 (95% CI 74.25–80.78) years. This was greater than the low and intermediate TIB groups, which had median age at conversion of 70.13 (95% CI 66.25–73.99) years and 68.28 (95% CI 64.66–71.89) years, respectively. Pairwise log rank comparisons were conducted to determine which TIB groups had different survival distributions. There was a statistically significant difference in survival distributions for the high vs. low TIB (χ2 = 9.205, p = 0.002) and intermediate vs. low TIB (χ2 = 9.094, p = 0.003). The survival distributions for high and intermediate TIB were not statistically significantly different (χ2 = 0.637, p = 0.425) (Table 2; Fig. 2).
Kaplan–Meier survival analysis for comparisons of age at conversion in SCD-c subjects ranked according to TIB score (low, n = 14; intermediate, n = 17; high, n = 13). Median age at conversion in high (77.52 years) and intermediate (70.13 years) TIB group was statistically significantly greater than median age at conversion in low TIB group (68.28 years)
Influence of cognitive reserve in MCI
In the MCI group, TIB positively correlated with age at baseline (χ2 = 0.257, p = 0.002) and age at onset (χ2 = 0.250, p = 0.002). No correlations were found neither among age at baseline, years of education and MMSE, nor between age at onset, years of education and MMSE. There were no differences between ε4 − and ε4 − MCI subjects neither in age at baseline (p = 0.383) nor in age at onset (p = 0.058).
To the evaluate the effect of TIB on the risk of progression from MCI to AD dementia and to ascertain that this effect was independent from other confounding factors, a backward Cox regression analysis was performed on MCI group considering follow-up time as time and including age at baseline, age at onset, TIB, MMSE, and APOE as covariates. The Cox regression model was statistically significant (χ2 = 28.39, p < 0.001) including age at onset (HR = 1.189, p < 0.001, I.C. 95% = 1.096:1.289) and APOE (HR = 3.538, p < 0.010, I.C. 95% = 1.351:9.264) were included in the final model.
Given this result, we divided the MCI group according to APOE genotype and repeated the Cox regression analysis. In the ε4 + MCI subgroup, TIB was included in the final model (χ2 = 5.789, p < 0.016) as a risk factor (HR = 1.125, p < 0.036, I.C. 95% = 1.088:1.256), while in the ε4 – subgroup, only age at baseline remained statistically significant in the final model.
No statistically significant correlation between TIB and age at conversion was found, neither in ε4 + (p = 0.775) nor in ε4 − subjects (p = 0.320). However, in MCI-c ε4 + subjects, TIB negatively correlates with conversion time (time from baseline evaluation to conversion to AD; χ2 = − 0.712, p = 0.001). No statistically significant correlations were found between age at conversion and other variables. There were no statistically significantly differences in conversion time between MCI-c who were SCD at baseline and MCI-c who were MCI at baseline (p = 0.856).
In the last step, MCI-c subjects were ranked into four groups according to TIB score and APOE genotype (low/ε4 −, n = 6; low/ε4 +, n = 4; high/ε4 −, n = 7; high/ε4 +, n = 13). Kaplan–Meier survival analysis was conducted to compare conversion time in the four different groups. Subjects in high TIB/ε4 + group had a median conversion time of 1.71 (95% CI, 1.054 to 2.364) years. This was longer than the low/ε4 +, low/ε4 −, and high/ε4 − groups, which had similar median conversion time of 5.49 (95% CI, 1.83 to 9.15), 3.49 (95% CI, 2.05 to 4.84), and 3.29 (95% CI, 1.62 to 4.95) years, respectively. Pairwise log rank comparisons were conducted to determine which TIB groups had different survival distributions. There was a statistically significant difference in survival distributions for the high/ε4 + vs. low/ε4 + (χ2 = 4.66, p = 0.031) and high/ε4 + vs. low/ε4 − (χ2 = 4.77, p = 0.029) (Table 3; Fig. 3). There were no statistically significant differences between high/ε4 − vs. low/ε4 + and high/ε4 − vs. low/ε4 −. The difference in survival distributions for the high/ε4 + vs. high/ε4 + did not reach statistical significance (χ2 = 2.76, p = 0.096).
Kaplan–Meier survival analysis for comparisons follow-up time in MCI-c subjects ranked according to TIB score and APOE genotype (low/ε4 −, n = 6; low/ε4 +, n = 4; high/ε4 −, n = 7; high/ε4 +, n = 13). Median conversion time in high TIB/ε4 + group (1.71 years) was statistically significantly shorter than median conversion time in low/ε4 + and low/ε4 − groups (5.49, 3.49, and 3.29 years, respectively)
Discussion
This study investigated the relationship between level of CR and long-term diagnostic outcomes among middle-aged and older individuals with SCD and MCI. The main finding was that the relationship between level of CR and longitudinal change in cognitive outcome differed as a function of the initial cognitive state and APOE profile.
In a recently published study in SCD and MCI [3], we demonstrated that intellectual activities play as protective factors, reducing the risk of conversion from SCD to MCI of about 30%. In the present study, we aimed to evaluate the role of premorbid intelligence, as a standard proxy of CR, on SCD and MCI onset and on progression to AD dementia. Premorbid intelligence has been measured by mean of the Test di Intelligenza Breve (TIB, i.e., Brief Intelligence Test) [46], functionally equivalent to the National Adult Reading Test [47], which assesses pronunciation of irregular, low-frequency words. These words are likely to be pronounced incorrectly using common phonetic interpretation rules if an individual does not already possess this linguistic knowledge. Based on the observation that reading ability is typically only mildly affected by aging and by many forms and degrees of cerebral injury or pathology [49,50,51], this measure has been successfully applied to evaluate premorbid abilities in MCI [52] and in mild-to-moderate dementia cases [53, 54] in a more precise manner than years of education.
We found that CR positively correlated with age at baseline of SCD. This means that subjects with higher CR complained cognitive decline later compared to subjects with lower CR. The previous studies on SCD subjects showed that lower CR is associated with greater overall memory concerns [55, 56], suggesting that SCD individuals with higher CR may perceive cognitive changes at relatively later stages in the neuropathological disease process. However, at the best of our knowledge, this is the first study, showing that the onset of SCD may be influenced by CR.
As regards progression from SCD to MCI, CR reduced the risk of progression by about 8%, while APOE played as risk factor, as ε4 carriers had a 2.5 times higher risk of progression compared to non-carriers.
The effect of APOE ε4 allele on memory decline and on risk of progression from SCD to objective cognitive decline (MCI or AD) over time has already been widely demonstrated by the previous study [26,27,28,29,30]. In particular, Jessen et al. [30] and Hong et al. [29] showed Hazard ratio (HR) values comparable to ours HR, ranging from 2.4 to 2.7. A number of studies have been conducted to ascertain the effect of CR on progression from normal cognition to a full-blown cognitive decline [23,24,24, 33, 36]. Carlson et al. [33] demonstrated that higher CR was associated with a 26% risk reduction for dementia onset. Pettigrew et al. [36] showed that CR reduced risk of progressing from normal cognition to MCI by about 50% in both ε4 carriers and non-carriers. A study by Lojo-Seoane et al. [24] first showed that CR had a positive influence on cognitive performance at follow-up in a sample of older adults with SCD. In a recent study by our group [3], we showed that greater participation in intellectual activities acted as a protective factor against progression from SCD to MCI, reducing risk of progression by about 30%. Our current results are in line with these previous evidences, even if the effect on risk of progression seems to be lower (8%).
Finally, CR also influence rate of progression from SCD to MCI postponing age at conversion. More to the point, SCD subjects with low TIB scores progressed to MCI 2 years before SCD subjects with intermediate TIB score and 9 years before SCD subjects with high TIB scores. A similar results was found in a study by Vemuri et al. [57] which showed that in cognitively healthy subjects with high CR, the onset of cognitive impairment was approximately 8 years later compared with subjects with low CR.
In summary, CR delayed the onset of earliest symptoms of cognitive decline, but the effect on risk of progression was low (only 8%). On the contrary, APOE has been confirmed as a strong risk factor for progression from SCD to objective cognitive decline, as demonstrated in the previous studies [58, 59], but did not show any effect on rate of progression from SCD to MCI.
In the second part of our work, we focused on MCI and progression to AD dementia. We have shown that higher CR is related to higher age at onset of MCI, in line with literature data [36, 60].A series of studies suggested that CR might have different effects according to APOE genotype, mainly demonstrating that the protective effect of CR against dementia was stronger in healthy subjects with ε4 allele [31, 33, 35]. However, at the best of our knowledge, there are no studies investigating the interaction between APOE and CR on rate of progression from MCI to AD.
According to our results, CR was a risk factor for progression from MCI to AD dementia only in the ε4 + group, increasing the risk of about 12%, while in the ε4 − group did not show any statistically significant effect.
A first explanation is that the lower amount of conversion in the ε4 − group might mask the negative effect that CR would play in MCI. Studies with longer follow-up on larger samples are needed to ascertain this evidence. Another hypothesis may be that subjects with a genetic predisposition might be more sensitive to the depletion of CR than ε4 non-carriers. Interestingly, a study by Arenaza-Urquijo et al. [61] on 72 healthy subjects showed that higher CR was related to higher fronto-temporal metabolism only in APOE ε4 carriers. Hence, we could speculate that, when CR is overcome, this compensatory mechanism runs out, leading to a more rapid decline only in the ε4 carriers. One more chance is that ε4 non-carriers groups included also ε2 allele carriers, which has been shown as a protective factor against dementia [62]. Indeed, a work by Pettigrew and colleagues [36] found that ε2 allele is protective only in individuals with high CR but not in individuals with low CR. Thence, we could hypothesize that detrimental effect of higher CR may be minimize by protective effect of ε2. Due to the low number of ε2 carrier subjects in our sample, we are not able to test this hypothesis at the time. However, this should by inspiration for further studies. Soldan et al. [23] recently showed that among individuals with normal cognition, higher CR was associated with better cognitive performance but with faster cognitive decline after onset of MCI. A number of the previous studies reported greater rates of cognitive decline [63, 64] or functional decline [65] among individuals affected by dementia with higher CR compared to those with low CR. In line with these evidences and according to Stern’s model, which predicts that when CR is overcome a faster rate of decline after the onset of clinical symptoms is evident [2, 4], we expected a faster rate of progression from MCI to AD in subjects with higher CR. Interestingly, our results confirmed these evidences only in APOE ε4 + subjects. In fact, median time of conversion in ε4 − carrier MCI subjects with high TIB was 4 years shorter than ε4 carriers with low TIB, while CR levels did not lead to different rate of progression in ε4 non-carriers. Thus, we demonstrated that CR influenced progression from MCI to AD dementia only in case of genetic predisposition. A number of clinical [31,32,33,34,35] and neuroimaging studies [38, 39] showed an interaction between CR and APOE, mainly demonstrating that high CR may modulate the detrimental role of ε4 effect on cognitive impairment or dementia risk in healthy subjects. In our previous study [66], we focused on the role of CR and APOE on cognitive performance and decline progression in mild-AD patients and found that higher CR was related to a more rapid progression of the cognitive impairment. Our present study supports and extends these findings, showing that CR might modulate progression of cognitive decline also in the MCI phase.
A limitation of the present study is the lack of biomarkers data. As CR hypothesis is based on the assumption that different grades of pathology load correspond to different grade of cognitive decline, estimation of grade disease by means of CSF biomarkers and functional and structural imaging may undoubtedly provide useful information. For future, studies are needed to test if biomarker levels at the time of symptom onset modify the relationship between CR and subsequent cognitive change, as Stern’s model would predict. We are presently in the process of studying these issues. Second, MCI subgroups ranked according to APOE genotype were very small, in particular low/ε4 − and low/ε4 + MCI-c groups. Moreover, as it is a single-center study, there may be estimator and analytical biases with regard to assessment and diagnosis procedures. Finally, as we considered a clinic-based cohort, sampling error might be possible.
A major strength of this study is the very long average follow-up time. In fact, follow-up time in the SCD-nc group is comparable to time of conversion in SCD-c, and MCI-nc is even much longer than conversion time in MCI-c. This information allows us to minimize the possible underestimation of conversion to AD dementia and the risk of classifying as stable subjects carrying an Alzheimer pathology who will convert later in the follow-up. Furthermore, at the best of our knowledge, this is one of the first studies evaluating the effect of CR on progression to MCI in people with carefully phenotyped SCD. The majority of the previous studies considered a whole sample without distinguishing between individuals with MCI. Finally, another strength is the use of reading level by means of TIB (an Italian version of the National Adult Reading Test) as CR estimator. Indeed, the ease of administration, patient tolerability, and low costs of this test allow, suggesting its use both in research and in clinical setting.
Conclusion
Stern’s model of CR consists of two points: (1) that high levels of CR allow one to better cope with brain pathology delaying the onset of cognitive symptoms and (2) that when CR is overcome a faster rate of decline after the onset of clinical symptoms is evident. The results of the present study are in line with this model showing that: (1) progression to MCI is delayed about 9 years in SCD subjects with high CR and (2) higher CR anticipates diagnosis of AD dementia up to 4 years in MCI subjects only, where there is a genetic predisposition (APOE ε4). In addition to informing theoretical models of CR, overall, our data provide evidence that higher levels of CR protect against the earliest clinical manifestations of AD by delaying the onset of symptoms associated with the disease. In the absence of effective medications that reduce underlying AD pathology, activities which increase CR throughout life should be promoted as preventive measures, to delay the onset of a potential future cognitive decline.
Abbreviations
- CR:
-
Cognitive reserve
- SCD:
-
Subjective cognitive decline
- TIB:
-
Test di Intelligenza Breve
- HDRS:
-
Hamilton Depression Rating Scale
- NART:
-
National adult reading test
References
Stern Y (2009) Cognitive reserve. Neuropsychologia 47:2015–2028. https://doi.org/10.1016/j.neuropsychologia.2009.03.004
Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R (1994) Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271:1004–1010
Bessi V, Mazzeo S, Padiglioni S, Piccini C, Nacmias B, Sorbi S, Bracco L (2018) From subjective cognitive decline to Alzheimer’s disease: the predictive role of neuropsychological assessment, personality traits, and cognitive reserve. A 7-year follow-up study. J Alzheimers Dis JAD. https://doi.org/10.3233/JAD-171180
Stern Y (2002) What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc JINS 8:448–460
Cecchetti L, Ricciardi E, Volpi L, Carlesi C, Pagni C, Handjaras G, Leo A, Tognoni G, Pietrini P (2013) Cognitive reserve modulates neural responses in subjective and mild cognitive impairment. Alzheimers Dement J Alzheimers Assoc 9:P490. https://doi.org/10.1016/j.jalz.2013.05.1003
Garibotto V, Borroni B, Sorbi S, Cappa SF, Padovani A, Perani D (2012) Education and occupation provide reserve in both ApoE ε4 carrier and noncarrier patients with probable Alzheimer’s disease. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 33:1037–1042. https://doi.org/10.1007/s10072-011-0889-5
Franzmeier N, Hartmann JC, Taylor ANW, Araque Caballero M, Simon-Vermot L, Buerger K, Kambeitz-Ilankovic LM, Ertl-Wagner B, Mueller C, Catak C, Janowitz D, Stahl R, Dichgans M, Duering M, Ewers M (2017) Left frontal hub connectivity during memory performance supports reserve in aging and mild cognitive impairment. J Alzheimers Dis JAD 59:1381–1392. https://doi.org/10.3233/JAD-170360
Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y (2011) Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc JINS 17:593–601. https://doi.org/10.1017/S1355617710001748
Stern Y, Alexander GE, Prohovnik I, Mayeux R (1992) Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol 32:371–375. https://doi.org/10.1002/ana.410320311
Scarmeas N, Levy G, Tang M-X, Manly J, Stern Y (2001) Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology 57:2236–2242
Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK (2002) Models of visuospatial and verbal memory across the adult life span. Psychol Aging 17:299–320
Wisdom NM, Mignogna J, Collins RL (2012) Variability in Wechsler Adult Intelligence Scale-IV subtest performance across age. Arch Clin Neuropsychol Off J Natl Acad Neuropsychol 27:389–397. https://doi.org/10.1093/arclin/acs041
Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 10:844–852. https://doi.org/10.1016/j.jalz.2014.01.001
Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256:183–194. https://doi.org/10.1111/j.1365-2796.2004.01388.x
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7:270–279. https://doi.org/10.1016/j.jalz.2011.03.008
Reisberg B, Gauthier S (2008) Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. Int Psychogeriatr 20:1–16. https://doi.org/10.1017/S1041610207006412
Mitchell AJ (2008) Is it time to separate subjective cognitive complaints from the diagnosis of mild cognitive impairment? Age Ageing 37:497–499. https://doi.org/10.1093/ageing/afn147
Mendonça MD, Alves L, Bugalho P (2016) From subjective cognitive complaints to dementia: who is at risk? A systematic review. Am J Alzheimers Dis Other Demen 31:105–114. https://doi.org/10.1177/1533317515592331
Lojo-Seoane C, Facal D, Guàrdia-Olmos J, Juncos-Rabadán O (2014) Structural model for estimating the influence of cognitive reserve on cognitive performance in adults with subjective memory complaints. Arch Clin Neuropsychol 29:245–255. https://doi.org/10.1093/arclin/acu007
Perquin M, Diederich N, Pastore J, Lair M-L, Stranges S, Vaillant M, Group on behalf of the M (2015) Prevalence of dementia and cognitive complaints in the context of high cognitive reserve: a population-based study. PLoS One 10:e0138818. https://doi.org/10.1371/journal.pone.0138818
João AA, Maroco J, Ginó S, Mendes T, Mendonça A de, Martins IP (2015) Education modifies the type of subjective memory complaints in older people. Int J Geriatr Psychiatry 31:153–160. https://doi.org/10.1002/gps.4305
Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC (2015) Relationship between education and age-related cognitive decline: a review of recent research. Psychogeriatrics 15:154–162. https://doi.org/10.1111/psyg.12083
Soldan A, Pettigrew C, Cai Q, Wang J, Wang M-C, Moghekar A, Miller MI, Albert M, BIOCARD Research Team (2017) Cognitive reserve and long-term change in cognition in aging and preclinical Alzheimer’s disease. Neurobiol Aging 60:164–172. https://doi.org/10.1016/j.neurobiolaging.2017.09.002
Lojo-Seoane C, Facal D, Guàrdia-Olmos J, Pereiro AX, Juncos-Rabadán O (2018) Effects of cognitive reserve on cognitive performance in a follow-up study in older adults with subjective cognitive complaints. The role of working memory. Front Aging Neurosci 10:189. https://doi.org/10.3389/fnagi.2018.00189
Aghjayan SL, Buckley RF, Vannini P, Rentz DM, Jackson JD, Sperling RA, Johnson KA, Amariglio RE (2017) The influence of demographic factors on subjective cognitive concerns and beta-amyloid. Int Psychogeriatr 29:645–652. https://doi.org/10.1017/S1041610216001502
Rawle MJ, Davis D, Bendayan R, Wong A, Kuh D, Richards M (2018) Apolipoprotein-E (Apoe) ε4 and cognitive decline over the adult life course. Transl Psychiatry 8:18. https://doi.org/10.1038/s41398-017-0064-8
Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DEC, Snyder CH, Alexander GE, Rademakers R, Reiman EM (2009) Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 361:255–263. https://doi.org/10.1056/NEJMoa0809437
Soldan A, Pettigrew C, Lu Y, Wang M-C, Selnes O, Albert M, Brown T, Ratnanather JT, Younes L, Miller MI, BIOCARD Research Team (2015) Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease. Hum Brain Mapp 36:2826–2841. https://doi.org/10.1002/hbm.22810
Hong YJ, Yoon B, Shim YS, Kim S-O, Kim HJ, Choi SH, Jeong JH, Yoon SJ, Yang DW, Lee J-H (2015) Predictors of clinical progression of subjective memory impairment in elderly subjects: data from the clinical research centers for dementia of South Korea (CREDOS). Dement Geriatr Cogn Disord 40:158–165. https://doi.org/10.1159/000430807
Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, Pentzek M, Riedel-Heller SG, Luck T, Fuchs A, Weyerer S, Werle J, van den Bussche H, Scherer M, Maier W, Wagner M, German Study on Aging, Cognition and Dementia in Primary Care Patients (2014) AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement J Alzheimers Assoc 10:76–83. https://doi.org/10.1016/j.jalz.2012.09.017
Kaup AR, Nettiksimmons J, Harris TB, Sink KM, Satterfield S, Metti AL, Ayonayon HN, Yaffe K (2015) Cognitive resilience to apolipoprotein E ε4: contributing factors in black and white older adults. JAMA Neurol 72:340–348. https://doi.org/10.1001/jamaneurol.2014.3978
López ME, Turrero A, Delgado ML, Rodríguez-Rojo IC, Arrazola J, Barabash A, Maestú F, Fernández A (2017) APOE ε4 genotype and cognitive reserve effects on the cognitive functioning of healthy elders. Dement Geriatr Cogn Disord 44:328–342. https://doi.org/10.1159/000481852
Carlson MC, Helms MJ, Steffens DC, Burke JR, Potter GG, Plassman BL (2008) Midlife activity predicts risk of dementia in older male twin pairs. Alzheimers Dement J Alzheimers Assoc 4:324–331. https://doi.org/10.1016/j.jalz.2008.07.002
Niti M, Yap K-B, Kua E-H, Tan C-H, Ng T-P (2008) Physical, social and productive leisure activities, cognitive decline and interaction with APOE-ε4 genotype Chinese older adults. Int Psychogeriatr 20:237–251. https://doi.org/10.1017/S1041610207006655
Wang H-X, Gustafson DR, Kivipelto M, Pedersen NL, Skoog I, Windblad B, Fratiglioni L (2012) Education halves the risk of dementia due to apolipoprotein ε4 allele: a collaborative study from the Swedish brain power initiative. Neurobiol Aging 33:1007.e1–1007.e7. https://doi.org/10.1016/j.neurobiolaging.2011.10.003
Pettigrew C, Soldan A, Li S, Lu Y, Wang M-C, Selnes OA, Moghekar A, O’Brien R, Albert M, The Biocard Research Team null (2013) Relationship of cognitive reserve and APOE status to the emergence of clinical symptoms in preclinical Alzheimer’s disease. Cogn Neurosci 4:136–142. https://doi.org/10.1080/17588928.2013.831820
Ferrari C, Lombardi G, Polito C, Lucidi G, Bagnoli S, Piaceri I, Nacmias B, Berti V, Rizzuto D, Fratiglioni L, Sorbi S (2018) Alzheimer’s disease progression: factors influencing cognitive decline. J Alzheimers Dis JAD 61:785–791. https://doi.org/10.3233/JAD-170665
Arenaza-Urquijo EM, Gonneaud J, Fouquet M, Perrotin A, Mézenge F, Landeau B, Egret S, De la Sayette V, Desgranges B, Chételat G (2015) Interaction between years of education and APOE ε4 status on frontal and temporal metabolism. Neurology 85:1392–1399. https://doi.org/10.1212/WNL.0000000000002034
Wirth M, Haase CM, Villeneuve S, Vogel J, Jagust WJ (2014) Neuroprotective pathways: lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol Aging 35:1873–1882. https://doi.org/10.1016/j.neurobiolaging.2014.02.015
Crook TH, Feher EP, Larrabee GJ (1992) Assessment of memory complaint in age-associated memory impairment: the MAC-Q. Int Psychogeriatr 4:165–176
Lawton MP, Brody EM (1969) Assessment of Older people: self-maintaining and instrumental activities of daily living. The Gerontologist 9:179–186. https://doi.org/10.1093/geront/9.3_Part_1.179
Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, Nordberg A, Bäckman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, De Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, Jack C, Jorm A, Ritchie K, Van Duijn C, Visser P, Petersen R c (2004) Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 256:240–246. https://doi.org/10.1111/j.1365-2796.2004.01380.x
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 7:263–269. https://doi.org/10.1016/j.jalz.2011.03.005
Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 43:250–260
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Colombo L, Sartori G, Brivio C (2002) Stima del quoziente intellettivo tramite l’applicazione del TIB (test breve di intelligenza). G Ital Psicol. https://doi.org/10.1421/1256
Nelson H (1982) National adult reading test (NART): for the assessment of premorbid intelligence in patients with dementia: test manual. NFER-Nelson, Windsor
Sorbi S, Nacmias B, Forleo P, Latorraca S, Gobbini I, Bracco L, Piacentini S, Amaducci L (1994) ApoE allele frequencies in Italian sporadic and familial Alzheimer’s disease. Neurosci Lett 177:100–102
Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S (2007) APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology 21:1–8. https://doi.org/10.1037/0894-4105.21.1.1
Casaletto KB, Cattie J, Franklin DR, Moore DJ, Woods SP, Grant I, Heaton RK (2014) The wide range achievement test-4 reading subtest “Holds” in HIV-infected individuals. J Clin Exp Neuropsychol 36:992–1001. https://doi.org/10.1080/13803395.2014.960370
Green REA, Melo B, Christensen B, Ngo L-A, Monette G, Bradbury C (2008) Measuring premorbid IQ in traumatic brain injury: an examination of the validity of the Wechsler Test of Adult Reading (WTAR). J Clin Exp Neuropsychol 30:163–172. https://doi.org/10.1080/13803390701300524
Oliveira MO de, Nitrini R, Yassuda MS, Brucki SMD (2014) Vocabulary is an appropriate measure of premorbid intelligence in a sample with heterogeneous educational level in Brazil. In: Behav. Neurol. https://www.hindawi.com/journals/bn/2014/875960/. Accessed 29 May 2018
Crawford JR, Deary IJ, Starr J, Whalley LJ (2001) The NART as an index of prior intellectual functioning: a retrospective validity study covering a 66-year interval. Psychol Med 31:451–458
McGurn B, Starr JM, Topfer JA, Pattie A, Whiteman MC, Lemmon HA, Whalley LJ, Deary IJ (2004) Pronunciation of irregular words is preserved in dementia, validating premorbid IQ estimation. Neurology 62:1184–1186
Stewart R, Godin O, Crivello F, Maillard P, Mazoyer B, Tzourio C, Dufouil C (2011) Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br J Psychiatry J Ment Sci 198:199–205. https://doi.org/10.1192/bjp.bp.110.078683
Derouesné C, Dealberto MJ, Boyer P, Lubin S, Sauron B, Piette F, Kohler F, Alpérovitch A (1993) Empirical evaluation of the “Cognitive Difficulties Scale” for assessment of memory complaints in general practice: a study of 1628 cognitively normal subjects aged 45–75 years. Int J Geriatr Psychiatry 8:599–607. https://doi.org/10.1002/gps.930080712
Vemuri P, Lesnick TG, Przybelski SA, Machulda M, Knopman DS, Mielke MM, Roberts RO, Geda YE, Rocca WA, Petersen RC, Jack CR (2014) Association of lifetime intellectual enrichment with cognitive decline in the older population. JAMA Neurol 71:1017–1024. https://doi.org/10.1001/jamaneurol.2014.963
Guerreiro R, Bras J (2015) The age factor in Alzheimer’s disease. Genome Med. https://doi.org/10.1186/s13073-015-0232-5
Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261:921–923
Soldan A, Pettigrew C, Wang M-C, Li S, Lu Y, Albert M, Selnes O (2013) Relationship of cognitive reserve and APOE status to clinical symptom onset of mild cognitive impairment: the BIOCARD Cohort. Alzheimers Dement J Alzheimers Assoc 9:P134. https://doi.org/10.1016/j.jalz.2013.04.072
Arenaza-Urquijo EM, Wirth M, Chételat G (2015) Cognitive reserve and lifestyle: moving towards preclinical Alzheimer’s disease. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2015.00134
Wu L, Zhao L (2016) ApoE2 and Alzheimer’s disease: time to take a closer look. Neural Regen Res 11:412–413. https://doi.org/10.4103/1673-5374.179044
Roselli F, Tartaglione B, Federico F, Lepore V, Defazio G, Livrea P (2009) Rate of MMSE score change in Alzheimer’s disease: influence of education and vascular risk factors. Clin Neurol Neurosurg 111:327–330. https://doi.org/10.1016/j.clineuro.2008.10.006
Scarmeas N, Albert SM, Manly JJ, Stern Y (2006) Education and rates of cognitive decline in incident Alzheimer’s disease. J Neurol Neurosurg Psychiatry 77:308–316. https://doi.org/10.1136/jnnp.2005.072306
de Oliveira IR, Seixas C, Osório FL, Crippa JAS, de Abreu JN, Menezes IG, Pidgeon A, Sudak D, Wenzel A (2015) Evaluation of the psychometric properties of the cognitive distortions Questionnaire (CD-Quest) in a sample of undergraduate students. Innov Clin Neurosci 12:20–27
Bracco L, Piccini C, Baccini M, Bessi V, Biancucci F, Nacmias B, Bagnoli S, Sorbi S (2007) Pattern and progression of cognitive decline in Alzheimer’s disease: role of premorbid intelligence and ApoE genotype. Dement Geriatr Cogn Disord 24:483–491. https://doi.org/10.1159/000111081
Acknowledgements
This research was funded by Ministero della Salute and Regione Toscana (Grants n° GR-2010-2316359—Longitudinal clinical–neuropsychological study of subjective memory complaints).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest to report.
Ethical standards
All procedures involving experiments on human subjects have been done in accordance with the ethical standards of the Committee on Human Experimentation of the institution in which the experiments were done or in accordance with the Helsinki Declaration of 1975. Specific national laws have been observed.
Rights and permissions
About this article
Cite this article
Mazzeo, S., Padiglioni, S., Bagnoli, S. et al. The dual role of cognitive reserve in subjective cognitive decline and mild cognitive impairment: a 7-year follow-up study. J Neurol 266, 487–497 (2019). https://doi.org/10.1007/s00415-018-9164-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9164-5