Abstract
Objective
We investigated if anodal transcranial direct current stimulation (A-tDCS), applied over the supplementary motor areas (SMAs), could improve gait initiation in Parkinson’s disease (PD) with freezing of gait (FOG).
Methods
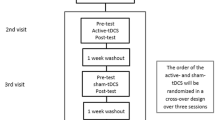
In this double-blinded cross-over pilot study, ten PD with FOG underwent two stimulation sessions: A-tDCS (1 mA, 10 min) and sham stimulation. Eight blocks of gait initiation were collected per session: (1) pre-tDCS, with acoustic cueing; (2) pre-tDCS, self-initiated (no cue); and (3–8) post-tDCS, self-initiated. Gait initiation kinetics were analyzed with two-way repeated measures ANOVAs for the effects of A-tDCS.
Results
A-tDCS did not significantly improve the magnitude or timing of anticipatory postural adjustments or the execution of the first step during self-initiated gait compared with baseline measures (p > .13). The lack of significant change was not due to an inability to generate functional APAs since external cueing markedly improved gait initiation (p < .01).
Conclusions
A single dose of A-tDCS over the SMAs did not improve self-initiated gait in PD and FOG. Alternative approaches using a different dose or cortical target are worthy of exploration since individuals demonstrated the capacity to improve.
Significance
Neuromodulation strategies tailored to facilitate SMA activity may be ineffective for the treatment of gait initiation impairment in people with PD and FOG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease leading to a progressive decline in motor function, including signs of akinesia, rigidity, tremor, bradykinesia, postural instability, and gait disorders. It has been estimated that more than one-third of all patients with idiopathic PD will develop the akinetic syndromes of start hesitation (episodic impairments in the ability to initiate gait) or freezing of gait (FOG, the spontaneous arrest of stepping during gait) [1]. Over time, these symptoms often become refractory to pharmacological (e.g., levodopa replacement) and neurosurgical (e.g., deep brain stimulation) treatments and are associated with a significant reduction in quality of life [2, 3]. Currently, there is no effective treatment for these symptoms.
Previous research has shown that individuals with PD who have start hesitation and FOG often demonstrate impairments in the anticipatory postural adjustments (APAs) that precede and accompany gait initiation, particularly when it is self-initiated (non-cued) [4,5,6]. APAs involve a sequence of shifts in the location and magnitude of forces beneath the feet that ensure the body’s center of mass is propelled forward and toward the initial stance leg prior to the step leg toe-off [7, 8]. In people with PD, APAs are often absent or reduced in amplitude and prolonged in duration, which results in the termination or shortening of the initial step [4,5,6]. Abnormal APAs are also associated with an increased risk of falls [9].
It has been hypothesized that reduced activity in the supplementary motor area (SMA) contributes to the pathogenesis of start hesitation and FOG in PD [10]. The SMA is believed to play a critical role in the preparation and initiation of uncued (self-initiated) movements [11,12,13,14,15] and the coupling of movement and posture during tasks such as reaching and gait initiation [16,17,18]. Movement-related activity in the SMA is significantly reduced in people with PD [13, 19]. Moreover, structural and functional connectivity between the SMA and mesencephalic locomotor region, a region that contributes to the control of posture and locomotion, is abnormal in people with PD and FOG [20, 21]. There is recent evidence that APAs in people with PD may be differentially affected in those with and without FOG. People with FOG tend to have reduced medial–lateral and anterior-posterior APAs, particularly during the transition phase from standing to toe-off, compared with those without FOG [22, 23]. Anodal transcranial direct current stimulation (A-tDCS) is a non-invasive brain stimulation method that has the potential to facilitate SMA function and thus improve gait initiation. Studies in healthy adults have shown that A-tDCS (at 1–2 mV, 10–20 min) is associated with changes in movement preparation, performance, and learning in the upper limb [24,25,26,27], including modulation of postural components of APAs [28]. In people with PD, a small trial of A-tDCS (eight sessions over 2 ½ weeks) applied over the motor, premotor or prefrontal cortex demonstrated that treatment was associated with improvements in the time to walk 10 m (starting from quiet standing) [29], suggesting that facilitation of the premotor regions may contribute to improved gait initiation. Similarly, repetitive transcranial magnetic stimulation (rTMS) over the SMA was shown to reduce the duration of the APA during the first trial following stimulation, but not subsequent trials, in a group of subjects with PD off-medication [18].
Recent studies have examined the effects of tDCS, applied to either the primary motor cortex alone, or in combination with stimulation of prefrontal regions involved in executive function, on motor behavior and freezing in people with PD [30,31,32]. These studies showed that single [30] or repeated sessions [31, 32] can reduce the incidence and duration FOG episodes and the time to complete timed up-and-go tasks. In particular, combined primary motor and dorsolateral prefrontal stimulation showed the most consistent results across subjects at reducing FOG events [30]. Yet, it is unclear if these interventions improved the capacity to generate APAs required for movement transitions. To date, no study has examined the effects of A-tDCS targeting the SMA on self-initiated gait in people with PD and FOG.
The purpose of this pilot study was to evaluate the effect of A-tDCS over the SMA on the APAs and initial steps associated with self-initiated gait in people with PD and FOG. Changes in gait initiation following A-tDCS were compared with improvements observed during acoustically cued gait initiation, since external cueing can evoke APAs comparable to those seen in healthy adults or following the administration of levodopa [5, 23, 33,34,35,36]. We hypothesized that (1) A-tDCS would significantly facilitate gait initiation by increasing the amplitude and decreasing the duration of APAs compared to sham stimulation, and (2) the effects of A-tDCS on APAs would be comparable to external cueing.
Methods
Subjects
Ten people with PD and FOG (mean ± SD, 66.3 ± 9.9 years; seven men) were included in this pilot study. All subjects were diagnosed with idiopathic PD by a movement disorders neurologist (Hoehn and Yahr scale of II–IIII) [37]. The mean ± SD score for the FOG questionnaire [38] was 18.5 ± 4.6, time since disease onset was 7.7 ± 4.0 years, ‘off drug’ Unified Parkinson’s Disease Rating Scale (UPDRS) part III was 39.2 ± 17.2, Montreal Cognitive Assessment (MoCA) was 24.5 ± 3.3, and the levodopa equivalent dosage was 761.0 ± 362.2 mg.
Subjects were tested in the morning after overnight withdrawal (12+ h) from antiparkinson’s medications (in the practically defined off-medication state). Subjects were excluded for a history of a musculoskeletal disorder affecting lower limb movement, a tremor score of greater than 2 on items 20 and 21 of the UPDRS, or a history of seizures or migraines. Subjects were excluded from the MRI portion of the study when unable to pass the MRI safety screening, but were allowed to participate in the remainder of the study. Written informed consent was obtained prior to participation in the study and all procedures were approved by the Institutional Review Board at the University of Minnesota.
MRI and neuronavigation
Eight of the ten subjects underwent an MRI scan acquired on a 3T scanner (MAGNETOM Prisma, Siemens, Erlangen, Germany) on the morning of their first tDCS session. (Two subjects were not approved for the MRI, but participated in the remainder of the study procedures.) Anatomical data of T1-weighted images were acquired using a Magnetization-Prepared Rapid Gradient-Echo (MPRAGE) sequence (TR = 2.54 s; TE = 3.65 ms; flip angle = 7°, FOV = 256 mm × 256 mm; 256 slices; voxel size = 1 × 1 × 1 mm). The acquisition time for the anatomical MRI scans was approximately 6 min. Anatomical scans were used to identify the SMA in each individual with a neuronavigation system (Brainsight Version 2, Rogue Research Inc., Montreal, Canada). The anatomical scan was first transformed into Talairach space and co-registered with the subject’s head. The center of the bilateral SMAs was initially set at [0, − 10, 80] mm in the Talairach space and the z coordinate was adjusted accordingly to be located on the surface of the scalp. The boundaries of the electrode placement were then marked so the long axis of the electrode was aligned with the mid-sagittal fissure. For the two subjects not eligible for the MRI scan, the stimulation site was targeted at 1.8 cm anterior to the location of Cz. This was based on the international 10–20 system for EEG electrode placement.
Transcranial direct current stimulation
The active electrode (Empi Inc., Dupel B.L.U.E—medium butterfly 2.0 cc, 8.1 cm2) was saturated with sterile saline (0.9% NaCl) and placed over the SMA using each subject’s anatomical MRI image as described above. It was held in place with an EEG cap. The “return” electrode was a self-adhesive carbon-foam electrode (Empi Inc.) measuring 51 cm2 (8.5 cm × 6 cm) that was placed centrally on the forehead directly above the eyebrows. The anodal stimulation was applied using a Dupel iontophoresis constant current delivery device (Empi Inc.) for 10 min with a current of 1 mA (current density = 0.123 mA/cm2). This intensity was chosen based on the findings of previous work showing stimulation with these parameters was associated with a significant improvement in movement preparation in young adults [26]. For the sham condition, the stimulation setup was the same as the anodal stimulation except that the stimulation device was only powered on while ramping up to 1 mA (< 15 s), then surreptitiously shut off. Stimulation type was blinded to subjects and all investigators except Dr. Lu. Stimulation for all subjects were monitored and delivered by Dr. Lu alone. The two testing sessions were conducted in a randomized order at least 1 week apart to ensure a complete washout of any residual tDCS effects.
Gait initiation task
Subjects stood with each foot on a force platform (Kistler, 9260AA) using their natural stance width. An outline of each foot was drawn on the platforms to ensure consistent initial foot placement across trials. Subjects initiated walking forward with their preferred step leg in eight blocks (five trials per block) in the following order: (block 1) pre-tDCS, externally cued; (block 2) pre-tDCS, baseline, self-initiated (no cue); and (blocks 3–8) post-tDCS, immediately after tDCS and then every 12 min. for a total of 1 h, self-initiated. For self-initiated stepping trials, subjects were instructed to hold still, then initiate walking forward “as fast as possible”, after waiting a minimum of 3–5 s following the verbal instruction of “Anytime.” Self-initiated trials were discarded and re-collected if the subject immediately initiated the step in response to the verbal instruction. For cued trials, an acoustic “warning” tone (80 dB, 1000 Hz for 100 ms) was followed 3 s later by a “go” tone (90 dB, 2000 Hz for 100 ms). The acoustic tones were presented to the subject using a speaker placed at eye level 3.3 m in front of the subject. Subjects were instructed to hold still, then walk forward as fast as possible in response to the go cue. The cued trials were added to demonstrate the performance capabilities of the individual, since sensory cueing can significantly improve the magnitude and timing of APAs [5, 23, 35]. The cued trials were conducted at the beginning of the experiment to ensure that this condition did not confound the subsequent self-initiated trials. We have previously shown that the order of externally cued and self-initiated conditions does not affect gait initiation performance in people with PD [23]. Between blocks of gait initiation trials, the subjects were seated in a chair placed at the starting position. Optional seated breaks were provided upon request within each gait initiation block.
Data analysis
All assessments, including the UPDRS part III and data analysis, were performed by experimenters blinded to the stimulation type. For each trial, the outcome variables were separated into components associated with the APA phase (weight shift onset to the end of step leg loading) and the execution of the step (end of step leg loading to step leg toe-off) (Fig. 1). These variables included the amplitude and timing of the peaks of the following: step leg loading force, stance leg unloading force, posterior excursion of the net center of pressure (CoP) during loading/unloading (CoPap1) and near the step leg toe-off (CoPap2), and lateral excursion of the CoP toward the step leg (CoPml). In addition, the times from the weight shift onset to the step and stance leg toe-offs were analyzed. Forces were normalized to a percentage of total body weight (BW). CoP movement and force onsets were identified when the signal crossed a threshold of ± 3 standard deviations from baseline quiet standing, verified by visual inspection, and adjusted manually.
Examples of anticipatory postural adjustments (APAs) during an acoustically cued gait initiation trial in a subject with Parkinson’s disease for a vertical ground reaction forces under the step (dashed line) and stance legs (solid line) and b center of pressure (CoP) displacement in the mediolateral (dashed line) and anteroposterior (solid line) directions. The main APA components are labeled. The vertical gray line designates the separation of the APA phase from the execution phase
Statistical analyses
To assess the effect of A-tDCS on APAs during self-initiated gait, two-way repeated measures analysis of variance (ANOVAs) were performed on the APA data with factors of stimulation type (anodal vs. sham) and trial block (seven levels: baseline self-initiated, post-tDCS at 0, 12, 24, 36, 48 and 60 min of stimulation). In addition, two-way repeated measures ANOVAs were used to test the effect of external cueing on APAs with stimulation type (anodal and sham) and trial block (two levels: baseline self-initiated and baseline cued blocks). Greenhouse–Geisser corrected degrees of freedom were used to correct for violations of the assumption of sphericity. Bonferroni procedures were used to correct for the multiple comparisons in the post hoc analysis of gait initiation blocks. A paired t test was used to examine the differences in UPDRS motor scores between the two stimulation sessions. Two-tailed p values were set at 0.05.
Results
All subjects completed the study and no adverse events were reported. There was no significant difference in the off-medication UPDRS motor scores between sessions (p = .645) and no significant effect of visit order for any outcome variable (p = .091–.951). The average peak amplitudes and timings of the vertical ground reaction forces and CoP posterior excursions during the APA are presented in Table 1 and Fig. 2 and the results of the ANOVAs are presented in Table 2 (see Supplementary Tables 1 and 2 for additional gait initiation variables).
A-tDCS did not significantly affect the amplitude of the forces or CoP shifts during the APA phase of gait initiation, as shown by the absence of a significant stimulation type × block interaction effect (p > .13). During the execution phase, there was a significant main effect of stimulation type (p = .017) and an interaction effect (p = .048) for the peak CoPap2 amplitude. Post hoc analysis showed that differences in CoPap2 amplitude between stimulation conditions were related to the change between 36 and 48 min, with a mean decrease of 0.4 cm during the A-tDCS visit and an increase of 0.5 cm during the sham visit. However, these effects were smaller than the differences (1.1 cm) observed between stimulation conditions at baseline. Significant main effects of trial block were seen for all amplitude components in the APA phase (Table 2). These effects were associated with a drift (usually worsening) of performance over the course of the experiment in both the A-tDCS and sham condition, but post hoc comparisons were not significant with the exception that the peak CoPml excursion at 48 min post stimulation was smaller than at 0 min (see Supplementary Tables 1 and 2).
Significant main effects of stimulation type were observed for many timing variables in the APA and step execution phases (p < .05), including time to step peak loading, time to stance peak unloading, time to CoPap2, and time to the second toe-off. Post hoc analysis of the main effect of stimulation type showed these effects were due to a generalized reduction in APA and execution phase durations during the A-tDCS visit. Most importantly, there were no significant stimulation × block interaction effects for any of these temporal variables. Differences in timing between stimulation conditions at each trial block post-tDCS were comparable to the differences observed at baseline (See Table 1).
The lack of an effect of A-tDCS on gait initiation could not be explained by a ceiling effect in performance ability since external cueing was associated with a significant increase in the magnitude of APAs (main effect of cue/trial block: p < .001) and reduced APA durations (main effect of cue/trial block: p < .05) relative to self-initiated gait. Cueing improved the amplitude of the initial loading and unloading forces by an average of 50% and CoP excursions by 48%. The time to peak loading and unloading were reduced by an average of 21% and the time to the CoP peaks by an average of 17%. In addition, we examined if the gait initiation-related sub-scores (mean ± SD, 4.9 ± 2.7) from the FOG questionnaire were correlated with the effect of A-tDCS. However, in our small sample pool, the difference between anodal and sham stimulation for all gait initiation variables did not systematically vary with the sub-scores from the FOG questionnaire.
Discussion
The aim of this study was to test if A-tDCS, applied over the SMA, could facilitate self-initiated gait in people with PD and FOG in the off-medication state. The results demonstrated that a single session of A-tDCS at 1 mA for 10 min had little effect on the performance of self-initiated (uncued) gait. In general, A-tDCS had no significant effect on the amplitude of the kinetic components contributing to the APA phase of gait initiation compared with sham stimulation. Significant main effects of stimulation condition were observed for many of the timing variables and the second posterior shift in the CoP, but no differences were seen between any post-tDCS blocks and baseline measures.
Our findings did not support the hypothesis that facilitation of the SMA, via A-tDCS, would improve self-initiated gait in people with PD and FOG. The SMA is a primary output target of the basal ganglia thalamocortical pathway [39] and is believed to play an important role in the preparation and execution of self-initiated movements [11,12,13,14,15]. Our hypothesis was derived from extensive evidence from neurophysiological and neuroimaging studies showing that the SMA is underactive in people with PD, particularly during self-initiated movements [13, 19], and the idea that SMA has been implicated in the pathogenesis of FOG [10]. In particular, FOG is associated with marked changes in the structural connectivity between the SMA and the region of the pedunculopontine nucleus [20], a structure associated with the control of locomotion. Accordingly, up-regulation of the SMA with non-invasive brain stimulation would be expected to improve self-initiated tasks such as reaching, gait, and gait initiation. Yet, the results of studies using techniques designed to facilitate SMA activity have been equivocal. Support for the idea of facilitating motor and premotor cortical activity comes from a study by Benninger et al. who demonstrated that eight sessions of A-tDCS (2 mA for 20 min) applied over the motor, premotor or prefrontal cortices (including the SMA) was associated with a reduced time to walk 10 m (starting from quiet standing) [29]. However, due to the size and orientation of the active electrode, it is unclear if these effects were produced by facilitation of the primary, premotor and/or SMA regions. Similarly, Costa-Ribeiro et al. found that ten sessions of A-tDCS (2 mA for 13 min), combined with visually cued gait training, was associated with a lasting enhancement (up to 1 month) in the Timed Up-and-Go task [40]. Facilitatory rTMS (e.g., 10 Hz) over the leg region of the motor cortex or dorsolateral prefrontal cortex has also been shown to improve Timed Up-and-Go and turning tasks in people with PD and FOG, but SMA rTMS at 10 Hz was ineffective [41]. Other studies have shown either no significant effect of 10 Hz rTMS over the SMA [42] or worsening of performance [43]. Paradoxically, two studies observed improvements in performance with low frequency (suppressive) rTMS over the SMA. Shirota et al. reported a more than 6-point reduction in motor UPDRS scores after an 8-week trial of 1 Hz SMA rTMS [42]. Similarly, Jacobs et al. showed that 1 Hz rTMS reduced the duration of APAs before step initiation; however, this effect was only seen in the first trial immediately after 30 min of stimulation [18]. Taken together, it remains ambiguous how up- or down-regulation of SMA activity through non-invasive brain stimulation modulates movement performance in people with PD.
A common finding across studies that have examined the effects of SMA stimulation on voluntary movement have been transient improvements in the temporal features of the task. A recent experiment using the same SMA tDCS protocol as the present study showed that A-tDCS was associated with a significant reduction in reaction times in healthy young adults [6]. Similarly, several studies in people with PD have shown that SMA stimulation, using either repetitive transcranial magnetic stimulation or tDCS, decreases the time to complete motor tasks (e.g., timed up and go, time to turn, walk, step) [18, 29, 41]. The effects were often transient, lasting for a single trial following stimulation [18] or reported only in a longer study of repeated stimulation (e.g., eight sessions over 2.5 weeks) [29]. Significant main effects of A-tDCS on movement timing were also observed in the present study, but there were no stimulation type x trial block interaction effects. Moreover, any improvements observed following A-tDCS were well within the variance observed during baseline measures.
The lack of change in gait initiation with A-tDCS could not be explained by an absence of functional APAs or small sample size since each of the individuals tested showed marked improvements in performance when stepping was cued by an acoustic tone. The results of the cueing condition demonstrate that the subjects in this study processed the capacity to improve the amplitude and timing of gait initiation. The acoustic cue was associated with an approximately 50% improvement in APA magnitude and 20% reduction in APA duration. These large effects were similar to the findings reported in previous studies comparing sensory cued versus self-initiated forward stepping [5, 23, 35]. Furthermore, the interaction effect observed in the APA timing during gait initiation indicates that the subjects who showed more prolonged APAs at baseline were more responsive to external cueing. This finding is consistent with our previous work showing that persons with smaller and slower self-initiated APAs can utilize an external cue to restore proper postural preparation to a greater extent [23]. Although the underlying mechanisms are different between externally cued and self-initiated gait initiations, our goal with comparing the self-initiated vs. externally cued conditions was to elucidate if each individual possessed the capacity to generate the forces and moments required to produce APAs. The cueing data demonstrated that the effectors (corticofugal pathways and spinal motoneurons) of all subjects could be organized and integrated in a manner that achieved a highly functional APA.
It should be noted that our protocol and testing were limited to the examination of the anticipatory postural adjustments (APAs) and first step components of gait initiation. Other behaviors that are affected in people with FOG that also require APAs such as obstacle avoidance and turning, may benefit from stimulation directed at the SMA, other targets, or combinations of targets. In keeping with this idea, Dagan et al. showed that tDCS applied simultaneously over the leg area of the primary motor cortex and left dorsolateral prefrontal cortex was associated with a significant reduction in freezing episodes (provoked by sit-to-stand, walking, turning, or walking through a doorway) and shortened duration of a timed up-and-go task [30]. Their results provide evidence that stimulation targeting frontal cortical regions involved in motor execution and executive function can influence the expression of freezing. However, it is unclear if these effects included improvement in the APA phases of the tasks, such as the transition from quiet standing to forward stepping.
There are several factors that may have contributed to the absence of facilitation of gait initiation with A-tDCS over the SMA in this study. These factors include: stimulation dose, SMA targeting, and a lack of paired stimulation and task training. It could be argued that the dose applied in this study was too low at 1 mA. The A-tDCS dose was chosen based on previous work in healthy adults showing significant changes in the preparation and initiation of a ballistic arm movements with 10 min of 1 mA applied over the SMA using the same electrode montage [26, 27]. Stimulation over the leg region of the primary motor cortex at current densities less than that used in this study (e.g., 60 µA/cm2;[44, 45]) has been shown to produce significant changes in corticospinal excitability, suggesting that the 123 µA/cm2 dose was sufficient to modulate regions along the banks of the longitudinal fissure. Duration may also be a critical factor, considering that studies that have shown robust lowering of UPDRS and FOG scores used tDCS for 20 min (and across multiple sessions) [30, 46, 47]. Nonetheless, we cannot fully discount the possibility that our stimulation protocol may have been too short to activate the leg regions of SMA. The electrode montage was designed to cover the medial–lateral extent of the SMA bilaterally, with minimal overlap over the premotor or primary motor cortices. The reference electrode was placed on the forehead to reduce current flow into these regions. However, this montage may have resulted in higher current densities near the anterior edge of the stimulating electrode, over more anterior regions of SMA or pre-SMA, and away from the leg region of the SMA proper. Our montage may also have decreased excitability in cognitive areas in the prefrontal cortex, which could have worsened the APAs during gait initiation [48]. However, due to the large surface area of the cathode (51 cm2), the relative simplicity of the task and the absence of change from baseline behavior, this was unlikely to be a confound. In addition, the efficacy of stimulation may have been improved if A-tDCS had been applied concurrently with practice of the gait initiation task. Task-specific training during tDCS has been shown to enhance efficacy of physical therapy in PD [49] and learning a balance task in young adults [50]. Finally, the use of dopaminergic medications may change the cortical excitability in PD and thus alter the effects of tDCS [46].
Changes in the function and structure of the SMA in people with PD may also affect the efficacy of tDCS. Neuroimaging studies have shown significant changes in the structural and functional connectivity between the SMA and brainstem locomotor regions (pedunculopontine nucleus) [20, 51]. Reduced functional connectivity between these regions was associated with prolonged APAs during gait initiation in a group of PD with postural instability [51]. In non-human primate models of parkinsonism, the loss of nigrostriatal dopaminergic neurons is also associated with marked changes in the morphology of thalamocortical connections, including the SMA [52]. These findings raise the possibility that the SMA of people with PD may be less responsive to the effects of SMA stimulation, particularly when applied at lower stimulation intensities.
Conclusions
Due to the relatively small sample size, the outcome of this study should be interpreted with caution. The results of our pilot study show that 10 min of 1 mA stimulation over the SMA does not produce clinically meaningful improvements in either the amplitude or timing of the APAs that precede self-initiated stepping in people with PD and FOG. The effects of external cueing demonstrate that individuals have the capacity to perform a good quality step. For this reason, future studies are warranted to investigate if changes in dose, target location (unilateral vs. bilateral SMA, primary motor cortex, premotor cortex), or simultaneous task-specific training can improve gait initiation and reduce the incidence of start hesitation and FOG.
References
Giladi N, McMahon D, Przedborski S et al (1992) Motor blocks in Parkinson’s disease. Neurology 42:333–339
Rahman S, Griffin HJ, Quinn NP, Jahanshahi M (2008) Quality of life in Parkinson’s disease: the relative importance of the symptoms. Mov Disord 23:1428–1434. https://doi.org/10.1002/mds.21667
Moore ST, MacDougall HG, Ondo WG (2008) Ambulatory monitoring of freezing of gait in Parkinson’s disease. J Neurosci Methods 167:340–348. https://doi.org/10.1016/j.jneumeth.2007.08.023
Elble RJ, Cousins R, Leffler K, Hughes L (1996) Gait initiation by patients with lower-half parkinsonism. Brain 119(Pt 5):1705–1716
Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA (1997) Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord 12:206–215. https://doi.org/10.1002/mds.870120211
Jacobs JV, Nutt JG, Carlson-Kuhta P et al (2009) Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol 215:334–341. https://doi.org/10.1016/j.expneurol.2008.10.019
Crenna P, Frigo C (1991) A motor programme for the initiation of forward-oriented movements in humans. J Physiol 437:635–653. https://doi.org/10.1113/jphysiol.1991.sp018616
Elble RJ, Moody C, Leffler K, Sinha R (1994) The initiation of normal walking. Mov Disord 9:139–146. https://doi.org/10.1002/mds.870090203
Callisaya ML, Blizzard L, Martin K, Srikanth VK (2016) Gait initiation time is associated with the risk of multiple falls—a population-based study. Gait Posture 49:19–24. https://doi.org/10.1016/j.gaitpost.2016.06.006
Nutt JG, Bloem BR, Giladi N et al (2011) Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10:734–744. https://doi.org/10.1016/S1474-4422(11)70143-0
Alexander GE, Crutcher MD (1990) Preparation for movement: neural representations of intended direction in three motor areas of the monkey. J Neurophysiol 64:133–150
Cunnington R, Iansek R, Bradshaw JL, Phillips JG (1995) Movement-related potentials in Parkinson’s disease. Presence and predictability of temporal and spatial cues. Brain 118(Pt 4):935–950
Jahanshahi M, Jenkins IH, Brown RG et al (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118(Pt 4):913–933
Deiber MP, Honda M, Ibañez V et al (1999) Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81:3065–3077
Jenkins IH, Jahanshahi M, Jueptner M et al (2000) Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123 (Pt 6):1216–1228
Hugon M, Massion J, Wiesendanger M (1982) Anticipatory postural changes induced by active unloading and comparison with passive unloading in man. Pflügers Arch Eur J Physiol 393:292–296. https://doi.org/10.1007/BF00581412
Viallet F, Massion J, Massarino R, Khalil R (1992) Coordination between posture and movement in a bimanual load lifting task: putative role of a medial frontal region including the supplementary motor area. Exp Brain Res 88:674–684. https://doi.org/10.1007/BF00228197
Jacobs JV, Lou JS, Kraakevik JA, Horak FB (2009) The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 164:877–885. https://doi.org/10.1016/j.neuroscience.2009.08.002
Sabatini U, Boulanouar K, Fabre N et al (2000) Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 123(Pt 2):394–403
Fling BW, Cohen RG, Mancini M et al (2013) Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136:2405–2418. https://doi.org/10.1093/brain/awt172
Fling BW, Cohen RG, Mancini M et al (2014) Functional reorganization of the locomotor network in parkinson patients with freezing of gait. PLoS One 9:e100291. https://doi.org/10.1371/journal.pone.0100291.s001
Schlenstedt C, Mancini M, Nutt J et al (2018) Are hypometric anticipatory postural adjustments contributing to freezing of gait in Parkinson’s disease? Front Aging Neurosci 10:36. https://doi.org/10.3389/fnagi.2018.00036
Lu C, Amundsen Huffmaster SL, Tuite PJ et al (2017) Effect of cue timing and modality on gait initiation in Parkinson disease with freezing of gait. Arch Phys Med Rehabil 98:1291–1299. https://doi.org/10.1016/j.apmr.2017.01.009
Vollmann H, Conde V, Sewerin S et al (2013) Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul 6:101–107. https://doi.org/10.1016/j.brs.2012.03.018
Carter MJ, Maslovat D, Carlsen AN (2014) Anodal transcranial direct current stimulation applied over the supplementary motor area delays spontaneous anti-phase to in-phase transitions. J Neurophysiol. https://doi.org/10.1152/jn.00662.2014
Carlsen AN, Eagles JS, MacKinnon CD (2015) Transcranial direct current stimulation over the supplementary motor area modulates the preparatory activation level in the human motor system. Behav Brain Res 279:68–75. https://doi.org/10.1016/j.bbr.2014.11.009
Hayduk-Costa G, Drummond NM, Carlsen AN (2013) Anodal tDCS over SMA decreases the probability of withholding an anticipated action. Behav Brain Res 257:208–214. https://doi.org/10.1016/j.bbr.2013.09.030
Bolzoni F, Bruttini C, Esposti R et al (2015) Transcranial direct current stimulation of SMA modulates anticipatory postural adjustments without affecting the primary movement. Behav Brain Res 291:407–413. https://doi.org/10.1016/j.bbr.2015.05.044
Benninger DH, Lomarev M, Lopez G et al (2010) Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1105–1111. https://doi.org/10.1136/jnnp.2009.202556
Dagan M, Herman T, Harrison R et al (2018) Multitarget transcranial direct current stimulation for freezing of gait in Parkinson’s disease. Mov Disord 33:642–646. https://doi.org/10.1002/mds.27300
Valentino F, Cosentino G, Brighina F et al (2014) Transcranial direct current stimulation for treatment of freezing of gait: a cross-over study. Mov Disord 29:1064–1069. https://doi.org/10.1002/mds.25897
Chang WH, Park E, Cho J-W et al (2017) P029 Effects of dual-mode non-invasive brain stimulation on freezing of gait in patients with Parkinson’s disease. Clin Neurophysiol 128:e23. https://doi.org/10.1016/j.clinph.2016.10.157
Dibble LE, Nicholson DE, Shultz B et al (2004) Sensory cueing effects on maximal speed gait initiation in persons with Parkinson’s disease and healthy elders. Gait Posture 19:215–225. https://doi.org/10.1016/S0966-6362(03)00065-1
Jiang Y, Norman KE (2006) Effects of visual and auditory cues on gait initiation in people with Parkinson’s disease. Clin Rehabil 20:36–45. https://doi.org/10.1191/0269215506cr925oa
Rogers MW, Kennedy R, Palmer S et al (2011) Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol 106:915–924. https://doi.org/10.1152/jn.00005.2010
Delval A, Moreau C, Bleuse S et al (2014) Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clin Neurophysiol. https://doi.org/10.1016/j.clinph.2013.12.101
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Nieuwboer A, Rochester L, Herman T et al (2009) Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 30:459–463. https://doi.org/10.1016/j.gaitpost.2009.07.108
Schell GR, Strick PL (1984) The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci 4:539–560
Costa-Ribeiro A, Maux A, Bosford T et al (2016) Dopamine-independent effects of combining transcranial direct current stimulation with cued gait training on cortical excitability and functional mobility in Parkinson’s disease. J Rehabil Med 48:819–823. https://doi.org/10.2340/16501977-2134
Lee SY, Kim M-S, Chang WH et al (2014) Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with Parkinsonism. Restor Neurol Neurosci 32:743–753. https://doi.org/10.3233/RNN-140397
Shirota Y, Ohtsu H, Hamada M et al (2013) Supplementary motor area stimulation for Parkinson disease: a randomized controlled study. Neurology 80:1400–1405. https://doi.org/10.1212/WNL.0b013e31828c2f66
Boylan LS, Pullman SL, Lisanby SH et al (2001) Repetitive transcranial magnetic stimulation to SMA worsens complex movements in Parkinson’s disease. Clin Neurophysiol 112:259–264. https://doi.org/10.1016/S1388-2457(00)00519-8
Jeffery DT, Norton JA, Roy FD, Gorassini MA (2007) Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Exp Brain Res 182:281–287. https://doi.org/10.1007/s00221-007-1093-y
Madhavan S, Stinear JW (2010) Focal and bidirectional modulation of lower limb motor cortex using anodal transcranial direct current stimulation. Brain Stimul 3:42–50. https://doi.org/10.1016/j.brs.2009.06.005
Broeder S, Nackaerts E, Heremans E et al (2015) Transcranial direct current stimulation in Parkinson’s disease: neurophysiological mechanisms and behavioral effects. Neurosci Biobehav Rev 57:105–117. https://doi.org/10.1016/j.neubiorev.2015.08.010
Ferrucci R, Bocci T, Cortese F et al (2016) Cerebellar transcranial direct current stimulation in neurological disease. Cerebellum Ataxias 3:16. https://doi.org/10.1186/s40673-016-0054-2
Tard C, Dujardin K, Bourriez J-L et al (2014) Attention modulates step initiation postural adjustments in Parkinson freezers. Park Relat Disord 20:284–289. https://doi.org/10.1016/j.parkreldis.2013.11.016
Kaski D, Allum JH, Bronstein AM, Dominguez RO (2014) Applying anodal tDCS during tango dancing in a patient with Parkinson’s disease. Neurosci Lett 568:39–43. https://doi.org/10.1016/j.neulet.2014.03.043
Kaminski E, Steele CJ, Hoff M et al (2016) Transcranial direct current stimulation (tDCS) over primary motor cortex leg area promotes dynamic balance task performance. Clin Neurophysiol 127:2455–2462. https://doi.org/10.1016/j.clinph.2016.03.018
Gallea C, Ewenczyk C, Degos B et al (2017) Pedunculopontine network dysfunction in Parkinson’s disease with postural control and sleep disorders. Mov Disord 32:693–704. https://doi.org/10.1002/mds.26923
Behnke J, Villalba R, Pare J-F et al (2017) Reorganization of thalamocortical glutamatergic synapses in the supplementary motor area (SMA) of MPTP-treated parkinsonian monkeys. In: Program No. 757.08. 2017 neuroscience meeting planner. Society for Neuroscience, Washington, DC
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke at the National Institutes of Health [Grant numbers R01 NS070264; R01 NS085188 and P50 NS098573]; the University of Minnesota Neuromodulation Innovations (MnDRIVE) and the National Center for Advancing Translational Sciences of the National Institutes of Health [grant number UL1TR000114]. We are grateful to Bradley Baker, Kyle Ballard, Christina Barth, and Annabel Bavage for their assistance in data post-processing, and our clinical research coordinators, Jacqueline Vachon and Joshua De Kam.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Additional information
Preliminary findings from this study were presented in abstract form at the Society for Neuroscience Meeting in 2015
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lu, C., Amundsen Huffmaster, S.L., Tuite, P.J. et al. The effects of anodal tDCS over the supplementary motor area on gait initiation in Parkinson’s disease with freezing of gait: a pilot study. J Neurol 265, 2023–2032 (2018). https://doi.org/10.1007/s00415-018-8953-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8953-1