Abstract
Background
Various randomized-controlled clinical trials (RCTs) have investigated the neuroprotective role of minocycline in acute ischemic stroke (AIS) or acute intracerebral hemorrhage (ICH) patients. We sought to consolidate and investigate the efficacy and safety of minocycline in patients with acute stroke.
Methods
Literature search spanned through November 30, 2017 across major databases to identify all RCTs that reported following efficacy outcomes among acute stroke patients treated with minocycline vs. placebo: National Institute of Health Stroke Scale (NIHSS), Barthel Index (BI), and modified Rankin Scale (mRS) scores. Additional safety, neuroimaging and biochemical endpoints were extracted. We pooled mean differences (MD) and risk ratios (RR) from RCTs using random-effects models.
Results
We identified 7 RCTs comprising a total of 426 patients. Of these, additional unpublished data was obtained on contacting corresponding authors of 5 RCTs. In pooled analysis, minocycline demonstrated a favorable trend towards 3-month functional independence (mRS-scores of 0–2) (RR = 1.31; 95% CI 0.98–1.74, p = 0.06) and 3-month BI (MD = 6.92; 95% CI − 0.92, 14.75; p = 0.08). In AIS subgroup, minocycline was associated with higher rates of 3-month mRS-scores of 0–2 (RR = 1.59; 95% CI 1.19–2.12, p = 0.002; I2 = 58%) and 3-month BI (MD = 12.37; 95% CI 5.60, 19.14, p = 0.0003; I2 = 47%), whereas reduced the 3-month NIHSS (MD − 2.84; 95% CI − 5.55, − 0.13; p = 0.04; I2 = 86%). Minocycline administration was not associated with an increased risk of mortality, recurrent stroke, myocardial infarction and hemorrhagic conversion.
Conclusions
Although data is limited, minocycline demonstrated efficacy and seems a promising neuroprotective agent in acute stroke patients, especially in AIS subgroup. Further RCTs are needed to evaluate the efficacy and safety of minocycline among ICH patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Minocycline is a semisynthetic second-generation tetracycline that provides neuroprotective effects in various neurological disease processes [1,2,3,4]. As a lipophilic agent that readily crosses the blood–brain barrier (BBB), minocycline has demonstrated improved outcomes in focal cerebral ischemia [5, 6], and further suggested reduction in the infarct size and hemorrhagic conversion in ischemic stroke. Minocycline does not affect the fibrinolytic effects of intravenous tissue-plasminogen-activator (tPA) [7], rendering a scope to extend the thrombolytic window in ischemic stroke patients [8]. Although the results seem to be promising, majority of the efficacy data on minocycline has involved animal studies, whereas data from human studies remain scarce.
A small number of phase II RCTs (randomized-controlled clinical trials) have investigated the efficacy of minocycline by assessing clinical recovery after acute ischemic stroke (AIS) without convincing results [9]. Recently, the efficacy and safety of minocycline administration has been analyzed in acute intracerebral hemorrhage (ICH) cohorts [10, 11]. Given the limited data available on the clinical efficacy and safety of minocycline on post-stroke functional outcomes, the objective of our systematic review and meta-analysis was to consolidate the evidence on the utilization, efficacy and safety of minocycline in ischemic and hemorrhagic strokes. We additionally analyzed the role of minocycline in reduction of hematoma and perihematomal edema (PHE) volumes among ICH patients.
Methods
Our study is reported in accordance to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines, and was exempt for approval from the Institutional Review Board of our Institution. Our study protocol for inclusion and exclusion criteria was designed a priori, however, was not registered.
Data sources and searches
All the RCTs that reported and compared the neuroprotective effects of minocycline with placebo were identified by systematic search in Ovid Medline, Ovid Embase, Scopus, ClinicalTrials.gov and Google Scholar databases from database inception to November 30, 2017. The combinations of search strings used to perform our search were “stroke”, “cerebrovascular disease”, “cerebral ischemia”, “hemorrhagic stroke”, “intracerebral hemorrhage”, “neuroprotection” and “minocycline”. The complete search algorithm used in MEDLINE is available in the online supplement. For any missing or unpublished data, corresponding authors were contacted and individual patient-level data was obtained. We restricted our search to human studies and publications in English language. We performed an additional manual search of conference abstracts and bibliographies of articles meeting study criteria for a comprehensive literature search.
Study selection and data extraction
We identified trials that investigated clinical outcomes in patients treated with minocycline and placebo. The inclusion criteria were: (1) randomized controlled design; (2) trials comparing neuroprotective effects of minocycline vs. placebo in patients with acute stroke; (3) availability of clinical outcome data including NIHSS (National Institutes of Health Stroke Scale), mRS (modified Rankin Scale) score, BI (Barthel Index), and mortality; (4) adult patients (> 18 years old).
Two authors (KM and AK) independently reviewed all the retrieved articles. In case of disagreements regarding study inclusion or exclusion, the remaining coauthors were consulted and disagreement was resolved with mutual consensus. The following information were extracted: name of the study, first author and year of publication, mean age, sex distribution, trial name, total number and type of study participants, prevalence of cerebrovascular risk factors, stroke clinical syndrome, clinical outcomes, and follow-up duration. Clinical outcomes (mRS, BI, NIHSS scores) were studied at baseline, day 5–7, 1-month, and 3-month time periods. We further dichotomized the clinical outcomes: mRS 0–1 (favorable functional outcome), mRS 0–2 (functional independence), and mRS 6 (mortality). Our primary objective was to assess the efficacy of minocycline in regards to the clinical recovery of acute stroke patients, and further evaluate among AIS and ICH subgroups by performing sensitivity analyses. Following efficacy outcomes were analyzed: functional independence (mRS 0–2), mean NIHSS and BI scores at 3 months. We also evaluated pooled and individual efficacy outcomes based on different time intervals of clinical assessment. Our secondary objective involved the assessment of safety and neuroprotective role of minocycline among AIS and ICH patients. We additionally assessed neuroimaging and biochemical endpoints in ICH patients by analyzing the reduction of matrix metalloproteinase (MMP)-9 levels, PHE and hematoma volumes. The corresponding authors of 5 RCTs [9,10,11,12,13] were contacted and were requested by KM to contribute unpublished original data regarding primary and secondary outcomes for this meta-analysis.
Risk of bias assessment
Cochrane risk of bias assessment was used to explore the potential sources of bias among the included RCTs. This scale evaluates the following criteria: (1) Randomized sequence generation, (2) allocation concealment, (3) blinding of participants, personnel and outcome assessors, (4) incomplete outcome data, (5) selective outcome reporting, and (6) other sources of bias. Risk of bias was labeled as high, low or unclear by 2 independent investigators (KM and AK). We assessed the symmetry of funnel plots to analyze the publication bias amongst the involved trials.
Data synthesis and statistical analysis
We calculated mean difference (MD) or relative risk (RR) and their corresponding 95% confidence interval (CI) of reported outcomes between minocycline and placebo groups for each RCT, and pooled separately using the random-effects model (DerSimonian Laird) [14]. We used inverse variance method to calculate MD for continuous outcomes, and Mantel–Haenszel (M-H) method to assess the difference in risks between the treatment arms for categorical variables. For studies with a zero cell, we used a continuity correction of 0.5 [15]. The RCTs with zero events in both cells were not depicted in the final plots; however, their data was used to obtain the final pooled RR using M-H method. We additionally performed shift analysis based on 3-month mRS-scores, and the effect corresponds to common OR (cOR) in ordinal logistic regression analyses (per 1-grade improvement in mRS-scores). As per the Cochrane Handbook for Systematic Reviews of Interventions [16], we assessed for heterogeneity using Cochran Q and I2 statistics. For the qualitative interpretation of heterogeneity, I2 > 50% and I2 > 75% indicated substantial and considerable heterogeneity, respectively. Publication bias across individual studies was graphically evaluated using a funnel plot [17], while formal assessment with Egger’s test could not be performed due to small number of studies (< 10 RCTs) [18]. We performed equivalent z test for each pooled RR, and a two-tailed p values < 0.05 was considered statistically significant [14]. Sensitivity analyses were conducted separately for the subgroups of AIS and ICH. All statistical analyses were carried out with Cochrane Collaboration’s Review Manager Software Package (RevMan 5.3) and the metafor package of R 3.3.2 (The R Foundation).
Results
Study selection and study characteristics
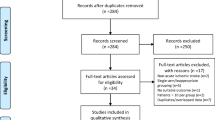
Systematic search of all the databases yielded a total of 2009 articles. After removing the duplicates, the titles and abstracts from the remaining 1122 studies were screened and 11 potentially eligible studies were retained for full-text evaluation. After retrieving the full-text version of the aforementioned 11 studies, 2 RCTs [19, 20] were excluded due to lack of comparator groups, whereas remaining 2 studies were duplicates among the 11 potentially eligible studies (Supplemental Table I). After careful evaluation and without disagreements amongst the 2 reviewers, 7 RCTs were included that met the study protocol’s inclusion criteria. The detailed study flow chart is presented in Supplemental Figure I. The corresponding authors of 5 RCTs [9,10,11,12,13] were individually contacted who contributed their original unpublished data for clinical and radiological outcomes from their respective RCTs.
Table 1 summarizes the 7 RCTs comprising of 426 patients with a median follow-up of 3 months. Three RCTs were conducted in Asia, two in Australia, and the remaining two RCTs were conducted in North America. The mean values for various clinical outcomes assessed at 3-months were available from 6 RCTs for mRS-scores [9,10,11,12, 21, 22], and 5 RCTs each for NIHSS [10, 11, 13, 21, 22] and BI [9, 10, 12, 21, 22] scores. The doses of minocycline varied from 200 mg/day in 4 RCTs, 400 mg/day in 1 RCT and 10 mg/kg/day in 1 RCT with a maximum allowed dosage up to 700 mg/day. The distribution of acute stroke subtypes across the 7 RCTs were AIS (n = 4), ICH (n = 2), AIS and ICH (n = 1). Although majority of the study cohort for Kohler and colleagues [9] comprised of AIS patients, approximately 12% of the cohort involved hemorrhagic strokes and separate clinical outcome data was not available for each subgroup of patients. The baseline characteristics (demographics and vascular risk factors) of the included studies are presented in Supplemental Table II.
Study quality and publication bias
The individual and pooled assessments of the risk of bias across included RCTs are presented in Supplemental Figure II. Random sequence generation and allocation concealment was low in half of the included trials, while the risk was unclear in the remaining trials. All the RCTs were noted to have a high risk of performance bias due to their open-label design, while only one RCT was noted to have detection-bias. Visual inspection of the funnel plots revealed no evidence of publication bias for the outcomes of 3-month mRS (Supplemental Figure III), 3-month NIHSS score (Supplemental Figure IV), and 3-month BI (Supplemental Figure V).
Efficacy outcomes
Modified Rankin Scale score
In the pooled analysis, minocycline demonstrated a trend towards higher likelihood of functional independence (defined as mRS-scores of 0–2) at 3 months (6 RCTs, 370 patients; RR = 1.31; 95% CI 0.98–1.74, p = 0.06; p for Cochran Q statistic = 0.002, I2 = 73%; Fig. 1). Minocycline was independently associated with increased mRS 0–2 in AIS patients (3 RCTs, 242 patients; RR = 1.59; 95% CI 1.19–2.12, p = 0.002; p for Cochran Q statistic = 0.09, I2 = 58%), whereas, no such association was observed in ICH patients (2 RCTs, 36 patients; RR = 1.01; 95% CI 0.76–1.35, p = 0.93; p for Cochran Q statistic = 0.40, I2 = 0%) or combined AIS and ICH patients (1 RCT, 92 patients; RR = 0.96; 95% CI 0.72–1.28, p = 0.77). No association was noted for mean 3-month mRS in the pooled analysis (6 RCTs, MD − 0.27; 95% CI − 0.89, 0.34; p = 0.38; Supplemental Figure VI) or in either subgroup, however, considerable heterogeneity was observed with an I2 = 80% (P for Cochran Q statistic = 0.0002). Minocycline, in comparison to placebo, was not associated with improvement in 3-month mRS-scores (shift analysis from 6 RCTs; cOR: 1.64; 95% CI 0.49–5.45; p = 0.42; p for Cochran Q statistic < 0.001, I2 = 87%; Supplemental Figure VII).
With regards to different time intervals, there was no association of minocycline with 5–7 day mRS (3 RCTs; MD= − 0.68; 95% CI − 1.78, 0.42, p = 0.23; p for Cochran Q statistic < 0.0001, I2 = 90%; Supplemental Figure VIII), 30-day mRS (5 RCTs; MD= − 0.36; 95% CI − 1.20, 0.48, p = 0.40; p for Cochran Q statistic < 0.0001, I2 = 89%; Supplemental Figure IX), and 3-month favorable functional outcome (defined as mRS-scores of 0–1) (6 RCTs; RR = 1.29; 95% CI 0.71–2.35, p = 0.40; p for Cochran Q statistic < 0.0001, I2 = 83%; Supplemental Figure X).
NIHSS score
Although no association was observed with mean 3-month NIHSS score (5 RCTs, 283 patients, MD − 1.93; 95% CI − 4.88, 1.02; p = 0.20; p for Cochran Q statistic < 0.0001, I2 = 92%; Fig. 2), minocycline was associated with a reduction in 3-month NIHSS-score in AIS subgroup (3 RCTs, 254 patients; MD= − 2.84; 95% CI − 5.55, − 0.13, p = 0.04; p for Cochran Q statistic = 0.001, I2 = 86%), whereas no association was observed in ICH subgroup (2 RCTs, 29 patients; MD = 0.70; 95% CI − 0.67, 2.08, p = 0.31; p for Cochran Q statistic = 0.34, I2 = 0%). No significant association was observed with minocycline for 5–7 day NIHSS score (7 RCTs; MD= − 0.74; 95% CI − 1.85, 0.37, p = 0.19; p for Cochran Q statistic = 0.26, I2 = 22%; Supplemental Figure XI) and 30-day NIHSS score (4 RCTs; MD= − 1.09; 95% CI − 5.16, 2.99, p = 0.60; p for Cochran Q statistic < 0.0001, I2 = 92%; Supplemental Figure XII).
Barthel Index score
Minocycline was associated with a higher likelihood of improved 3-month BI amongst AIS patients (3 RCTs, 242 patients; MD = 12.37; 95% CI 5.60, 19.14, p = 0.0003; p for Cochran Q statistic = 0.15, I2 = 47%; Fig. 3); however, there was no beneficial effect in the ICH (1 RCT, 20 patients; MD= − 1.00; 95% CI − 11.51, 9.51, p = 0.85) or combined AIS and ICH (1 RCT, 92 patients; MD= − 1.62; 95% CI − 12.69, 9.45, p = 0.77) subgroups. Additionally, a trend towards significance was noted for a higher likelihood of mean 3-month BI in pooled analysis (5 RCTs, 354 patients; MD = 6.92; 95% CI − 0.92, 14.75; p = 0.08; p for Cochran Q statistic = 0.007, I2 = 72%). No significant association was observed with minocycline for 5–7 day BI (3 RCTs; MD = 11.26; 95% CI − 3.97, 26.49, p = 0.15; p for Cochran Q statistic = 0.004, I2 = 82%; Supplemental Figure XIII), and 30-day BI (5 RCTs; MD = 5.45; 95% CI − 6.49, 17.40, p = 0.37; p for Cochran Q statistic = 0.0004, I2 = 80%; Supplemental Figure XIV).
Safety outcomes
Mortality
In the pooled analysis, minocycline administration did not increase the risk of 3-month mortality (7 RCTs involving 426 patients; RR: 0.70; 95% CI 0.29–1.72, p = 0.44; Supplemental Figure XV) after using the continuity correction (Supplemental Table III). There was no evidence of heterogeneity, with an I2 = 10% (p for Cochran Q statistic = 0.34).
Recurrent stroke, myocardial infarction and hemorrhagic conversion
After using the continuity correction, no significant association of minocycline was observed for recurrent stroke or myocardial infarction (5 RCTs; RR = 0.51; 95% CI 0.20–1.31; p = 0.16; p for Cochran Q statistic = 0.95, I2 = 0%; Supplemental Figure XVI) and hemorrhagic transformation (4 RCTs; RR = 0.84; 95% CI 0.13–5.33; p = 0.85; p for Cochran Q statistic = 0.25, I2 = 23%; Supplemental Figure XVII).
Neuroimaging and biochemical endpoints in ICH patients
Although the number of patients included in 2 RCTs involving ICH patients was low (n = 36 patients), we sought to assess the neuroprotective effect of minocycline among ICH patients by analyzing serum biomarker MMP-9 levels, and neuroimaging metrics including PHE and ICH volumes. Minocycline was not associated with a reduction of MMP-9 at days 1–3 (2 RCTs; MD 28.87; 95% CI − 57.81, 115.55, p = 0.51; p for Cochran Q statistic = 1.00, I2 = 0%; Supplemental Figure XVIII), PHE volume at days 1–3 (2 RCTs; MD 5.45; 95% CI − 6.12, 17.02, p = 0.36; p for Cochran Q statistic = 0.74, I2 = 0%; Supplemental Figure XIX), PHE volume at days 5–11 (2 RCTs; MD 10.11; 95% CI − 5.47, 25.68, p = 0.20; p for Cochran Q statistic = 0.98, I2 = 0%; Supplemental Figure XX), ICH volume at days 1–3 (2 RCTs; MD 3.00; 95% CI − 8.43, 14.43, p = 0.61; p for Cochran Q statistic = 0.99, I2 = 0%; Supplemental Figure XXI) and ICH volume at days 5–11 (2 RCTs; MD − 2.94; 95% CI − 15.66, 9.78, p = 0.65; p for Cochran Q statistic = 0.70, I2 = 0%; Supplemental Figure XXII).
Subgroup analyses
We compared the RCTs for their reported clinical outcomes based on the route of minocycline administration. In the subgroup analysis of RCTs comparing oral and intravenous minocycline administration, oral route was significantly associated with a higher likelihood of 3-month functional independence (3 RCTs involving 217 patients; RR = 1.81; 95% CI 1.47–2.24, p < 0.001; p for Cochran Q statistic = 0.96, I2 = 0%; Supplemental Figure XXIII). Similarly, oral route was associated with higher clinical improvement for mean 3-month NIHSS score (4 RCTs involving 263 patients; MD= − 2.93; 95% CI − 5.45, − 0.42, p = 0.02; p for Cochran Q statistic = 0.003, I2 = 78%; Supplemental Figure XXIV) and mean 3-month BI (2 RCTs involving 201 patients; MD = 13.14; 95% CI 4.34–21.93, p = 0.003; p for Cochran Q statistic = 0.07, I2 = 69%; Supplemental Figure XXV).
Discussion
To the best of our knowledge, this is the first comprehensive systematic review and meta-analysis to evaluate the efficacy and safety profiles of minocycline in comparison to placebo involving acute stroke patients. Among 7 RCTs involving 426 patients, minocycline suggested a trend towards improved 3-month functional independence and BI scores. This association was significantly noted in our sensitivity analyses involving AIS patients for 3-month functional independence, BI and NIHSS scores. Additionally, minocycline was safe in both ischemic and hemorrhagic stroke patients, and was not related to increased mortality, recurrent stroke or adverse events. Finally, we observed no association of minocycline with any of the neuroimaging outcomes including hemorrhagic conversion, PHE, and hematoma volume.
Prior human studies have investigated the efficacy of minocycline in patients with ischemic stroke. However, the results were conflicting to assess the efficacy and further stratify its neuroprotective role. Kohler and colleagues [9] performed a multicenter RCT to assess the efficacy and safety of minocycline in both ischemic and hemorrhagic stroke patients. Although the trial demonstrated that intravenous administration of minocycline is safe, its efficacy could not be established, as the study was underpowered to analyze the treatment effect of minocycline. On the contrary, three additional RCTs [13, 21, 22] have demonstrated the efficacy of minocycline among ischemic stroke patients by demonstrating the improvement in NIHSS score [13, 21, 22], mRS score [21, 22] and BI [21, 22]. Our detailed analyses highlights the net clinical benefit of minocycline in AIS patients for early stroke recovery. With the significant clinical benefit observed with minocycline in AIS subgroup, it is reasonable to anticipate that minocycline could be routinely used for clinical recovery of AIS patients in future. Although the results are reassuring, the included trials were observed to have inherent heterogeneities amongst the comparator groups, especially in relation to the dosing, route of delivery, and therapeutic window for mincocyline. At this stage, our results likely serve for the design of future adequately powered phase III RCTs that will eventually establish potential efficacy and neuroprotective role of minocycline in AIS patients.
Minocycline is a semisynthetic antibiotic with additional neuroprotective properties. Apart from inhibition of MMP-9 levels, data suggests that minocycline has other pleiotropic properties including anti-inflammation, anti-oxidation, anti-apoptosis, vascular protection, and reduction of harmful bleeding effects from IV tPA [7, 8]. Increase in MMP-9 levels has been associated with the disruption of BBB [23], poor functional recovery [24], worsening of hematoma [25] and perihematomal edema [26] volumes in patients with hemorrhagic strokes. Switzer et al. have previously assessed the levels of MMP-9 in AIS patients receiving IV tPA, and corroborated these findings with a trend for reduced MMP-9 levels within an hour of minocycline infusion [19]. These findings underscore the potency of minocycline as an adjunctive therapy with IV tPA, with a potential to expand the narrow thrombolytic window period in ischemic stroke patients [7, 8].
Recently, few studies have investigated the clinical efficacy and safety of minocycline administration in ICH patients. Chang et al. [10] conducted a pilot RCT for the assessment of MMP-9 inhibition and safety profile of minocycline in patients with acute spontaneous ICH. Although the trial was underpowered to assess for functional recovery or radiological improvement based on PHE and ICH volumes, the authors noted a trend towards reduced MMP-9 levels, especially after days 3–5 of minocycline administration. A similar trial assessed the pharmacokinetic and safety profile of minocycline in ICH patients [11]. However, due to limitations in sample size and incomplete enrollment, no clinical benefit was detected with minocycline in any of the primary and secondary clinical, neuroimaging and biochemical endpoints. Additionally, these RCTs primarily targeted to assess the safety of minocylcine and accordingly included lower hematoma volumes (mean 14.8 ml and 20.1 ml, respectively). This might have introduced type II error since minocycline would require substantial perihematomal volumes to demonstrate neuroprotection via MMP inhibition [27]. However, these RCTs coupled with the present meta-analysis highlight the safety of intravenous minocycline at doses as high as 10 mg/kg/day among patients with acute ICH. Future phase II RCTs will provide additional insights regarding the potential benefit of minocycline in hematoma expansion, PHE and functional improvement. Further, the safety of minocycline in ICH would permit its use in the prehospital setting, facilitating ultra-early treatment in the field by paramedics, potentially salvaging the penumbra for thrombolytic or endovascular treatment, and increasing the likelihood of functional recovery.
Our study has certain limitations that need to be acknowledged. First, the design of all included RCTs was open-label and this may have resulted in performance bias. Second, majority of the included RCTs were small and underpowered, and this could have introduced random error in our analyses. Third, heterogeneity in the majority of the included trials could have imposed further limitations. We attempted to address these methodological shortcomings by conducting sensitivity analyses separately in ischemic and hemorrhagic stroke patients. Additionally, we used random-effects model to assess the heterogeneity in our analyses. Fourth, the included RCTs evaluated heterogeneous doses and routes for minocycline administration that could have affected the pharmacokinetic profile, and thus may account for the heterogeneity documented in our meta-analysis. Finally, the variable therapeutic time window ranging between 6 and 24 h following symptom onset may represent another source of heterogeneity across the included studies.
In conclusion, our systematic review and meta-analysis consolidates all available clinical data on minocycline investigated in acute stroke patients. Although the data is limited, our results suggest minocycline as an efficacious and a safe agent in acute stroke patients. Our study underscores minocycline as a promising neuroprotective agent in AIS subgroup. Future adequately powered RCTs are warranted to establish the potential efficacy of minocycline separately in AIS and ICH subtypes.
References
Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM (2002) Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 417(6884):74–78. https://doi.org/10.1038/417074a
Popovic N, Schubart A, Goetz BD, Zhang SC, Linington C, Duncan ID (2002) Inhibition of autoimmune encephalomyelitis by a tetracycline. Ann Neurol 51(2):215–223
Brundula V, Rewcastle NB, Metz LM, Bernard CC, Yong VW (2002) Targeting leukocyte MMPs and transmigration: minocycline as a potential therapy for multiple sclerosis. Brain: J Neurol 125(Pt 6):1297–1308
Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM (2000) Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nature Med 6(7):797–801. https://doi.org/10.1038/77528
Fan X, Lo EH, Wang X (2013) Effects of minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats. Stroke; J Cereb Circ 44(3):745–752. https://doi.org/10.1161/strokeaha.111.000309
Soliman S, Ishrat T, Fouda AY, Patel A, Pillai B, Fagan SC (2015) Sequential therapy with minocycline and candesartan improves long-term recovery after experimental stroke. Transl Stroke Res 6(4):309–322. https://doi.org/10.1007/s12975-015-0408-8
Machado LS, Sazonova IY, Kozak A, Wiley DC, El-Remessy AB, Ergul A, Hess DC, Waller JL, Fagan SC (2009) Minocycline and tissue-type plasminogen activator for stroke: assessment of interaction potential. Stroke 40(9):3028–3033. https://doi.org/10.1161/strokeaha.109.556852
Murata Y, Rosell A, Scannevin RH, Rhodes KJ, Wang X, Lo EH (2008) Extension of the thrombolytic time window with minocycline in experimental stroke. Stroke 39(12):3372–3377. https://doi.org/10.1161/strokeaha.108.514026
Kohler E, Prentice DA, Bates TR, Hankey GJ, Claxton A, van Heerden J, Blacker D (2013) Intravenous minocycline in acute stroke: a randomized, controlled pilot study and meta-analysis. Stroke 44(9):2493–2499. https://doi.org/10.1161/strokeaha.113.000780
Chang JJ, Kim-Tenser M, Emanuel BA, Jones GM, Chapple K, Alikhani A, Sanossian N, Mack WJ, Tsivgoulis G, Alexandrov AV, Pourmotabbed T (2017) Minocycline and matrix metalloproteinase inhibition in acute intracerebral hemorrhage: a pilot study. Eur J Neurol 24(11):1384–1391. https://doi.org/10.1111/ene.13403
Fouda AY, Newsome AS, Spellicy S, Waller JL, Zhi W, Hess DC, Ergul A, Edwards DJ, Fagan SC, Switzer JA (2017) Minocycline in acute cerebral hemorrhage: an early phase randomized trial. Stroke 48(10):2885–2887. https://doi.org/10.1161/strokeaha.117.018658
Blacker DJ, Prentice D, Alvaro A, Bates TR, Bynevelt M, Kelly A, Kho LK, Kohler E, Hankey GJ, Thompson A, Major T (2013) Reducing haemorrhagic transformation after thrombolysis for stroke: a strategy utilising minocycline. Stroke Res Treat 2013:362961. https://doi.org/10.1155/2013/362961
Amiri-Nikpour MR, Nazarbaghi S, Hamdi-Holasou M, Rezaei Y (2015) An open-label evaluator-blinded clinical study of minocycline neuroprotection in ischemic stroke: gender-dependent effect. Acta Neurol Scand 131(1):45–50. https://doi.org/10.1111/ane.12296
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Sweeting MJ, Sutton AJ, Lambert PC (2004) What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Stat Med 23(9):1351–1375. https://doi.org/10.1002/sim.1761
Higgins JP, Green S (2011) Cochrane handbook for systematic reviews of interventions, vol 4. Wiley, Chichester
Sterne JAC, Sutton AJ, Ioannidis JPA, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JPT (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. https://doi.org/10.1136/bmj.d4002
Sterne JA, Gavaghan D, Egger M (2000) Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol 53(11):1119–1129
Switzer JA, Hess DC, Ergul A, Waller JL, Machado LS, Portik-Dobos V, Pettigrew LC, Clark WM, Fagan SC (2011) Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke 42(9):2633–2635. https://doi.org/10.1161/strokeaha.111.618215
Fagan SC, Waller JL, Nichols FT, Edwards DJ, Pettigrew LC, Clark WM, Hall CE, Switzer JA, Ergul A, Hess DC (2010) Minocycline to improve neurologic outcome in stroke (MINOS): a dose-finding study. Stroke 41(10):2283–2287. https://doi.org/10.1161/strokeaha.110.582601
Lampl Y, Boaz M, Gilad R, Lorberboym M, Dabby R, Rapoport A, Anca-Hershkowitz M, Sadeh M (2007) Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology 69(14):1404–1410. https://doi.org/10.1212/01.wnl.0000277487.04281.db
Padma Srivastava MV, Bhasin A, Bhatia R, Garg A, Gaikwad S, Prasad K, Singh MB, Tripathi M (2012) Efficacy of minocycline in acute ischemic stroke: a single-blinded, placebo-controlled trial. Neurol India 60(1):23–28
Barr TL, Latour LL, Lee KY, Schaewe TJ, Luby M, Chang GS, El-Zammar Z, Alam S, Hallenbeck JM, Kidwell CS, Warach S (2010) Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke 41(3):e123–e128. https://doi.org/10.1161/strokeaha.109.570515
Li N, Liu YF, Ma L, Worthmann H, Wang YL, Wang YJ, Gao YP, Raab P, Dengler R, Weissenborn K, Zhao XQ (2013) Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke 44(3):658–663. https://doi.org/10.1161/strokeaha.112.673590
Castellazzi M, Tamborino C, De Santis G, Garofano F, Lupato A, Ramponi V, Trentini A, Casetta I, Bellini T, Fainardi E (2010) Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir Suppl 106:137–140. https://doi.org/10.1007/978-3-211-98811-4_24
Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J, Ribo M, Santamarina E, Quintana M, Monasterio J, Montaner J (2004) Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke 35(6):1316–1322. https://doi.org/10.1161/01.str.0000126827.69286.90
Wu J, Yang S, Hua Y, Liu W, Keep RF, Xi G (2010) Minocycline attenuates brain edema, brain atrophy and neurological deficits after intracerebral hemorrhage. Acta Neurochir Suppl 106:147–150. https://doi.org/10.1007/978-3-211-98811-4_26
Funding
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KM: Study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. JJC: Acquisition and interpretation of data, critical revision of the manuscript for important intellectual content. AK: Analysis and interpretation, critical revision of the manuscript for important intellectual content. DB: Acquisition and interpretation of data, critical revision of the manuscript for important intellectual content. JAS: Acquisition and interpretation of data, critical revision of the manuscript for important intellectual content. NG: Acquisition and interpretation of data, critical revision of the manuscript for important intellectual content. AVH: Analysis and interpretation, critical revision of the manuscript for important intellectual content. VP: Analysis and interpretation, critical revision of the manuscript for important intellectual content. AVA: Acquisition and interpretation of data, critical revision of the manuscript for important intellectual content. GT: Study concept and design, study supervision, critical revision of the manuscript for important intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Malhotra reports no disclosures. Dr. Chang reports no disclosures. Dr. Khunger reports no disclosures. Dr. Blacker reports no disclosures. Dr. Switzer reports no disclosures. Dr. Goyal reports no disclosures. Dr. Hernandez reports no disclosures. Dr. Pasupuleti reports no disclosures. Dr. Alexandrov reports no disclosures. Dr. Tsivgoulis reports no disclosures.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Malhotra, K., Chang, J.J., Khunger, A. et al. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J Neurol 265, 1871–1879 (2018). https://doi.org/10.1007/s00415-018-8935-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8935-3