Abstract
Objective
We examined the anatomical involvement related to cognitive impairment in patients with multiple system atrophy (MSA).
Methods
We examined 30 patients with probable MSA and 15 healthy controls. All MSA patients were assessed by the Unified MSA-Rating scale and Addenbrooke’s Cognitive Examination-Revised (ACE-R). We classified 15 MSA patients with ACE-R scores > 88 as having normal cognition (MSA–NC) and 15 with scores ≤ 88 as having cognitive impairment (MSA–CI). All subjects underwent 3 T MRI scanning and were investigated using voxel-based morphometry and diffusion tensor imaging.
Results
Both the MSA–NC and MSA–CI patients exhibited cerebellar but not cerebral atrophy in voxel-based morphometry compared to controls. In contrast, tract-based spatial statistics revealed widespread and significantly decreased fractional anisotropy (FA) values, as well as increased mean diffusivity, radial diffusivity, and axial diffusivity in both the cerebrum and cerebellum in MSA–CI patients compared to controls. MSA–NC patients also exhibited similar involvement of the cerebellum but less extensive involvement of the cerebrum compared with the MSA–CI patients. In particular, FA values in MSA–CI patients were significantly decreased in the anterior part of the left corpus callosum compared with those in MSA–NC patients. The mean FA values in the left anterior part of the corpus callosum were significantly correlated with total ACE-R scores and subscores (memory, fluency, and language) in MSA patients.
Conclusions
Decreased FA values in the anterior corpus callosum showed a significant correlation with cognitive impairment in MSA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple system atrophy (MSA) is a sporadic neurodegenerative disease characterized by cerebellar ataxia, parkinsonism, and autonomic failure to various degrees during the course of the illness [1, 2]. Pathologically, glial cytoplasmic inclusion (GCI) with immunoreactivity for α-synuclein is a pathological hallmark of MSA [2, 3]. Although dementia that fulfills the criteria set forth in the Diagnostic and Statistical Manual of Mental Disorders is not a supporting feature [4], it has been widely recognized that patients with MSA often present significant declines in attentional function, working memory, recognition, delayed recall, and visuospatial functions [5]. Imaging studies revealed that MSA patients with cognitive decline have frontal lobe-dominant atrophic changes as well as reduced blood flow in the frontal, temporal, and parietal lobes [5]. Nevertheless, the anatomical background of cognitive impairment in MSA patients remains unclear.
Diffusion tensor imaging is a promising neuroimaging technique that can assess the microstructural white matter tract in healthy and diseased populations [6]. Because MSA patients exhibit both gray and white matter involvement [7,8,9], combination studies of brain structural changes and anatomical network abnormalities have the potential to provide a new insight into the anatomical alterations causing cognitive impairment in MSA. In this study, we examined differences in the findings of voxel-based morphometry (VBM) and diffusion tensor imaging between MSA patients with cognitive impairment and those with normal cognition.
Methods
Study population and patient selection
We initially examined 39 consecutive MSA patients from August 1, 2013 to December 31, 2016, at Nagoya University Hospital. The diagnosis was based on the clinical criteria of the second consensus statement on the diagnosis of MSA [4]. We then excluded nine of these patients who did not fulfill the diagnostic criteria for probable MSA or did not complete all cognitive function and imaging tests due to severe motor dysfunction. Thus, 30 MSA patients were included in this study. All participants were assessed by the unified MSA rating scales (UMSARS) [10], Mini-Mental State Examination (MMSE), and Addenbrooke’s Cognitive Examination-Revised (ACE-R) [11]. ACE-R is an established battery of tasks that evaluates five cognitive domains: orientation/attention, memory, verbal fluency, language, and visuospatial ability [11]. ACE-R is comparable to MoCA, and there is a significant correlation of scores between the two batteries [12]. A systematic review and meta-analysis showed that ACE-R is one of the best screening tests for dementia compared to the Montreal Cognitive Assessment [13]. The optimal cut-score of the ACE-R for discriminating mild cognitive impairment and normal cognition was 89 (impairment = score of 88 or less) [11]. The translated versions of the ACE-R, including the Japanese version, showed good to excellent sensitivity and specificity for detecting dementia [14]. The MMSE score could be obtained simultaneously. Recently, ACE-R has been used for the assessment of cognitive impairment in MSA patients [15, 16].
In this study, 15 MSA patients showed ACE-R scores > 88 and were defined as having MSA–normal cognition (MSA–NC) (8 males and 7 females; mean age, 61.5 ± 8.0 years; disease duration, 2.7 ± 1.2 years), and 15 patients showed scores ≤ 88 and were defined as having MSA–cognitive impairment (MSA–CI) (10 males and 5 females; mean age, 65.5 ± 6.7 years; disease duration, 2.9 ± 1.7 years) [17]. Of the patients with MSA–NC, nine had MSA with predominant cerebellar ataxia (MSA–C) and six had MSA with predominant parkinsonism (MSA–P). Of the MSA–CI patients, eight had MSA–C and seven had MSA–P. We also investigated 15 age- and gender-matched non-demented control subjects who had a normal neurological examination and no medical history of stroke, traumatic brain injury, psychiatric disorder, or neurological disorders (9 males and 6 females; mean age, 63.3 ± 7.9 years).
This study conformed to the Ethical Guidelines for Medical and Health Research Involving Human Subjects endorsed by the Japanese government and was approved by the ethics committee of the Nagoya University Graduate School of Medicine, and written informed consent was obtained from all participants before participating in the study.
Magnetic resonance imaging
All MRI scans were performed using a Siemens Magnetom Verio (Siemens, Erlangen, Germany) 3.0 T scanner with a 32-channel head coil at Nagoya University’s Brain and Mind Research Center. A high-resolution T1-weighted image (TR = 2.5 s, TE = 2.48 ms, 192 sagittal slices with a distance factor of 50% and 1 mm thickness, FOV = 256 mm, 256 × 256 matrix size, and an in-plane voxel resolution of 1 × 1 mm2) was acquired from each participant. Diffusion tensor images were also acquired using the following parameters: TR = 13,600 ms, TE = 92 ms, Voxel size 2.0 × 2.0 × 2.0 mm, FOV = 196 mm, b = 0, 1000 s/mm2, 64 directions.
Voxel-based morphometry
Three-dimensional T1-weighted images were analyzed using Statistical Parametric Mapping (SPM8; Wellcome Trust Center for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm8) running on MATLAB (R2014a, Math Work, Natick, Mass, USA). The T1-weighted images were first segmented into component images including gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF), among others, using SPM8’s new segmentation approach. The segmented images were then normalized to the standard Montreal Neurological Institute space using DARTEL (Diffeomorphic Anatomical Registration using Exponentiated Lie algebra) [18]. Normalized images were modulated to preserve the total amount of signal from each region and resampled to an isotropic voxel resolution of 2 × 2 × 2 mm3. To facilitate an unbiased comparison among regions of interest in different patients, the GM images were spatially smoothed using an 8-mm full-width-at-half-maximum isotropic Gaussian filter. The preprocessed GM images were further corrected for age and gender before group comparisons were performed. In all comparisons, we included the total intracranial volume, computed as the sum of gray matter, white matter, CSF volumes, and age as covariates of no interest to account for differences in brain sizes. The obtained statistical maps were thresholded at p < 0.05 and corrected for multiple comparisons using a cluster-level family wise error correction (FWEc) with a cluster-forming threshold set at p = 0.001. From the VBM results, we generated ROIs (sphere, 4 mm radius) centered at the peak coordinates from all comparisons and then extracted the mean GM value within the ROI and computed Pearson’s correlations with behavioral measures.
Tract-based spatial statistics
Voxel-wise statistical analysis of the fractional anisotropy (FA) data was carried out using the tract-based spatial statistics (TBSS) [19] part of FSL [20]. First, the brain image was skull stripped using BET [21]. FA images were then created by fitting a tensor model to the raw diffusion data using FDT. All subjects’ FA data were then aligned into a common space using the nonlinear registration tool FNIRT [22, 23], which uses a b-spline representation of the registration warp field [24]. Next, a mean FA image was created and projected into the main cortical tracts to create a mean FA skeleton, which represents the centers of all tracts common to the group. Each subject’s aligned FA data were then projected onto this skeleton and the resulting data fed into voxel-wise, cross-subject statistics. TBSS analysis was repeated for mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) maps. The results were overlaid on the mean FA image. We performed two-sample t tests using randomization with 5000 permutations. Specifically, the following comparisons were made: controls vs MSA–NC, controls vs MSA–CI, and MSA–NC vs MSA–CI. Using an in-house Matlab script, a mask was created that included only voxels showing a significant difference in the TBSS results. Using this mask, the FA values from each participant’s processed FA data were then extracted and averaged. The mean FA values were correlated with ACE-R scores.
Statistical analysis
Group results were expressed as the mean ± standard deviation. Comparisons of the differences in gender and motor type (MSA–C or MSA–P) were performed using the Chi-square test. The Shapiro–Wilk test was used to assess the normality of continuous variables. To compare the age at examination, we used one-way analysis of variance. The UMSARS I and UMSARS II scores were compared using Student’s t test. To assess differences in disease durations and UMSARS IV scores, we used the Mann–Whitney U test. To evaluate differences in the total scores or five subdomains of the ACE-R and the MMSE scores among controls and MSA–NC and MSA–CI patients, we used the Kruskal–Wallis test. When the p value was significant (< 0.05), post hoc tests were conducted with the Tukey’s test for data with normal distributions or the Bonferroni test for data with non-normal distributions. Statistical analyses were performed in PASW Statistics 18.0 for Windows using SPSS version 18 (SPSS Inc., Chicago, IL, USA).
Results
Comparisons of neuropsychological tests in patients with MSA–NC, those with MSA–CI, and controls
Table 1 shows the demographic and clinical data of the subjects studied. There were no significant differences in age; sex; scores on the UMSARS I, UMSARS II, and UMSARS IV; or the proportion of MSA–C and MSA–P between the MSA–NC and MSA–CI groups. The average MMSE and ACE-R scores of the MSA–CI patients were significantly lower than those of controls and MSA–NC patients. ACE-R attention/orientation, memory, fluency, and language subdomain scores in MSA–CI patients were significantly lower than those of controls and MSA–NC patients (p < 0.05). The visuospatial subdomain scores of the ACE-R were significantly lower in MSA–CI patients than in the controls (p < 0.05).
VBM findings in MSA–NC and MSA–CI patients compared with controls
The VBM findings demonstrated that both MSA–NC and MSA–CI patients exhibited significant reductions in gray matter volume in the cerebellum compared to controls (FWEc p < 0.05, cluster defining threshold p = 0.001, Fig. 1). There was no significant relationship between ACE-R scores and gray matter volume changes. Neither MSA–NC nor MSA–CI patients exhibited any significant reductions in cerebral gray matter volume.
Voxel-based morphometry analyses. a Blue areas indicate regions with brain atrophy in patients with MSA–NC compared to controls (controls > MSA–NC). b Blue areas indicate regions with brain atrophy in patients with MSA–CI compared to controls (controls > MSA–CI). MSA–NC multiple system atrophy– normal cognition, MSA–CI multiple system atrophy– cognitive impairment
FA, MD, RD and AD values in MSA–NC and MSA–CI patients compared with controls
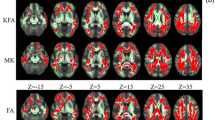
There were widespread significantly decreased FA values and increased MD, RD, and AD values in the cerebral and cerebellar white matter tracts in patients with MSA–CI compared with controls (FWE p < 0.05, Fig. 2). Patients with MSA–NC also showed abnormal MD, RD, and AD values compared to controls. However, patients with MSA–NC had more limited MD, RD, and AD values in the cerebral hemisphere than those with MSA–CI. Particularly, there were no significant differences in FA values in the cerebral hemisphere between MSA–NC patients and controls. Cerebral hemispheres showed abnormal MD, RD, and AD values that were similar but more restricted in MSA–NC than MSA–CI. There were no significant differences in FA values in the cerebral hemispheres between MSA–NC patients and controls.
Tract-based spatial statistics analyses. Maps of differences in fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) for MSA–NC (a) and MSA–CI (b) patients compared to controls. Blue lines indicate reduced FA values (controls > MSA–NC) and red lines indicate increased MD, RD, and AD values (controls < MSA–CI), compared to controls. MSA–NC multiple system atrophy– normal cognition, MSA–CI multiple system atrophy– cognitive impairment
FA, MD, RD and AD values in MSA–NC compared with MSA–CI patients
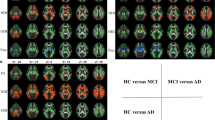
Comparing MSA–CI to MSA–NC, there were significant reductions in FA values in the left corpus callosum in patients with MSA–CI (FDR q < 0.05 Fig. 3a). However, MD, RD, and AD values did not show any significant differences between MSA–CI and MSA–NC.
Associations between mean fractional anisotropy in the corpus callosum and cognition scores. a Maps of differences in mean fractional anisotropy (FA) in the corpus callosum for MSA–NC patients compared to MSA–CI patients. b–e Correlations between mean FA values in the corpus callosum and the ACE-R total (b) and subdomain scores for memory (c), fluency (d), and language (e) in MSA–CI (blue) and MSA–NC (red) patients
Relationship between FA values in the corpus callosum and ACE-R scores in MSA patients
We investigated the relationship between ACE-R scores and mean FA values in the left corpus callosum, which were significantly different in MSA–CI and MSA–NC patients. There was a significant association between mean FA values of these areas in the left anterior part of the corpus callosum and the total ACE-R scores (r = 0.69, p = 2.60 × 10−5) as well as the subscores for memory (r = 0.55, p = 0.0015), fluency (r = 0.59, p = 6.47 × 10−4), and language (r = 0.46, p = 0.0099) (Fig. 3b–e) but not attention and orientation in all MSA patients. There was no significant association between the mean FA values and age or disease duration.
Discussion
VBM findings showed that both MSA–NC and MSA–CI patients exhibited significant reductions in gray matter volume in the cerebellum compared to controls. Neither MSA–NC nor MSA–CI patients exhibited any significant reductions in cerebral gray matter volume. On the other hand, TBSS images indicated that there were widespread significantly decreased FA values and increased MD, RD, and AD values in cerebral and cerebellar white matter tracts in patients with MSA–CI compared with controls. There were also decreased FA in the cerebellum in patients with MSA–NC. They also showed abnormal MD, RD, and AD values compared to controls, although they were less extensive compared with MSA–CI. In other words, compared with controls, MSA–CI patients exhibited significant widespread microstructural cerebral white matter involvement assessed by FA, MD, RD, and AD values in contrast to reduced cerebral gray matter volume. In particular, FA values were significantly decreased in the anterior part of the left corpus callosum in MSA–CI patients compared with MSA–NC patients. Moreover, the mean FA values in the anterior part of the left corpus callosum were significantly correlated with both total and subscores (memory, fluency, and language) of the ACE-R in MSA patients.
The corpus callosum is the largest collection of white matter in the brain and is composed of axons directly connecting the bilateral cerebral hemispheres. Moreover, there was a significant correlation between the severity of the anterior part of the corpus callosum involvement assessed by FA values and ACE-R scores. Although other demented patients also show decreased FA values in the corpus callosum, this is the first study to demonstrate the significant correlation between FA values in the corpus callosum and ACE-R scores in MSA patients [25]. Previously, we reported progressive atrophic changes in the corpus callosum of MSA patients based on MR images analyzed via region-of-interest methods [26]. MSA is thought to be a primary oligodendrogliopathy with abundant GCI and secondary neuronal multisystem degeneration. A pathological study of MSA revealed myelin involvement and Wallerian degeneration in the cerebrum [27]. Thus, it is plausible that the corpus callosum is a vulnerable region in MSA involved not only via primary involvement but also via secondary pathological changes due to widespread cerebral white matter involvement, contributing to cognitive impairment.
Frontal lobe atrophy may be associated with dementia in advanced MSA cases, as previously reported [28,29,30,31]. It was reported that reduced gray matter volume was observed in orbitofrontal and mid-frontal regions as well as in temporomesial and insular areas of both hemispheres in MSA–C patients [28], while in MSA-P patients, reduced gray matter volume was reported in the primary bilateral sensorimotor cortices, supplementary bilateral motor areas, right premotor cortex, prefrontal cortex bilateral (middle frontal gyri), and bilateral insular cortices [29]. A longitudinal study of MSA–P patients revealed that cortical atrophy increased during the course of the illness [30]. The left superior and inferior frontal regions, left caudate, right hippocampus, and right inferior temporal regions were correlated with memory scores [31]. This paper mostly complemented these findings, showing that diffusion changes in the anterior corpus callosum connecting the frontal lobes were correlated with cognitive impairment. These underlying mechanisms may be associated with reduced FA values in the left corpus callosum in MSA–CI.
In this study, the changes between MSA–CI and MSA–NC were only observed in the left, but not the right, corpus callosum. It is unclear why the left, but not right, contributed to the cognitive decline in MSA. A section of the anterior part of the body of the corpus callosum can be associated with the deficits of memory, the dysexecutive cognitive and behavioral syndrome, and disturbances in interhemispheric transfer of learning [32]. However, it has not been determined whether the left and right corpus callosum work in different ways. Further longitudinal study will be needed to clarify the meanings of the laterality.
Detailed relationships between FA, MD, AD, and RD values and pathological conditions have not been elucidated. Although we should consider the influence of the non-Gaussian behavior water motion on these parameters, the reduced FA values we observed were considered to correspond to several pathological conditions, including chronic white matter degeneration, demyelination, subtle microstructural alterations, secondary Wallerian degeneration, and gliosis or early axonal injury [6]. In contrast, increased MD is observed in chronic white matter degeneration, while AD and RD mainly express axonal and myelination involvement, respectively [6]. Since the relationships between FA, MD, RD, and AD values and pathological conditions are not so simple, further comparative study between pathological and radiological findings will be needed to clarify this issue.
Recent neuropsychological studies report dementia in MSA patients [33,34,35]. Fiorenzato et al. reported that at a 15-month follow-up, the proportion of mild cognitive impairment increased up to 80% [36]. Executive dysfunction, including semantic and phonemic word list generation, is a prominent cognitive disturbance in MSA patients [35, 36], and memory disturbances are also observed, presenting with delayed recall [35]. In this study, patients with MSA–CI had significantly lower ACE-R subscores in the four domains of attention and orientation, memory, fluency, and language, in accordance with previous findings [35, 36]. The interval from MSA diagnosis to clinical cognitive symptoms is estimated to be 6.2 ± 2.4 years [33]; however, earlier cognitive impairments have also been described [34]. The mean disease duration in this study was 2.9 ± 1.7 years in the MSA–CI patients and 2.7 ± 1.2 years in the MSA–NC patients. Interestingly, there were no significant differences in disease duration, motor phenotype, or UMSARS scores between the MSA–CI and MSA–NC patients. Since the mean age at the onset of MSA–CI was 4 years older than the onset of MSA–NC, although not significant, we might consider the influence of aging on the results. However, according to previous studies, widespread white matter can show a significant decline in FA values with age in normal healthy subjects [6], but there were significant differences to a limited extent in the corpus callosum between MSA–CI and MSA–NC in this study. Thus, it would be difficult to explain our results exclusively based on the aging effect. Our results indicate that there may be a discrete phenotype showing mild cognitive involvement during the early course of the illness in MSA.
Limitation
There are several limitations in our study. First, the number of MSA–CI patients was relatively low and influenced on VBM and TBSS results. Particularly, this could be due to no significant cortical gray matter in patients with MSA–CI compared to controls by VBM, although some previous studies showed the relationship between cognitive decline and cortical atrophy in MSA. Second, analysis techniques in this study are rather complex and operator dependent. Some types of blinding of the assessors and future development of automated and observer-independent methods will be needed for clinical use and individual assessment. Finally, because our MSA cases were not pathologically confirmed, concomitant pathology such as tau and βamyloid may be associated with cognitive decline. Further study will be needed to clarify these issues.
Conclusion
In MSA, cognitive impairment appears to be associated with white matter tract involvement, and the anterior part of the corpus callosum in particular may be the target for neuroimaging and pathological study to detect early cognitive impairment.
References
Graham JG, Oppenheimer DR (1969) Orthostatic hypotension and nicotine sensitivity in a case of multiple system atrophy. J Neurol Neurosurg Psychiatry 32:28–34
Papp MI, Kahn JE, Lantos PL (1989) Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy–Drager syndrome). J Neurol Sci 94:79–100
Nakazato Y, Yamazaki H, Hirato J, Ishida Y, Yamaguchi H (1990) Oligodendroglial microtubular tangles in olivopontocerebellar atrophy. J Neuropathol Exp Neurol 49:521–530
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Stankovic I, Krismer F, Jesic A, Antonini A, Benke T, Brown RG, Burn DJ, Holton JL, Kaufmann H, Kostic VS, Ling H, Meissner WG, Poewe W, Semnic M, Seppi K, Takeda A, Weintraub D, Wenning GK; Movement Disorders Society MSA (MODIMSA) Study Group (2014) Cognitive impairment in multiple system atrophy: a position statement by the Neuropsychology Task Force of the MDS Multiple System Atrophy (MODIMSA) study group. Mov Disord 29:857–867
Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR (2010) Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage 49:2104–2112
Ito M, Watanabe H, Kawai Y, Atsuta N, Tanaka F, Naganawa S, Fukatsu H, Sobue G (2007) Usefulness of combined fractional anisotropy and apparent diffusion coefficient values for detection of involvement in multiple system atrophy. J Neurol Neurosurg Psychiatry 78:722–728
Ito M, Watanabe H, Atsuta N, Senda J, Kawai Y, Tanaka F, Naganawa S, Fukatsu H, Sobue G (2008) Fractional anisotropy values detect pyramidal tract involvement in multiple system atrophy. J Neurol Sci 271:40–46
Ji L, Zhu D, Xiao C, Shi J (2014) Tract based spatial statistics in multiple system atrophy: a comparison between clinical subtypes. Parkinsonism Relat Disord 20:1050–1055
Wenning GK, Tison F, Seppi K, Sampaio C, Diem A, Yekhlef F, Ghorayeb I, Ory F, Galitzky M, Scaravilli T, Bozi M, Colosimo C, Gilman S, Shults CW, Quinn NP, Rascol O, Poewe W; Multiple System Atrophy Study Group (2004) Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 19:1391–1402
Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR (2006) The Addenbrooke’s Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21:1078–1085
Siciliano M, Raimo S, Tufano D, Basile G, Grossi D, Santangelo F, Trojano L, Santangelo G (2016) The Addenbrooke’s Cognitive Examination Revised (ACE-R) and its subscores: normative values in an Italian population sample. Neurol Sci 37:385–392
Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC (2015) Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med 175:1450–1458
Habib N, Stott J (2017) Systematic review of the diagnostic accuracy of the non-English versions of Addenbrooke’s Cognitive Examination-Revised and III. Aging Ment Health. https://doi.org/10.1080/13607863.2017.1411882
Cao B, Wei QQ, Ou R, Yang J, Shang HF (2015) Association of serum uric acid level with cognitive function among patients with multiple system atrophy. J Neurol Sci 359:363–366
Cao B, Zhao B, Wei QQ, Chen K, Yang J, Ou R, Wu Y, Shang HF (2015) The global cognition, frontal lobe dysfunction and behavior changes in chinese patients with multiple system atrophy. PLoS One 10:e0139773
Yoshida H, Terada S, Honda H, Kishimoto Y, Takeda N, Oshima E, Hirayama K, Yokota O, Uchitomi Y (2012) Validation of the revised Addenbrooke’s Cognitive Examination (ACE-R) for detecting mild cognitive impairment and dementia in a Japanese population. Int Psychogeriatr 24:28–37
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31:1487–1505
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:208–219
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Andersson (2007a). http://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja1/tr07ja1.pdf
Andersson (2007b). http://www.fmrib.ox.ac.uk/datasets/techrep/tr07ja2/tr07ja2.pdf
Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ (1999) Non-rigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging 18:712–721
Elahi FM, Marx G, Cobigo Y, Staffaroni AM, Kornak J, Tosun D, Boxer AL, Kramer JH, Miller BL, Rosen HJ (2017) Longitudinal white matter change in frontotemporal dementia subtypes and sporadic late onset Alzheimer’s disease. Neuroimage Clin 16:595–603
Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, Aiba I, Abe Y, Tamakoshi A, Doyu M, Hirayama M, Sobue G (2002) Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain 125:1070–1083
Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM, Parisi JE (2015) Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138:2293–2309
Brenneis C, Boesch SM, Egger KE, Seppi K, Scherfler C, Schocke M, Wenning GK, Poewe W (2006) Cortical atrophy in the cerebellar variant of multiple system atrophy: a voxel-based morphometry study. Mov Disord 21:159–165
Brenneis C, Seppi K, Schocke MF, Müller J, Luginger E, Bösch S, Löscher WN, Büchel C, Poewe W, Wenning GK (2003) Voxel-based morphometry detects cortical atrophy in the Parkinson variant of multiple system atrophy. Mov Disord 18:1132–1138
Brenneis C, Egger K, Scherfler C, Seppi K, Schocke M, Poewe W, Wenning GK. (2007) Progression of brain atrophy in multiple system atrophy. A longitudinal VBM study. J Neurol 254:191–206
Chang CC, Chang YY, Chang WN, Lee YC, Wang YL, Lui CC, Huang CW, Liu WL (2009) Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration. Eur J Neurol 16:1144–1150
Peltier J, Roussel M, Gerard Y, Lassonde M, Deramond H, Le Gars D, De Beaumont L, Godefroy O (2012) Functional consequences of a section of the anterior part of the body of the corpus callosum: evidence from an interhemispheric transcallosal approach. J Neurol 259:1860–1867
O’Sullivan SS, Massey LA, Williams DR, Silveira-Moriyama L, Kempster PA, Holton JL, Revesz T, Lees AJ (2008) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131:1362–1372
Kitayama M, Wada-Isoe K, Irizawa Y, Nakashima K (2009) Assessment of dementia in patients with multiple system atrophy. Eur J Neurol 16:589–594
Brown RG, Lacomblez L, Landwehrmeyer BG, Bak T, Uttner I, Dubois B, Agid Y, Ludolph A, Bensimon G, Payan C, Leigh NP; NNIPPS Study Group (2010) Cognitive impairment in patients with multiple system atrophy and progressive supranuclear palsy. Brain 133:2382–2393
Fiorenzato E, Antonini A, Wenning G, Biundo R (2007) Cognitive impairment in multiple system atrophy. Mov Disord 32:1338–1339
Acknowledgements
This research is partially supported by a Grant-in-Aid from the Research Committee of Central Nervous System Degenerative Diseases by the Ministry of Health, Labour, and Welfare, Integrated Research on Neuropsychiatric Disorders project carried out by SRBPS and a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control 26117002) from the MEXT of Japan, Integrated Research on Neuropsychiatric Disorders carried out under the Strategic Research Program for Brain Sciences, Scientific Research on Innovative Areas (Comprehensive Brain Science Network), and Integrated Research on Depression, Dementia and Development Disorders by the Strategic Research Program for Brain Sciences from the Japan Agency for Medical Research and Development, AMED.
Funding
KH reports Grants from the Ministry of Education, Culture, Sports, Science and Technology (Grant number JP 15K19484). He also reports a salary from Nagoya University. HW reports Grants-in-Aid from the Research Committee of Central Nervous System Degenerative Diseases by the Ministry of Health, Labour, and Welfare and from the Integrated Research on Neuropsychiatric Disorders project carried out under the Strategic Research for Brain Sciences by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. He also reports a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan (Grant number 80569781) and a Grant-in-Aid for Scientific Research on Innovative Areas (Brain Protein Aging and Dementia Control) (26117002) from MEXT. He reports a salary from the Brain and Mind Research Center, Nagoya University. EB reports Grants-In-Aid for Scientific Research (KAKENHI number 26350993) funded by the Japan Society for the Promotion of Science and a salary from the Brain and Mind Research Center, Nagoya University. KK reports no disclosures. NY reports no disclosures. RO reports no disclosures. KI reports no disclosures. MM reports no disclosures. TY reports no disclosures. AO reports no disclosures. TT reports Grants from the Japan Society for the Promotion of Science (Grant number JP16K19507), GlaxoSmithKline, and Novartis. He also reports a salary from Nagoya University. MI is funded by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. NA is funded by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan. HN reports no disclosures. TT reports no disclosures. SM reports no disclosures. SN reports no disclosures. MK is supported by KAKENHI Grants from MEXT/JSPS, Japan (nos. 17H04195 and 16K15480); Grants from the Japan Agency for Medical Research and Development (nos. 17ek0109221h0001, 16dk0207026h0001, and 15ek0109165); and a Grant from the Naito Foundation. GS reports KAKENHI Grants from MEXT/JSPS, Japan (nos. 26117001); Grants from the Japan Science and Technology Agency; Grants from the Japan Agency for Medical Research and Development (AMED) (nos. 15ek0109025, 15ek0109048, and 15ek0109165); and Grants from the Ministry of Welfare, Health, and Labor of Japan.
Author information
Authors and Affiliations
Contributions
(1) Research project: (A) conception, (B) organization, (C) execution; (2) statistical analysis: (A) design, (B) execution, (C) review and critique; (3) manuscript: (A) writing of the first draft, (B) review and critique. KH: 1A, 1B, 1C, 2A, 2B, 2C, 3A. HW: 1A, 1B, 1C, 2A, 2C, 3B. EB: 1B, 1C, 2A, 2B, 2C. KK: 1A, 1C. NY: 1A, 1C. RO: 1B, 1C. KI: 1B, 1C. MM: 1B, 1C. TY: 1B, 1C. AO: 1B, 1C. TT: 1A, 1B, 1C. MI: 1A, 1B, 1C, 2A. NA: 1A, 1B, 2A. HN: 1C. TT: 1C, 2A, 2B, 2C. SM: 1B, 1C. SN: 1C, 2A, 2B, 2C, 3A. MK: 1A, 2C, 3B. GS: 1A, 2A, 2C, 3B.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declared no conflict of interest.
Ethical standards
This study conformed to the Ethical Guidelines for Medical and Health Research Involving Human Subjects endorsed by the Japanese government and was approved by the Ethics Committee of Nagoya University Graduate School of Medicine.
Rights and permissions
About this article
Cite this article
Hara, K., Watanabe, H., Bagarinao, E. et al. Corpus callosal involvement is correlated with cognitive impairment in multiple system atrophy. J Neurol 265, 2079–2087 (2018). https://doi.org/10.1007/s00415-018-8923-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8923-7