Abstract
Erectile function (EF) is frequently compromised in men with multiple sclerosis (MS). Functional neuroimaging in healthy men identified a network of brain areas, such as the insula, visual and somatosensory association areas, cingulate gyrus, prefrontal cortex, as well as subcortical regions, contributing to EF. This study intended to determine associations between EF deterioration during MS and cerebral MS-associated lesion sites. In 31 men with MS (mean age 38.2 ± 11.2 years), we evaluated MS-related EF deterioration by comparing scores of the 5-item International Index of Erectile Function-5 questionnaire (IIEF5) at the time of study and retrospectively, 3 months prior to MS diagnosis, by calculating score differences as DeltaIIEF5 (DeltaIIEF5 score < 0 indicated EF deterioration). To assess the impact of confounding factors of EF, patient age, disease duration, disease severity, depressiveness, bladder and bowel symptoms, and total cerebral MS lesion volume were correlated with DeltaIIEF5 scores (Spearman rank correlation) and compared between patients with and without EF deterioration (t tests or Mann–Whitney U test). MS lesions were assessed on T2-weighted magnetic resonance imaging (MRI; 1.5 or 3 T). We determined the lesion overlap (prevalence of identical lesion sites among patients), subtracted lesion overlaps in patients without EF deterioration from overlaps in patients with EF deterioration, and compared DeltaIIEF5 scores voxel-wise between patients with and without lesions in a given voxel (t test; significance: p < 0.05). In 14 patients (45.2%), DeltaIIEF5 scores indicated EF deterioration. DeltaIIEF5 scores were not associated with age (ρ = 0.06; p = 0.74), disease duration (ρ = 0.26; p = 0.15), disease severity (ρ = − 0.19; p = 0.31), depressiveness (ρ = 0.07; p = 0.72), bladder symptoms (ρ = − 0.11; p = 0.57), bowel symptoms (ρ = 0.17; p = 0.37), and total lesion volume (ρ = − 0.13; p = 0.47). The voxel-wise analysis showed associations between EF deterioration and MS lesions primarily in the bilateral, and predominantly left juxtacortical insular region. In conclusion, MS lesions particularly in the left insular region, which is activated with sexual arousal, contribute to erectile dysfunction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sexual dysfunction is common in patients with multiple sclerosis (MS) [1,2,3]. Among men with MS, erectile dysfunction (ED) is a particularly frequently reported symptom compromising quality of life [3, 4]. The MS-associated pathology has a predilection for the cerebral periventricular white matter and juxtacortical U-fibers, but also the cerebral cortex can be afflicted by MS lesions [5, 6]. In addition, MS lesions can affect the cerebellum, the brainstem and the spinal cord [5, 6].
Originally, ED associated with MS was thought to result mainly from MS lesions in the spinal cord [4]. Functional neuroimaging studies of healthy men identified cortical areas, such as the insula, the visual and somatosensory association areas, the cingulate gyrus, the prefrontal cortex, as well as subcortical regions (hypothalamus, thalamus, amygdala, and basal ganglia) as being activated during male sexual arousal and consecutive penile erection [7,8,9,10]. Hence, MS lesions may afflict brain areas that are important modulators of EF, thereby leading to deterioration of EF. We hypothesize that deterioration of EF occurring upon the onset of MS is associated with MS lesions in brain areas that contribute to male sexual function. Therefore, we assessed the cerebral lesion pattern of male MS patients and correlated clinical scores of EF with the MS-associated lesion sites using voxel-based lesion symptom mapping (VLSM) [11,12,13,14].
Methods
Patients
Among outpatients seen between February 2011 and February 2012 at the Multiple Sclerosis Clinic at the University Hospital Erlangen of the Friedrich-Alexander University Erlangen-Nuremberg, we screened male patients who had been diagnosed with relapsing–remitting or secondary progressive MS according to the 2010 revised McDonald criteria [15]. For the voxel-wise analysis, we studied men with MS who fulfilled the following inclusion criteria: (1) axial T2-weighted and fluid-attenuated inversion recovery (FLAIR) scan of sufficient quality available, (2) aged 18–65 years, and (3) sexually active. Men with the following conditions were excluded from the study: (1) evidence of structural cerebral diseases other than MS; (2) patients with diseases interfering with autonomic nervous system function, such as diabetes mellitus; (3) patients on phosphodiesterase type 5-inhibitors or any other drugs modifying EF; (4) inability to give informed consent or to adequately cooperate in the study. The study has been approved by the institutional ethics committee of the Friedrich-Alexander University Erlangen-Nuremberg. Prior to the study, all patients gave written informed consent according to the Declaration of Helsinki.
We took the medical history with particular emphasis on disease course, co-morbidities and medication, and performed a physical and neurological examination. We determined the degree of physical disability using the Expanded Disability Status Scale (EDSS) scores [16]. Since depressive mood changes are frequently associated with ED, we rated depressiveness using the abridged Beck’s Depression Index (BDI), a 20-item questionnaire, with ratings from 0 to 5 per item and a maximum score of 100 [17]. An overall BDI score above 35 indicates with a certainty of 90% that the patient has clinically manifest depression [17]. Because bladder and urinary symptoms as well as bowel dysfunction might be associated with ED, we determined the severity of bladder or urinary symptoms using scores of item 2 of the 19-item Multiple Sclerosis Intimacy and Sexuality Questionnaire (MSISQ-19) as well as the severity of bowel symptoms using scores of item 3 of the MSISQ-19 [18]. Each of both items scores symptoms over the last 6 months with ratings from 1 (never) to 5 (always) [18].
Assessment of erectile function
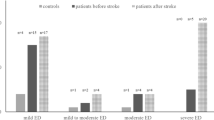
We assessed EF at the time of our study, i.e., during MS, and—retrospectively—3 months prior to diagnosis of MS [19]. To assess ED prevalence and severity, we used the validated abridged International Index of Erectile Function-5 (IIEF5) questionnaire that consists of five items. The items of the IIEF5 evaluate the patient’s confidence to maintain an erection, the level of penile tumescence, the ability to maintain an erection at the beginning of sexual intercourse, the ability to maintain the erection until completion of sexual intercourse, and the overall sexual satisfaction with a maximum score of 5 per item [19]. The IIEF5-score ranges from a minimum of 1 to a maximum of 25 [19]. For each of the five items a score of 5 indicates that (1) the patient is very confident to get an erection; (2) penile erections are almost always hard enough for vaginal penetration; (3) the patient is almost always able to maintain erection after penetration; (4) the patient is almost always able to maintain erection until the end of sexual intercourse, and (5) the patient is almost always satisfied with the sexual interaction. A score of 0 indicates no sexual activity. An IIEF5-score of 21 or below indicates ED [19]. The IIEF5 classifies EF, as no ED (22–25 points; grade 1), mild ED (17–21 points; grade 2), mild-to-moderate ED (12–16 points; grade 3), moderate ED (8–11 points; grade 4), and severe ED (1–7 points; grade 5). We calculated the difference between the IIEF5-scores assessed prior to and after the diagnosis of MS (DeltaIIEF5) by subtracting IIEF5 scores prior to diagnosis of MS from IIEF5 scores upon MS diagnosis.
Brain imaging and lesion analysis
All patients underwent 1.5 or 3 T magnetic resonance imaging (MRI) of the brain according to a standard protocol. MRI scans of the brain included axial or 3D FLAIR sequences for analyzing cortically located or supratentorial MS lesions and axial T2-weighted images (voxel size = 0.5 × 0.5 × 5 mm3) for detection of the infratentorial MS lesions. To analyze MS lesions in the callosal radiation or corpus callosum, sagittal FLAIR or T2-weighted scans were performed. Two experienced investigators (K.W. and F.S.) manually delineated the boundaries of the MS lesions on anonymized axial T2-weighted MRI scans using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/) [20]. To ensure that no perivascular spaces were scored as MS lesions, lesions were only delineated if they were detectable on T2 as well as on FLAIR scans as a hyperintense signal [11]. To avoid observer bias, both raters were blinded to clinical parameters during imaging analyses. The MRI scan and the MS lesion shape were transferred into stereotaxic space using the normalization algorithm of SPM8 (http://www.fil.ion.ucl.ac.uk/spm/) and the Clinical Toolbox for SPM8 (http://www.mricro.com/clinical-toolbox/spm8-scripts) [20]. We applied the MR-segment-normalize algorithm of the Clinical Toolbox to transform the MRI-derived lesion shape and the MR images to the standardized T1 template based on younger individuals with a resampled voxel size of 1 × 1 × 1 mm3 [20]. The pathological T2-weighted scan was used to co-register the lesion map to the T1-weighted anatomical scan [20]. To warp the anatomical scan and the lesion shape into stereotaxic space, the unified-segmentation normalization algorithm implemented in SPM8 was performed on the anatomical scan [20, 21]. The normalized lesion map was then analyzed with non-parametric mapping software implemented in the MRIcron software package [20].
Since increasing total cerebral MS lesion volume might be associated with an increased risk of afflicting brain areas that are strategically relevant for EF [12, 13, 22], we calculated the total volume of cerebral MS lesions using nonparametric mapping (NPM) software implemented in the MRIcron software package [14].
Statistical analysis
We determined the lesion overlap, i.e., the prevalence of identical lesion sites among different patients [11,12,13]. To determine whether the lesion overlap of patients in whom EF deteriorated during MS differed from the lesion overlap of patients without changes in EF, we performed a subtraction analysis of lesion overlaps subtracting lesion overlaps in patients with DeltaIIEF5 scores ≥ 0 (indicating no EF deterioration), from overlaps in patients with DeltaIIEF5 scores < 0 (indicating EF deterioration) [12, 23].
For each voxel that showed lesions in at least three patients, we compared DeltaIIEF5 scores between patients with and without lesions in this particular voxel using t tests. In voxel-wise lesion analyses it is common to exclude voxels from the analysis that show too few lesions to assure stable comparison of lesioned versus non-lesioned performance. In the present study, we only evaluated associations of lesioned voxels with deterioration of EF if there was a lesion overlap in at least 10%, i.e., in 3 of the 31 enrolled patients to assure stable comparison of lesioned and non-lesioned performance and to limit outlier effects [24]. To control for multiple comparisons during voxel-wise t tests, we applied a false discovery rate (FDR) correction of q < 0.05 based on corresponding z-scores calculated by the MRIcron software.
To determine damaged brain regions, affected voxels were overlaid on the Automated Anatomical Labeling (AAL) atlas [25] or the John Hopkins University (JHU)-White-matter-labels atlas (1 mm). The peak coordinates of the involved regions are presented in Montreal Neurological Institute (MNI) space.
To further determine parameters possibly contributing to MS-related deterioration of EF, we compared age, disease duration, EDSS scores, BDI scores, MSISQ-19 bladder and bowel dysfunction scores, IIEF5 scores at baseline, and the total cerebral lesion volume between patients with DeltaIIEF5 scores ≥ 0 (indicating no EF deterioration), and patients with DeltaIIEF5 scores < 0 (indicating EF deterioration) using t tests for normally distributed data and the Mann–Whitney U test for not normally distributed data. In addition, we correlated DeltaIIEF5 scores of all study participants with their age, disease duration, EDSS scores, BDI scores, MSISQ-19 bladder and bowel dysfunction scores, and the total volume of cerebral MS lesions using the Spearman rank correlation coefficient. Using the Mann–Whitney U test, we furthermore determined whether DeltaIIEF5 scores differed between patients with and without paraparesis (i.e., the presence/absence of sensorimotor lower limb affection). Using the Mann–Whitney U test, we also evaluated whether cerebral MS lesion volumes differed between patients who had 1.5 T MRIs and patients who had 3 T MRIs. To test for normal distribution of data, we used the Shapiro–Wilk test. Normally distributed data are presented as mean ± standard deviation (SD) and non-normally distributed data as median and interquartile ranges (IQRs). Statistical significance was assumed for p < 0.05. For statistical calculations, we used a commercially available statistic program (SPSS 20.0; IBM, Armonk, NY, USA).
Results
Patients
Of 35 screened patients with MS, four patients were excluded from VLSM analysis. One of the four patients was excluded because of poor MRI quality. The three other patients were excluded because they reported not to have had any sexual activity prior to nor after MS manifestation. Thus, a total of 31 patients fulfilled the inclusion criteria and were eligible to be included in the VLSM analysis. Mean age was 38.2 ± 11.2 years, median disease duration was 45 months (IQR 11–81 months), median EDSS score was 3.3 (IQR 2–4.4), median BDI score was 28 (IQR 16–42), and mean total volume of cerebral MS lesions was 21.0 ± 14.6 ml. At the time of examination, 27 patients (87.1%) had a stable disease course. Only four patients (12.9%) had a relapse at the time of the examination with two patients experiencing isolated symptoms of retrobulbar neuritis, one patient presenting with pure hypaesthesia of the right arm and leg and one patient having sensorimotor symptoms of the right leg. The median time since the last relapse was 316 days (IQR 101–643 days). IIEF5 scores prior to MS diagnosis ranged from 12 to 25 (median 22, IQR 19–25), IIEF5 scores at the time of the study, during MS ranged from 5 to 25 (median 19, IQR 15–24). DeltaIIEF5 scores ranged from − 17 to + 3 (median DeltaIIEF5 score = 0, IQR – 5 to 0). In 14 patients (45.2%), IIEF5 scores were lower during than 3 months before MS (DeltaIIEF5 < 0); in 17 patients, IIEF5 scores did not change or even improved (DeltaIIEF5 ≥ 0). Five of the 31 patients (16.1%) showed an improvement of IIEF5 scores during MS (DeltaIIEF5 score > 0) ranging between 1 and 2 points; only one of the five patients had an IIEF5 score improvement of 3 points. Patient age, disease duration, EDSS scores, BDI scores, MSISQ-19 bladder and bowel dysfunction scores, IIEF5 scores at baseline, and total volume of cerebral MS lesions did not differ between patients with Delta IIEF5 < 0 and patients with DeltaIIEF5 ≥ 0 (Table 1). A total of 10/31 MS patients (32.3%) had sensorimotor lower limb affection. DeltaIIEF5 scores did not differ between patients with and without paraparesis (i.e., sensorimotor lower limb affection) (Mann–Whitney U test; p = 0.29). 22 of the 31 patients (71.0%) had 1.5 T MRIs while 9 patients (29.0%) had 3 T MRIs. The total cerebral MS lesion volumes did not differ between patients who had 1.5 T MRIs (20.5 ± 15.1 ml) and patients who had 3 T MRIs (22.1 ± 14.4 ml; p = 0.72).
Correlation between DeltaIIEF5 scores and clinical parameters
DeltaIIEF5 scores did not correlate with patient age, disease duration, EDSS scores, BDI scores, MSISQ-19 bladder and bowel dysfunction scores, and total volume of cerebral MS lesions (Spearman rank correlation coefficient; p > 0.05).
Voxel-based lesion symptom mapping
Figure 1 shows the lesion overlap and distribution of lesioned voxels of all 31 patients. The highest lesion overlap, i.e., highest number of individuals with lesions prevalent in a given voxel, was seen in the periventricular region, especially the parietal white matter, the bilateral callosal radiation, and the periaqueductal midbrain gray.
Overlap and distribution of T2-hyperintense MS lesions of all patients. The number of overlapping lesions is illustrated by different colors coding increasing frequencies from dark red to yellow. Regions with higher lesion overlap counts are found symmetrically in periventricular regions, most prominently in the parietal periventricular white matter, as well as in the subinsular regions, and periaqueductal midbrain gray. Montreal Neurological Institute (MNI) z-coordinates of each transverse section are given. L left hemisphere, N number of individuals, R right hemisphere
Subtraction analysis of lesion overlap in patients with DeltaIIEF5 score ≥ 0 and in patients with Delta IIEF5 score < 0 showed that patients with Delta IIEF5 scores < 0 had larger clusters of lesioned voxels in the juxtacortical insular white matter, most prominently in the left hemisphere (Fig. 2). Table 2 shows the number of lesion overlaps in a given area after lesion-overlap subtraction analysis as well as the peak coordinates of each lesioned area—outlined in the Montreal Neurological Institute (MNI) space.
Subtraction map of lesion overlap in patients with DeltaIIEF5 scores ≥ 0 indicating unchanged or improved erectile function from the lesion overlap in patients with DeltaIIEF5 score < 0, indicating deteriorated erectile function. After subtraction of lesion overlap in patients with unchanged erectile function from the lesion overlap in patients with deteriorated erectile function, lesioned voxels were most prevalent in the insular, particularly left insular region. To reduce noise only voxels that were lesioned in at least three individuals after subtraction are shown. L left hemisphere, N number of individuals with a lesion in a given voxel after subtraction, R right hemisphere
Results of the voxel-wise t test statistics comparing DeltaIIEF5 scores between patients with and without lesions in a given voxel are shown in Table 3 and Fig. 3. A total of 1049 voxels correlated with decreasing DeltaIIEF5 scores. A total of 233 voxels were located in the gray matter and 810 voxels in the white matter. In short, decreasing DeltaIIEF5 scores correlated with a large cluster of MS lesions in the insular region including the adjacent juxtacortical white matter, most prominently in the left-hemispheric insular region. Only smaller clusters of lesioned voxels in the right fusiform gyrus and thalamus correlated with decreasing DeltaIIEF5 scores. Table 3 shows lesion sites associated with DeltaIIEF5 scores < 0, the corresponding number of damaged voxels, and peak coordinates outlined in MNI space.
Results of the voxel-wise t test statistics comparing DeltaIIEF5 scores between patients with and without lesions in a given voxel. Lesioned voxels in the insular juxtacortical white matter, again most prominently in the left hemisphere were associated with decreasing DeltaIIEF5 scores. Only voxels that were damaged in at least three individuals were included in the analysis. A false discovery rate (FDR) correction of q < 0.05 was applied (z-score = 3.2). L left hemisphere, R right hemisphere, z z-score
Discussion
Our study showed that 14 of the 31 men (45.2%) afflicted by MS also experienced deterioration of EF and thus confirms that ED is a common complication of MS [3, 4].
Moreover, the VLSM analysis identified specific sites of MS lesions to be associated with the EF deterioration. Both, the voxel-wise subtraction analysis of lesioned voxels in patients with and without EF deterioration (Table 2, Fig. 2) and the voxel-wise comparison of DeltaIIEF5 scores between patients with and without lesions in a given voxel (Table 3, Fig. 3) showed associations between MS-related EF deterioration and T2-weighted MS lesions in the insular region, with a left-hemispheric predominance, as well as associations with smaller lesions sites in the hippocampus and occipital cortex.
Although ED is rather common among MS patients and may compromise the patients’ quality of life, self-esteem, and partnership [3, 11, 22], studies assessing the impact of isolated central lesions on EF are scarce and moreover yielded discrepant results [1, 2, 26, 27]. Most previous studies used region-of-interest analyses to determine interactions between cerebral sexual dysfunction and lesion sites in male and female MS patients [1, 2, 26, 27] and assessed the MS lesion load in the whole brain, the frontal cortex, and the pons [1, 2]. While Zorzon et al. found no association between sexual dysfunction and MS lesions in these regions [2], Zivadinov et al. applied a multivariate regression analysis and found associations between sexual dysfunction and pontine MS lesions [1]. Barak et al. found correlations between male and female anorgasmia and the total volume of cerebral MS lesions, and more specifically of brainstem and corticospinal tract lesions [26].
In the present study, we assessed more detailed associations between EF deterioration and MS lesions, first by voxel-wise identification of the specific sites of MS lesions and then by assessing associations between lesioned sites and EF deterioration using the above voxel-wise subtraction analysis and voxel-wise t test statistics of DeltaIIEF5 scores [11,12,13]. While the VLSM analysis showed the above associations between specific lesion sites and EF deterioration, the analysis of correlations between EF deterioration and the potential confounding factors age, disease duration, disease severity, depressiveness, bladder and bowel dysfunction, sensorimotor lower limb affection, as well as total volume of cerebral MS lesions, showed no impact of these factors on EF. Among previous studies in female or male MS patients, some showed associations between these confounders and sexual dysfunction [1, 2] while others could not confirm such associations [26]. We cannot fully rule out a general impact of the above confounders in MS patients in general, but our patients were rather young and had rather low EDSS scores (median 3.3, IQR 2–4.4). Still, 45.2% of our patients experienced EF deterioration which supports the conclusion that lesioning at the above-mentioned sites has a predominant impact on EF deterioration. We therefore conclude that the identified areas, particularly, the insular juxtacortical region, are main contributors and modulators of EF [11, 12].
Our findings, moreover, confirm hemispheric differences in the modulation of sexual function [22]. In our patients, EF deterioration was more prominently associated with MS lesions in the left than the right insular juxtacortical white matter. The insula contributes significantly to generating and integrating visceral autonomic arousal that includes penile erection [12, 13, 28,29,30]. In healthy men, functional neuroimaging studies showed activation of the insular cortex during male sexual arousal and penile erection [7,8,9,10]. Penile erection is mainly mediated by parasympathetic activation that leads to penile trabecular smooth muscle relaxation and tumescence [12, 22]. We hypothesize that MS lesions in the left insular region compromise parasympathetic modulation and thereby contribute to ED [11, 12, 22]. In animal experiments, insular cortex lesioning induced autonomic imbalance depending on the lesion side [31]. Oppenheimer et al. showed associations between left insular cortex lesions and an increase in cardiac sympathetic tone after ischemic stroke [32]. Hemispheric inactivation as well as insular stimulation in humans also demonstrated hemispheric dominance of autonomic cardiovascular modulation, with more prominent sympathetic influence arising from the right hemisphere or insula, and more cardiovagal modulation arising from the left hemisphere [33,34,35]. Functional neuroimaging in healthy individuals and voxel-wise lesion studies in stroke patients further confirmed the hemispheric lateralization of autonomic arousal [13, 30, 36].
Using VLSM, our group recently studied associations between ED after ischemic stroke and the sites of ischemic lesions [12]. Again, lesions in the left insular and opercular region were associated with EF deterioration [12]. From these findings, we concluded that a stroke-induced disruption of central autonomic pathways that assure sexual function contributes to EF deterioration [12].
While EF deteriorated with MS in 14 of our 31 patients, there was a slight improvement of EF in five (16.1%) of the 31 patients. This improvement cannot be attributed to the effects of any medication such as phosphodiesterase-5-inhibitors since none of the patients took drugs enhancing EF. Therefore, we can only speculate that there might have been an improvement in the patients’ partnership or that patients had MS lesions in brain areas that are normally associated with inhibition of sexual function, but were disinhibited in the five patients due to the lesions [27].
There are several limitations to our study. The VLSM technique only supports conclusions regarding associations between EF deterioration and sites of MS lesions for those brain areas that are compromised in a high enough number of individual patients, i.e., if there is a high enough lesion overlap among patients [11, 12]. Thus, associations between MS-related EF deterioration and lesions in areas that are known to usually contribute to autonomic and emotional processing, such as the prefrontal or orbitofrontal cortices, the hypothalamus, the cingulate gyrus or infratentorial areas, cannot be adequately determined by the VLSM approach unless the sample size is significantly larger than in our study [11, 37]. However, we could not easily increase the number of participating patients, as our exclusion criteria ruled out the evaluation of patients with other possible causes of ED, and thus limited the number of MS patients who were suited for this study. A further limitation of our study results from the fact that we performed spinal cord imaging only in 19 of the 31 patients who had signs and symptoms of possible spinal cord lesioning. Yet, spinal cord lesioning may be a major cause of ED. Areas of afferent and efferent spinal sexual pathways are known to be located in the anterolateral columns and sacral segments [22].
While cerebral lesion volumes and DeltaIIEF5 scores were similar between patients with and patients without spinal cord lesions, we cannot rule out that possible spinal cord lesions in the remaining 12 patients without spinal cord imaging might have resulted in a significant difference of cerebral lesion volumes and DeltaIIEF5 scores between patients with and without spinal cord lesions. However, the VLSM method applied in our study is not validated for determining correlations between spinal cord lesions and symptom deterioration. Thus, spinal cord lesions might be a major contributor to EF deterioration in our patients. Still, our data support our conclusion that insular juxtacortical lesioning is a prominent contributing factor of EF deterioration.
A further limitation of our study is the retrospective evaluation of our patients’ EF status, 3 months prior to MS diagnosis. Especially for patients with long disease duration, it may be difficult to accurately recall and report their EF before MS onset. Finally, the patients’ subjective answers to the EF self-questionnaire might have had some effect on our findings [12]. Another limitation of our study results from the fact that the MS lesions were assessed with 1.5 T MRIs in 22 of the 31 patients and with 3 T MRIs in 9 patients. Although the comparison of MS-lesion volumes between these two groups showed no significant difference, we cannot rule out that the 22 patients with the 1.5 T MRI might have shown higher lesion volumes if they had undergone 3 T MRI. Moreover, we used a 5-mm imaging slice thickness which might have been too coarse to identify smaller lesions. Thus, a smaller slice thickness and uniform 3 T MRI might have refined our analysis and improved results of our VLSM analysis. Moreover, T2/FLAIR-hyperintense lesions may reflect several different pathological substrates. While we excluded patients with a history of other neurological diseases which might cause hyperintense T2/FLAIR lesions, the hyperintense T2-lesions seen in our MS patients may represent various pathological substrates such as demyelination, remyelination or destructive axonal lesions with possible regenerative potential [38, 39]. Therefore, we decided to use a FDR correction of q < 0.05 corresponding to a z-score of ≥ 3.2 which yields mild-to-moderate correlations between the clinical measure of deteriorating EF and lesional findings. The sensitivity of T2/FLAIR sequences might be reduced particularly in detecting cortical lesions. Moreover, we cannot rule out that we might have missed particularly small cortical or juxtacortical lesions with the T2 and FLAIR sequences since these sequences have limited sensitivity to detect small lesions with 1.5 T MRI. However, these sequences still showed cortical and juxtacortical lesions in many of our patients, as evidenced in the lesion overlap shown in Fig. 1. Finally, we cannot rule out that some of the lesions seen in the gray matter regions of the normalized template might be caused by the registration of the recorded T2-weighted brain MRI sequences and lesion masks into the normalized template space.
In summary, our finding of associations between EF deterioration and cerebral MS lesions in the juxtacortical insular regions, particularly the left insular region, is also of clinical relevance. Although autonomic dysfunction is common among MS patients [11, 40, 41], particularly sexual dysfunction is often overlooked despite its impact on the patients’ quality of life, family planning, and self-esteem [1, 2, 11]. Awareness of the risk of sexual dysfunction in MS patients with lesions in the insular, particularly left insular region will enable physicians to thoughtfully address the issue of possible sexual dysfunction and offer therapeutic advice which may significantly improve the patient’s psychological prospect.
References
Zivadinov R, Zorzon M, Locatelli L, Stival B, Monti F, Nasuelli D, Tommasi MA, Bratina A, Cazzato G (2003) Sexual dysfunction in multiple sclerosis: a MRI, neurophysiological and urodynamic study. J Neurol Sci 210:73–76
Zorzon M, Zivadinov R, Locatelli L, Stival B, Nasuelli D, Bratina A, Bosco A, Tommasi MA, Pozzi Mucelli RS, Ukmar M, Cazzato G (2003) Correlation of sexual dysfunction and brain magnetic resonance imaging in multiple sclerosis. Mult Scler 9:108–110
Rees PM, Fowler CJ, Maas CP (2007) Sexual function in men and women with neurological disorders. Lancet 369:512–525
Betts CD, Jones SJ, Fowler CG, Fowler CJ (1994) Erectile dysfunction in multiple sclerosis. Associated neurological and neurophysiological deficits, and treatment of the condition. Brain 117(Pt 6):1303–1310
Vigeveno RM, Wiebenga OT, Wattjes MP, Geurts JJ, Barkhof F (2012) Shifting imaging targets in multiple sclerosis: from inflammation to neurodegeneration. J Magn Reson Imaging 36:1–19
Ge Y (2006) Multiple sclerosis: the role of MR imaging. AJNR Am J Neuroradiol 27:1165–1176
Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, Lue TF, Atlas SW (2002) Brain activation and sexual arousal in healthy, heterosexual males. Brain 125:1014–1023
Ferretti A, Caulo M, Del Gratta C, Di Matteo R, Merla A, Montorsi F, Pizzella V, Pompa P, Rigatti P, Rossini PM, Salonia A, Tartaro A, Romani GL (2005) Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage 26:1086–1096
Miyagawa Y, Tsujimura A, Fujita K, Matsuoka Y, Takahashi T, Takao T, Takada S, Matsumiya K, Osaki Y, Takasawa M, Oku N, Hatazawa J, Kaneko S, Okuyama A (2007) Differential brain processing of audiovisual sexual stimuli in men: comparative positron emission tomography study of the initiation and maintenance of penile erection during sexual arousal. Neuroimage 36:830–842
Moulier V, Mouras H, Pélégrini-Issac M, Glutron D, Rouxel R, Grandjean B, Bittoun J, Stoléru S (2006) Neuroanatomical correlates of penile erection evoked by photographic stimuli in human males. Neuroimage 33:689–699
Winder K, Linker RA, Seifert F, Deutsch M, Engelhorn T, Dörfler A, Lee DH, Hösl KM, Hilz MJ (2016) Neuroanatomic correlates of female sexual dysfunction in multiple sclerosis. Ann Neurol 80:490–498
Winder K, Seifert F, Köhrmann M, Crodel C, Kloska S, Dörfler A, Hösl KM, Schwab S, Hilz MJ (2017) Lesion mapping of stroke-related erectile dysfunction. Brain 140:1706–1717
Winder K, Seifert F, Ohnemus T, Sauer EM, Kloska S, Dörfler A, Hilz MJ, Schwab S, Köhrmann M (2015) Neuroanatomic correlates of poststroke hyperglycemia. Ann Neurol 77:262–268
Rorden C, Karnath HO, Bonilha L (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19:1081–1088
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Richter P, Werner J, Heerlein A, Kraus A, Sauer H (1998) On the validity of the Beck Depression Inventory. A review. Psychopathology 31:160–168
Sanders AS, Foley FW, LaRocca NG, Zemon V (2000) The Multiple Sclerosis Intimacy and Sexuality Questionnaire-19 (MSISQ-19). Sex Disabil 18:3–26
Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Pena BM (1999) Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 11:319–326
Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO (2012) Age-specific CT and MRI templates for spatial normalization. Neuroimage 61:957–965
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26:839–851
Hilz MJ (2008) Female and male sexual dysfunction. In: Low PA, Benarroch EE (eds) Clinical autonomic disorders, 3rd edn. Lippincott Williams and Wilkins, Philadelphia, pp 657–711
Seifert CL, Schönbach EM, Magon S, Gross E, Zimmer C, Förschler A, Tölle TR, Mühlau M, Sprenger T, Poppert H (2016) Headache in acute ischaemic stroke: a lesion mapping study. Brain 139:217–226
Schwartz MF, Faseyitan O, Kim J, Coslett HB (2012) The dorsal stream contribution to phonological retrieval in object naming. Brain 135:3799–3814
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Barak Y, Achiron A, Elizur A, Gabbay U, Noy S, Sarova-Pinhas I (1996) Sexual dysfunction in relapsing-remitting multiple sclerosis: magnetic resonance imaging, clinical, and psychological correlates. J Psychiatry Neurosci 21:255–258
Winder K, Seifert F, Koehn J, Deutsch M, Engelhorn T, Dörfler A, Lee DH, Linker RA, Hilz MJ (2015) Site and size of multiple sclerosis lesions predict enhanced or decreased female orgasmic function. J Neurol 262:2731–2738
Saper CB (2002) The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25:433–469
King AB, Menon RS, Hachinski V, Cechetto DF (1999) Human forebrain activation by visceral stimuli. J Comp Neurol 413:572–582
Cechetto DF, Shoemaker JK (2009) Functional neuroanatomy of autonomic regulation. Neuroimage 47:795–803
Zhang ZH, Rashba S, Oppenheimer SM (1998) Insular cortex lesions alter baroreceptor sensitivity in the urethane-anesthetized rat. Brain Res 813:73–81
Oppenheimer SM, Kedem G, Martin WM (1996) Left-insular cortex lesions perturb cardiac autonomic tone in humans. Clin Auton Res 6:131–140
Yoon BW, Morillo CA, Cechetto DF, Hachinski V (1997) Cerebral hemispheric lateralization in cardiac autonomic control. Arch Neurol 54:741–744
Hilz MJ, Dütsch M, Perrine K, Nelson PK, Rauhut U, Devinsky O (2001) Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann Neurol 49:575–584
Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC (1992) Cardiovascular effects of human insular cortex stimulation. Neurology 42:1727–1732
Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ (2000) Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. J Physiol 523(Pt 1):259–270
Benarroch EE (1997) The central autonomic network. In: Low PA (ed) Clinical autonomic disorders. Lippincott-Raven Publishers, Philadelphia, pp 17–23
van Walderveen MA, Kamphorst W, Scheltens P, van Waesberghe JH, Ravid R, Valk J, Polman CH, Barkhof F (1998) Histopathologic correlate of hypointense lesions on T1-weighted spin-echo MRI in multiple sclerosis. Neurology 50:1282–1288
De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL (1998) Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain 121(Pt 8):1469–1477
Hilz MJ (2016) Cardiac stunning as first manifestation of multiple sclerosis: a case report reminding us not to overlook cardiovascular autonomic dysfunction in multiple sclerosis. Mult Scler 22:847–848
Kaplan TB, Berkowitz AL, Samuels MA (2015) Cardiovascular dysfunction in multiple sclerosis. Neurologist 20:108–114
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MJH reports grants and personal fees from Bayer HealthCare pharmaceuticals, during the conduct of the study; grants and personal fees from Genzyme, a Sanofi company, grants and personal fees from Novartis Pharma GmbH, outside the submitted work. DHL reports grants and personal fees from Bayer HealthCare Pharmaceuticals, grants and personal fees from Biogen Idec, grants and personal fees from Merck Serono, grants and personal fees from Novartis Pharma GmbH, grants and personal fees from TEVA Pharmaceutical Industries LTD, outside the submitted work. RAL reports grants and personal fees from Bayer HealthCare Pharmaceuticals, grants and personal fees from Biogen Idec, grants and personal fees from Merck Serono, grants and personal fees from Novartis Pharma GmbH, grants and personal fees from Roche, grants and personal fees from TEVA Pharmaceutical Industries LTD, and from Novartis foundation, outside the submitted work. RAL holds an endowed professorship supported by the Novartis Foundation.
Ethical approval
The study has been approved by the local ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Written informed consent has been obtained for all study participants.
Rights and permissions
About this article
Cite this article
Winder, K., Linker, R.A., Seifert, F. et al. Insular multiple sclerosis lesions are associated with erectile dysfunction. J Neurol 265, 783–792 (2018). https://doi.org/10.1007/s00415-018-8763-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8763-5