Abstract
Dentatorubral–pallidoluysian atrophy (DRPLA) is an autosomal dominant spinocerebellar ataxia caused by CAG triplet expansion in atrophin 1 and is frequently associated with cerebral white matter lesions. To elucidate the clinical features of elderly onset DRPLA and the key radiological findings for differentiating DRPLA from physiological white matter lesions in healthy elderly subjects, we reviewed the clinical and magnetic resonance imaging (MRI) features of ten patients with elderly onset genetically confirmed DRPLA (> 60 years) and compared their MRI findings with those of age- and sex-matched ten healthy subjects with asymptomatic cerebral white matter lesions. The initial symptom was cerebellar ataxia in all DRPLA patients, and five of them did not have any symptoms other than ataxia at the time of MRI examination. Atrophy of the brainstem, superior cerebellar peduncle, and cerebellum was detected in all DRPLA patients and none of the healthy subjects. Abnormal signals in the brainstem (inferior olive, pons, and midbrain), thalamus, and cerebellar white matter were frequently observed in elderly onset DRPLA patients but not in healthy subjects. In conclusion, elderly onset DRPLA presents as cerebellar ataxia alone in the early stage of disease. Atrophy of the brainstem, superior cerebellar peduncle, and cerebellum and abnormal signals in the brainstem, cerebellum, and thalamus are key findings for differentiating elderly onset DRPLA from asymptomatic cerebral white matter lesions in healthy subjects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dentatorubral–pallidoluysian atrophy (DRPLA) is an autosomal dominant neurodegenerative disorder caused by CAG triplet expansion in atrophin 1 [1, 2]. Although DRPLA is thought to exhibit a marked ethnic predilection and is considered to be detected almost exclusively among Japanese populations, there may be more patients with DRPLA than has been estimated in the western populations [3, 4]. DRPLA can be classified into two major forms according to the age at onset. Adult-onset DRPLA (age at onset of > 20 years) usually presents as ataxia, choreoathetosis, and dementia, whereas seizure and myoclonus typically occur in juvenile-onset DRPLA (age at onset of < 20 years) [5]. Adult-onset DRPLA is often divided into two groups according to the age at onset: early adult type (age at onset between 20 and 40 years) and late-adult type (age at onset of > 40 years) [6].
Several reports have demonstrated an inverse correlation between the size of the expanded CAG repeats and the age at onset and clinical severity of the disease [5,6,7]; furthermore, the clinical symptoms of elderly onset DRPLA with mildly expanded CAG repeats are expected to be mild and might be misdiagnosed as other neurological disorders or age-related changes. In such a situation, brain magnetic resonance (MR) imaging findings are expected to aid in differential diagnosis, as well as the survey of patient backgrounds and accurate neurological examination. However, the clinical features and brain MR imaging findings of elderly onset DRPLA have not yet been fully evaluated.
Previous studies have demonstrated that T2-weighted images (T2WI)/fluid-attenuated inversion recovery (FLAIR) high-intensity signals in the cerebral white matter, brainstem, and thalamus and atrophy of the cerebrum, brainstem, and cerebellum are characteristic MR imaging findings of adult-onset DRPLA [7,8,9,10,11]. In particular, T2WI high-intensity signals in the cerebral white matter have been observed more frequently in DRPLA patients with mildly expanded CAG repeats than in DRPLA patients with largely expanded CAG repeats [7]. However, some degree of white matter lesions on MR imaging are often found in elderly individuals without neurological disorders [12].
Herein we present the clinical features of elderly onset DRPLA patients and compared the frequency of atrophy and abnormal signals in the brain MR images of elderly onset DRPLA patients with those of healthy subjects with asymptomatic cerebral white matter lesions.
Materials and methods
Subjects
Ten genetically confirmed elderly onset (> 60 years of age at initial manifestation) DRPLA patients who underwent brain MR imaging from June 2008 to November 2016 at our institutions were identified (4 males and 6 females). We also recruited ten age- and sex-matched healthy controls with high-intensity signals in the cerebral white matter on brain T2WI/FLAIR images (grade 2–3 of deep white matter hyperintensity rated using the criteria outlined by Fazekas et al. [13]) in this study (4 males and 6 females).
The medical records of the ten DRPLA patients were reviewed for disease duration, age at onset and age at scan, CAG repeat length, family history, initial manifestation, and mini-mental state examination (MMSE) scores. This retrospective study was approved by our institutional review board, and the need for patient informed consent was waived. All of the healthy controls provided written informed consent for their data to be used in the study.
MR imaging analysis
All MR studies of DRPLA patients were performed during routine clinical care using 1 T (n = 1), 1.5 T (n = 7), and 3 T (n = 2) MR scanners. All MR studies of healthy controls were performed using the 3 T MR scanner. T2WI axial, T1WI sagittal, and FLAIR axial or coronal images were available for all patients and healthy controls. The MR data of each subject were independently evaluated by two neuroradiologists blinded to all clinical data other than age. For findings where the evaluation of the two examiners was different, the final results were determined by third examiner.
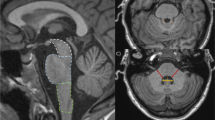
We evaluated the following findings: high-intensity signals in the cerebral white matter, thalamus, midbrain, pontine tegmentum, pontine base, inferior olive, and cerebellar white matter on T2WI/FLAIR images and atrophy of the cerebrum, midbrain tegmentum, pontine tegmentum, pontine base, superior cerebellar peduncle, and cerebellum (Fig. 1). The high-intensity signals in cerebral white matter on T2WI/FLAIR images were evaluated using the criteria for deep white matter intensity outlined by Fazekas et al. [13]. All findings other than the high-intensity signals in cerebral white matter were rated on a two-point scale: “−” indicates no lesions and “+” indicates some lesions. Furthermore, cerebral atrophy was judged to be present in cases where the severity of the lesions exceeded the influence of aging.
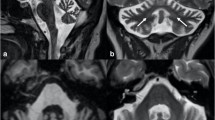
Brain magnetic resonance imaging findings of healthy controls with abnormal signals in the cerebral white matter (a–e). The pontine base was defined as the ventral part of the pons delineated by the ventral margin of the pons and the medial lemniscus (a, star). The pontine tegmentum was defined as the dorsal part of the pons and the base of the fourth ventricle (a, asterisk). No abnormal signals were observed in the brainstem (b, c) or thalamus (d). Axial T2-weighted images show high-signal intensity in the cerebral white matter (Fazekas grade 3; e, white arrows)
Results
There were no significant differences in age at MRI between DRPLA patients (70.4 ± 22.0 years) and healthy controls (70.9 ± 18.3 years). The DRPLA patients’ clinical presentations are summarized in Table 1. The initial symptom in all patients with DRPLA was ataxia. At the time of MRI, five of the ten patients with DRPLA (50%) did not have any symptoms other than ataxia. Furthermore, dementia was observed in five of the ten patients (50%), psychiatric symptoms in two of the ten patients (20%), and choreoathetoid and epilepsy in one of the ten patients (10%) at the time of MRI. In nine of the ten DRPLA patients (90%), the period from disease onset to the development of symptoms other than ataxia was > 5 years. The number of CAG repeats in the ten DRPLA patients was 52–58 (54.8 ± 4.2, mean ± SD). Among the ten DRPLA patients, three had family members in successive generations who had already been diagnosed with DRPLA, and nine had family members with symptoms similar to those of DRPLA.
The MRI findings of the ten DRPLA patients are summarized in Table 2. Among the healthy controls, abnormal signals other than those in the cerebral white matter were not observed in any case (Fig. 1). Among the DRPLA patients, high-intensity signals in the cerebral white matter were observed in all cases (Figs. 2, 3, 4). Seven of the DRPLA patients (70%) showed abnormally high signals in the brainstem (pontine tegmentum, 70%; pontine base, 70%; midbrain, 50%; and inferior olive, 20%) on T2WI/FLAIR images (Fig. 2). Seven of the DRPLA patients (70%) showed abnormally high signals in the thalamus (Fig. 2) and cerebellar white matter (Fig. 3) on T2WI/FLAIR images. Of the two DRPLA patients without abnormal T2WI/FLAIR high signals in the brainstem or thalamus, one exhibited abnormal signals in the cerebellar white matter (Fig. 3), whereas the other exhibited no abnormal signals other than those in the cerebral white matter (Fig. 4). With regard to atrophy, neither brainstem nor cerebellar atrophy was observed in healthy controls (Fig. 1). By contrast, atrophy of the superior cerebellar peduncle and cerebellum were observed in all cases of DRPLA (Figs. 2, 3, 4). Atrophy of the midbrain tegmentum, pontine base, and pontine tegmentum was observed in nine of the DRPLA patients (90%; Figs. 2, 3, 4), and atrophy of the cerebrum was detected in eight of the DRPLA patients (80%).
Brain magnetic resonance imaging findings of a dentatorubral–pallidoluysian atrophy patient with diffuse brain atrophy and abnormal signals in the brainstem, thalamus, and cerebral white matter (patient 1, a–e). Sagittal T1-weighted images a show atrophy of the cerebrum, cerebellum, midbrain tegmentum, and pontine tegmentum and the pontine base. Axial T2-weighted images b–e show atrophy of the superior cerebellar peduncle (c, black arrowheads) and high-signal intensity in the inferior olive (b, black arrows), pons (c), bilateral thalami (d, white arrows), and cerebral white matter (e)
Brain magnetic resonance imaging findings of a dentatorubral–pallidoluysian atrophy patient with abnormal cerebellar signals and brain stem and cerebellar atrophy (Patient 8, a–e). Sagittal T1-weighted images (a) and axial T2-weighted images (b–e) show atrophy of the cerebrum, cerebellum, superior cerebellar peduncle, midbrain tegmentum, pontine tegmentum, and pontine base. Axial T2-weighted images d show high-signal intensity in the bilateral cerebral white matter. Coronal fluid-attenuated inversion recovery images display high-signal intensities in the cerebellar white matter around the dentate nucleus (white arrows). Abnormal signals in the brainstem are not observed (b, c)
Brain magnetic resonance imaging findings of a dentatorubral–pallidoluysian atrophy patient without abnormal signals other than those in the cerebral white matter (patient 10, a–e). Sagittal T1-weighted images a show slight atrophy of the cerebellum and basilar pons. Axial T2-weighted images (c) and coronal fluid-attenuated inversion recovery images e show atrophy of the superior cerebellar peduncle (white arrows). Axial T2-weighted images d show high-signal intensity in the bilateral cerebral white matter. Abnormal signals in the brainstem are not observed (b, c)
Discussion
Here we report the clinical features of ten patients with elderly onset (age > 60 years at initial manifestation) genetically confirmed DRPLA and compared their MR imaging findings with those of age- and sex-matched elderly healthy subjects with cerebral white matter lesions. All elderly onset DRPLA patients presented with cerebellar ataxia as the initial manifestation, and 50% of the elderly onset DRPLA patients did not have any symptoms other than ataxia at the time of MRI. Atrophy of the brainstem, superior cerebellar peduncle, and cerebellum, and high-intensity signals in the brainstem and thalamus on T2WI were considered as characteristic MR imaging findings of elderly onset DRPLA, as previously reported in adult type DRPLA. In some elderly onset DRPLA cases, no abnormal signals were observed in the thalamus or brainstem, and it seems difficult to differentiate this condition from other neurological disorders that present as atrophy of both the brainstem and cerebellum. Several case reports have examined elderly onset DRPLA patients; however, to the best of our knowledge, the present study is the first to review both the clinical and MR imaging findings of elderly onset DRPLA patients.
DRPLA has been divided into juvenile, early adult, and late-adult types with respect to 20-year intervals of age at onset [5, 6]. Accordingly, in this study, we defined elderly onset DRPLA as involving an age at onset of > 60 years. Patients with elderly onset DRPLA often present with only cerebellar ataxia as a predominant feature in the early stage and might be misdiagnosed with another neurological disorder or age-related changes. In our study, the initial symptom of all patients with elderly onset DRPLA was ataxia, and five of the ten patients (50%) did not have any symptoms other than ataxia at the time of MRI. Three of the ten patients were initially diagnosed with alcohol-induced cerebellar degeneration, familial spinocerebellar ataxia, and cortical cerebellar atrophy and not with DRPLA. A previous report has described three DRPLA cases that were characterized by disease onset in the sixth to seventh decades of life, cerebellar ataxia as the first manifestation, and predominant cerebellar deficits in the ambulatory stage [14]. In all the three cases, the initial diagnosis was hereditary olivopontocerebellar atrophy, and dementia and choreoathetoid movements did not develop for > 10 years after onset.
Taking a detailed family history is considered important for the diagnosis of elderly onset DRPLA. In our study, three of the ten patients had family members in successive generations who had already been diagnosed with DRPLA, and six of the ten patients had family members with symptoms similar to those of DRPLA. In DRPLA, there is a tendency for earlier onset and increased severity of the disease in successive generations (anticipation), especially when DRPLA is transmitted paternally [5, 6, 15].
On brain MR imaging, the key feature for discriminating elderly onset DRPLA from cerebral white matter lesions in healthy subjects is atrophy of the brainstem, superior cerebellar peduncle, and cerebellum. In our study, atrophy of the brainstem, superior cerebellar peduncle, and cerebellum was detected in all cases of DRPLA and none of the healthy subjects. In DRPLA, a correlation between age at MR imaging and atrophy of the cerebellum and brainstem has been reported [7]. Regarding the distribution of atrophy in the brainstem, DRPLA presents as overall brain stem atrophy. In eight of the ten DRPLA patients in our study, atrophy was observed in all the evaluated regions of the brainstem, such as the midbrain tegmentum, basilar pons, and pontine tegmentum. A previous paper also reported that overall atrophy of the midbrain tegmentum, basilar pons, and pontine tegmentum was observed in patients with DRPLA [7].
Regarding the discrimination of DRPLA from other neurological disorders based on MR images, brainstem and cerebellar atrophy is also observed in spinocerebellar ataxia (SCA), and this is termed olivopontocerebellar atrophy [16]. Moreover, progressive supranuclear palsy with predominant cerebellar ataxia (PSP-C) also presents as atrophy in the midbrain, superior cerebellar peduncle, and cerebellum [17]. In such cases, abnormal signals in the thalamus and brainstem are useful findings for distinguishing DRPLA from these disorders involving atrophy of both the brainstem and cerebellum. In our study, abnormal signals in the thalamus and brainstem, including the inferior olive, pons, and midbrain, were observed frequently in patients with elderly onset DRPLA and not in healthy subjects with cerebral white matter lesions. A previous study has shown that abnormally high signals in the brainstem and thalamus were characteristic findings of DRPLA patients and were useful for differentiating this condition from SCA1 and Machado–Joseph disease, which usually present as olivopontocerebellar atrophy [10]. Abnormal signals on MR imaging of the brainstem and cerebellum has not been described in PSP-C [17]. The detailed assessment of atrophy distribution in the brainstem may also be useful for differentiating DRPLA from these disorders, but further studies using quantitative assessment of atrophy in the brainstem are considered necessary.
In MR imaging of some elderly onset DRPLA patients, abnormal signals were not recognized in the brainstem and thalamus [14], and in such cases, it is difficult to distinguish elderly onset DRPLA from other neurological disorders, such as SCA, with olivopontocerebellar atrophy. In our study, two of the ten elderly onset DRPLA patients did not show abnormal signals in the brainstem and thalamus on MR imaging. One of these two patients showed abnormal signals in the cerebellar white matter on MR imaging, and this finding may be useful for differentiating DRPLA from other disorders (Fig. 3). However, this finding has not been described in past reports on MR findings in DRPLA, and therefore, further study is needed.
In conclusion, elderly onset DRPLA presents with cerebellar ataxia as the initial manifestation and often presents only ataxia as a predominant feature in the early stage. On MR imaging, atrophy of the brainstem, superior cerebellar peduncle, and cerebellum and abnormal signals in the brainstem and thalamus are key features for differentiating elderly onset DRPLA from cerebral white matter lesions in healthy subjects. In some elderly onset DRPLA cases, abnormal signals in the brainstem and thalamus are not observed, and it is difficult to distinguish elderly onset DRPLA from other neurological disorders such as SCA in these cases. In such cases, it may be necessary to pay attention to other clinical and radiological features, such as the family history of successive generations and abnormal signals in the cerebellar white matter on MR imaging, for making a diagnosis.
References
Koide R, Ikeuchi T, Onodera O, Tanaka H, Igarashi S, Endo K, Takahashi H, Kondo R, Ishikawa A, Hayashi T, Saito M, Tomoda A, Miike T, Naito H, Ikuta F, Tsuji S (1994) Unstable expansion of CAG repeat in hereditary dentatorubral–pallidoluysian atrophy (DRPLA). Nat Genet 6:9–13
Nagafuchi S, Yanagisawa H, Sato K, Shirayama T, Ohsaki E, Bundo M, Takeda T, Tadokoro K, Kondo I, Murayama N, Tanaka Y, Kikushima H, Umino K, Kurosawa H, Furukawa T, Nihie K, Inoue T, Sano A, Komure O, Takahashi M, Yoshizawa T, Kanazawa I, Yamada M (1994) Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet 6:14–18
Wardle M, Majounie E, Williams NM, Rosser AE, Morris HR, Robertson NP (2008) Dentatorubral pallidoluysian atrophy in South Wales. J Neurol Neurosurg Psychiatry 79:804–807
Wardle M, Morris HR, Robertson NP (2009) Clinical and genetic characteristics of non-Asian dentatorubral–pallidoluysian atrophy: a systematic review. Mov Disord 24:1636–1640
Komure O, Sano A, Nishino N, Yamauchi N, Ueno S, Kondoh K, Sano N, Takahashi M, Murayama N, Kondo I, Nagafuchi S, Yamada M, Kanazawa I (1995) DNA analysis in hereditary dentatorubral–pallidoluysian atrophy: correlation between CAG repeat length and phenotypic variation and the molecular basis of anticipation. Neurology 45:143–149
Ikeuchi T, Koide R, Tanaka H, Onodera O, Igarashi S, Takahashi H, Kondo R, Ishikawa A, Tomoda A, Miike T, Sato K, Ihara Y, Hayabara T, Isa F, Tanabe H, Tokiguchi S, Hayashi M, Shimizu N, Ikuta F, Naito H, Tsuji S (1995) Dentato-Pallidoluysian atrophy: clinical features are closely related to unstable expansions of trinucleotide (CAG) repeat. Ann Neurol 37:769–775
Koide R, Onodera O, Ikeuchi T, Kondo R, Tanaka H, Tokiguchi S, Tomoda A, Miike T, Isa F, Beppu H, Shimizu N, Watanabe Y, Horikawa Y, Shimohata T, Hirota K, Ishikawa A, Tsuji S (1997) Atrophy of the cerebellum and brainstem in dentatorubral pallidoluysian atrophy: influence of CAG repeat size on MRI findings. Neurology 49:1605–1612
Uyama E, Kondo I, Uchino M, Fukushima T, Murayama N, Kuwano A, Inokuchi N, Ohtani Y, Ando M (1995) Dentatorubral–pallidoluysian atrophy (DRPLA): clinical, genetic, and neuroradiologic studies in a family. J Neurol Sci 130:146–153
Yoshii F, Tomiyasu H, Shinohara Y (1998) Fluid attenuation inversion recovery (FLAIR) images of dentatorubropallidoluysian atrophy: case report. J Neurol Neurosurg Psychiatry 65:396–399
Tomiyasu H, Yoshii F, Ohnuki Y, Ikeda JE, Shinohara Y (1998) The brainstem and thalamic lesions in dentatorubral pallidoluysian atrophy: an MRI study. Neurology 50:1887–1890
Yoon WT, Youn J, Cho JW (2012) Is cerebral white matter involvement helpful in the diagnosis of dentatorubral–pallidoluysian atrophy? J Neurol 259:1694–1697
de Leeuw FE, de Groot JC, Achten E, Oudkerk M, Ramos LM, Heijboer R, Hofman A, Jolles J, van Gijn J, Breteler MMB (2001) Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 70:9–14
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 149:351–356
Yabe I, Sasaki H, Kikuchi S, Nonaka M, Morikawa F, Tashiro K (2002) Late onset ataxia phenotype in dentatorubro-pallidoluysian atrophy (DRPLA). J Neurol 249:432–436
Sano A, Yamauchi N, Kakimoto Y, Komure O, Kawai J, Hazama F, Kuzume K, Sano N, Kondo I (1994) Anticipation in hereditary dentatorubral–pallidoluysian atrophy. Hum Genet 93:699–702
Mascalchi M, Vella A (2012) Magnetic resonance and nuclear medicine imaging in ataxias. Handb Clin Neurol 103:85–110
Shimohata T, Kanazawa M, Yoshida M, Saito Y, Iwai K, Yasuda T, Inukai A, Takahashi H, Nishizawa M, Aiba I (2016) Clinical and imaging findings of progressive supranuclear palsy with predominant cerebellar ataxia. Mov Disord 31:760–762
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sugiyama, A., Sato, N., Nakata, Y. et al. Clinical and magnetic resonance imaging features of elderly onset dentatorubral–pallidoluysian atrophy. J Neurol 265, 322–329 (2018). https://doi.org/10.1007/s00415-017-8705-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8705-7