Abstract
As there are scarce data regarding the outcomes of acute ischemic stroke (AIS) patients treated with intravenous thrombolysis (IVT) within 60 min from symptom onset (“golden hour”), we sought to compare outcomes between AIS patients treated within [GH(+)] and outside [GH(−)] the “golden hour” by analyzing propensity score matched data from the SITS-EAST registry. Clinical recovery (CR) at 2 and 24 h was defined as a reduction of ≥10 points on NIHSS-score or a total NIHSS-score of ≤3 at 2 and 24 h, respectively. A relative reduction in NIHSS-score of ≥40% at 2 h was considered predictive of complete recanalization (CREC). Symptomatic intracranial hemorrhage (sICH) was defined using SITS-MOST criteria. Favorable functional outcome (FFO) was defined as a mRS-score of 0–1 at 3 months. Out of 19,077 IVT-treated AIS patients, 71 GH(+) patients were matched to 6882 GH(−) patients, with no differences in baseline characteristics (p > 0.1). GH(+) had higher rates of CR at 2 (31.0 vs. 12.4%; p < 0.001) and 24 h (41 vs. 27%; p = 0.010), CREC at 2 h (39 vs. 21%; p < 0.001) and FFO (46.5 vs. 34.0%; p = 0.028) at 3 months. The rates of sICH and 3-month mortality did not differ (p > 0.2) between the two groups. GH(+) was associated with 2-h CR (OR: 5.34; 95% CI 2.53–11.03) and CREC (OR: 2.38; 95% CI 1.38–4.09), 24-h CR (OR: 1.88; 95% CI 1.08–3.26) and 3-month FFO (OR: 2.02; 95% CI 1.15–3.54) in multivariable logistic regression models adjusting for potential confounders. In conclusion, AIS treated with IVT within the GH seems to have substantially higher odds of early neurological recovery, CREC, 3-month FFO and functional improvement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although time from stroke symptom onset to the initiation of intravenous thrombolysis (IVT) is known to be associated with both the likelihood of successful recanalization and 3-month functional outcome [1–5], there are scarce data regarding the outcomes of acute ischemic stroke (AIS) patients treated with IVT within the ultra-early time window of 60 min from symptom onset, termed as the “golden hour” (GH) [6], when IVT is presumed to have its greatest benefit [7, 8]. Moreover, the number of patients who were treated with intravenous alteplase during the GH window in pivotal randomized-controlled clinical trials (RCTs) that established the safety and efficacy of IVT for AIS was negligible [8].
In view of the former considerations, we sought to compare outcomes between AIS patients treated within (onset-to-treatment time, OTT ≤60 min) and outside (OTT: 61–270 min) the “golden hour” [GH (+) and GH (−) groups, respectively], by analyzing propensity score matched data from the SITS-EAST registry.
Methods
We analyzed prospectively collected data from the Safe Implementation of Treatments in Stroke-East registry (SITS-EAST) on consecutive AIS patients treated with IVT during a 12-year period (October 2003 to December 2015). SITS-EAST Register data were collected on patients treated with IVT using the general SITS-ISTR register platform as previously described [9]. The participating countries in the SITS-EAST registry include Croatia, Czech Republic, Estonia, Greece, Hungary, Lithuania, Poland, Russia, Slovakia, Slovenia and Turkey, representing approximately 30% of the population of SITS registry [10].
We included all AIS patients treated with intravenous tissue plasminogen activator (tPA) if they: (1) had available data on the time interval between symptom onset and tPA bolus (onset-to-treatment time, OTT), (2) had no significant disability prior to stroke onset (modified Rankin Scale score, mRS ≤1), (3) had available 3-month functional outcome assessment using the mRS-score. Included patients were dichotomized according to their OTT as GH(+) if they were treated within the first “golden” hour (GH) after symptom onset (OTT ≤60 min) or GH(−) if IVT administration was initiated after the time interval of 60 min from symptom onset. After dichotomization according to treatment initiation time, GH(+) were matched to GH(−) using a structured, iterative propensity score model with the primary objective to maximize the balance in the distribution of possible confounders between GH(+) and GH(−) groups.
Baseline characteristics including demographics, vascular risk factors, admission stroke severity, admission blood pressure and serum glucose levels of the study population were documented as previously described [9, 10]. The primary outcome events of interest were the percentage of patients with: (1) clinical recovery with a total NIHSS-score of ≤3 or NIHSS reduction ≥10 points at 2 h and (2) clinical recovery with a total NIHSS-score of ≤3 or NIHSS reduction ≥10 points at 24 h [11]. Other secondary efficacy and safety outcomes included: (1) symptomatic intracerebral hemorrhage (sICH) according to the SITS-MOST definition (local or remote parenchymatous hemorrhage type 2 combined with NIHSS-score increase ≥4 points or leading to death <22–36 h) [9, 12], (2) relative reduction in NIHSS-score of ≥40% at 2 h after IVT, which was considered as predictive of complete recanalization (CREC) [13, 14], (3) neurological improvement at discharge, quantified as both the absolute (NIHSSadm − NIHSSdis) and relative decrease in NIHSS-score at discharge (NIHSSdis) in comparison to hospital admission (NIHSSadm) [(NIHSSadm − NIHSSdis)/NIHSSadm × 100%] [15, 16], (4) mortality at 3 months, (5) favorable functional outcome (FFO) at 3 months (defined as mRS-score of 0 or 1) [15, 17], and (6) the distribution of the 3-month mRS-scores between GH(+) and GH(−) groups [18].
Statistical analyses
In the propensity score matching algorithm all baseline characteristics, except those reporting time intervals and thus having a clear association with the OTT, were included. The corresponding propensity score of the variable GH was then calculated for each subject and a nearest neighbor matching algorithm was then used to match GH(+) patients to GH(−) patients within 0.2*SD of the logit of the propensity score according their OTT status (GH±). To determine whether the propensity score approach achieved balance in all potential confounders, we compared all baseline characteristics of GH(+) patients to their propensity-matched GH(−) patients.
Statistical comparisons were performed between the aforementioned propensity-matched groups using the χ 2 test (or the Fisher’s exact test) and the unpaired t test (or Mann–Whitney U test), where appropriate. We also compared baseline characteristics between the unmatched and propensity-matched subgroups to detect potential imbalances between the two populations. Association of IVT administration within the 1st hour after stroke onset (GH+) with efficacy endpoints was evaluated with two individual univariable and multivariable binary logistic regression models adjusting for potential confounders. In the first model we used as potential confounders age, NIHSSadm and admission glucose, as these variables have been reported to be independently associated with both the risk of sICH (SEDAN score) [19] and favorable outcome (DRAGON score) [20] after IVT. In the second model we additionally included all variables that differed significantly between the unmatched and propensity-matched populations: history of diabetes, history of smoking, history of atrial fibrillation and stroke unit admission and care following IVT. In both aforementioned models those factors that contributed to the outcome of interest in the initial univariable analyses at p values <0.1 were included in the multivariable model as candidate variables. The final variables that were independently associated in the multivariable logistic regression analyses with the outcome of interest were selected by backward stepwise selection procedure using a p value <0.05. Finally, the distribution on the mRS-scores at 3 months among AIS patients was compared between different GH subgroups using both the Cochran–Mantel–Haenszel test and univariable/multivariable ordinal logistic regression models (shift analysis), as previously described [18].
All statistical analyses were performed with the use of the Stata Statistical Software Release 13 for Windows (College Station, TX, StataCorp LP).
Results
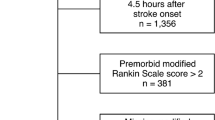
A total of 19,077 patients were treated with IVT in SITS-EAST registry from October 2003 to December 2015. Patients with missing data on OTT (n = 1346), missing data on baseline functional status (n = 1200), disability prior to index even (n = 1730) or missing data on 3-month mRS score (4888) were excluded from further analysis (Fig. 1). The total number of patients fulfilling the inclusion criteria was 9913 (86 within the GH and 9827 outside the GH). Propensity score matching resulted in two groups of 71 GH(+) patients and 6882 GH(−) patients that were balanced (p > 0.1) for all potential confounding variables, except for the times from symptom onset to admission, imaging and treatment which are all directly related to the matching variable of OTT (Table 1). Supplemental Table I in the online-only Data Supplement presents the comparative analyses of baseline characteristics between propensity-matched patients used for analysis and all patients included in the SITS-EAST registry during the aforementioned period. The two groups differed in terms of the following variables that were included as potential confounders in model II of statistical analyses: age, history of diabetes, history of smoking, history of atrial fibrillation and stroke unit admission and care following IVT.
The time course of serial NIHSS-score assessments at admission, 2 and 24 h following tPA bolus is displayed in Fig. 2a. Although the two groups did not differ in terms of median NIHSSadm-scores [12 points (IQR 7–17) in GH(+) vs. 11 points (IQR 7–16) in GH(−); p = 0.292], patients in the GH(+) had lower median NIHSS-scores at 24 h [7 points (IQR 1–13) vs. 8 (IQR 4−14); p = 0.042]. Thus, the absolute and relative NIHSS reduction at 2 and 24 h were more pronounced in the GH(+) subgroups (Fig. 2b, c). The GH(+) subgroup exhibited a more pronounced absolute and relative NIHSS decrease at both 2 and 24 h in comparison to GH(−) subgroup (Fig. 2b, c). More specifically, the mean relative NIHSS reduction of GH(+) patients was 32.3 and 41.7% at 2 and 24 h, respectively, while the corresponding relative NIHSS reductions in GH(−) patients were 13.3% (p < 0.001) and 27.3% (p = 0.002) at 2 and 24 h, respectively.
Serial assessments of National Institutes of Health Stroke Scale (NIHSS) scores at baseline, 2 and 24 h following tPA bolus in acute ischemic stroke patients treated within (blue line) and outside (red line) the golden hour (a). Statistical comparisons between groups were performed using Mann–Whitney U test. Absolute reductions in NIHSS-score at 2 and 24 h from tPA from tPA bolus in acute ischemic stroke patients treated within (blue bar) and outside (red bar) the golden hour (b). Relative reductions in NIHSS-score at 2 and 24 h from tPA from tPA bolus in acute ischemic stroke patients treated within (blue bar) and outside (red bar) the golden hour (c)
We also documented higher rates of clinical recovery in both the first 2 (31.0 vs. 12.4%, p < 0.001) and 24 h (40.8 vs. 27.2%, p = 0.010) following tPA bolus in the GH(+) subgroup (Table 2). Similarly, higher rates of CREC were documented in GH(+) patients (39.4 vs. 21.4%, p < 0.001). Additionally, GH(+) patients achieved higher rates of 3-month FFO (46.5 vs. 34.0%, p = 0.028) and greater 3-month functional improvement compared to their GH(−) propensity-matched counterparts (p = 0.033 by Cochran–Mantel–Haenszel test Fig. 3). Finally, no differences were recorded between the two groups in the rates of sICHs (0 vs. 1.8%, p = 0.264) and 3-month mortality (8.4 vs. 12.7%, p = 0.288).
In univariable and multivariable analyses adjusting for age, NIHSSadm and admission glucose (Table 3, Model 1), IVT treatment in the first hour after AIS onset (GH+) was independently associated with a higher likelihood of clinical recovery in both the first 2 (OR = 6.36; 95% CI 3.08–13.11; p < 0.001) and 24 h (OR = 2.15; 95% CI 1.27–3.66; p = 0.005), CREC at 2 h (OR = 2.71; 95% CI 1.62–4.55; p < 0.001), 3-month FFO (OR = 1.91; 95% CI 1.11–3.29; p = 0.020) and 3-month functional improvement defined as shift in mRS-scores (common OR = 1.92; 95% CI 1.25–3.03; p = 0.004). In univariable and multivariable analyses adjusting for age, NIHSSadm, admission glucose, gender, history of diabetes, history of smoking, history of atrial fibrillation and stroke unit care (Table 3, Model 2), GH(+) was also independently associated with a higher likelihood of clinical recovery in both the first 2 (OR = 5.34; 95% CI 2.53–11.30; p < 0.001) and 24 h (OR = 1.88; 95% CI 1.08–3.26; p = 0.026), CREC at 2 h (OR = 2.38; 95% CI 1.38–4.09; p = 0.002), 3-month FFO (OR = 2.02; 95% CI 1.15–3.54; p = 0.015) and 3-month functional improvement (common OR = 2.04; 95% CI 1.27–3.23; p = 0.003).
Discussion
Our study showed that IVT delivered within the first hour after symptom onset, namely the “golden hour”, is independently associated with higher odds of early neurological improvement at 2 and 24 h following tPA bolus as well as higher likelihood of FFO or functional improvement at 3 months. Moreover, ultra-early delivery of alteplase was independently related to ≥40% reduction in NIHSS-score at 2 h which in turn has been shown to be highly predictive of tPA-induced recanalization documented by Transcranial Doppler in real-time [13]. Consequently, the association between IVT within the GH and higher chance of early reversal of neurological deficits may be attributed to the higher rates of CREC with ultra-early delivery of tPA [14]. Our results are in accordance with a very recent report from the Get With The Guidelines-Stroke (GWTG-Stroke) registry suggesting that IVT delivery within 60 min from stroke onset is associated with increased odds of discharge to home and independent ambulation at discharge, without increased rates of hemorrhagic complications or in-hospital mortality, when compared with IVT received within 61–270 min [21].
In the SITS-EAST registry, 0.8% of the total 19,077 IVT-treated AIS patients were found to receive treatment within 60 min from symptom onset. This percentage is in line with those previously reported by both the SITS-International Stroke Treatment Registry (SITS-ISTR) registry (1.4% out of 12,529 total IVT-treated AIS patients) [22] and the GWTG-Stroke registry (<1% of the total 58,353 AIS patients) [22]. In another analysis of the GWTG-Stroke registry less than one-third of AIS patients were found to receive IVT with door-to-needle times (DTN) ≤60 min, with only a modest improvement over time [23]. In a similar pooled analysis from 10 European stroke care centers a median DNT of 55 min was observed in AIS presenting within the first 30 min from stroke onset with significant in-hospital treatment delays, especially for female and elderly patients, stressing thus the need for further improvements in the prompt IVT initiation of reperfusion treatments in the AIS setting [24].
As it has been suggested than even under optimal conditions, a large proportion of the US population will be unable to have access to a comprehensive stroke center within 60 min after stroke onset [25], the introduction of mobile stroke units (MSUs) arise as an attractive option for the earlier evaluation and treatment of AIS with the target of the “golden hour” after symptom onset [26]. In randomized clinical trials (RCTs) the implication of MSUs in Germany was found to be related with decreased OTT with no increase in adverse events [27, 28], while in its run-in phase the first MSU introduced in USA was able to deliver IVT in one out of three AIS within 60 min from symptom onset, with no complications [29]. Thus, our findings underscore the importance of wide implementation of MSUs in the settings of comprehensive stroke centers (CSC) since this will result in greater rates of tPA delivery within the GH window, which in turn will translate in greater number of patients with early reversal of their neurological deficits and fewer patients with emergent large vessel occlusions requiring endovascular reperfusion therapies when they reach the CSCs [30].
Apart from reducing prehospital delay times, there is also an urge to intensify institutional efforts to minimize avoidable in-hospital delays [31]. Pre-notification of the Emergency Department, adequate preparations prior to patient’s arrival and the use of a standardized operational algorithm on arrival seem to drastically reduce DNT [32–34]. Increased awareness and efforts should also focus on rapid neuroimaging evaluation [35], together with the prompt identification of eligible patients with very mild neurological deficits [10] or patients with posterior circulation ischemia [36].
Even though the two groups that were compared in the present manuscript derive from an international, multi-center registry with standardized protocol and are adequately balanced for numerous baseline characteristics after propensity matching for potential confounders, certain limitations should also be acknowledged. First, SITS-EAST registry is an observational multinational cohort with self-reported safety and effectiveness outcomes and no central adjudication of imaging or clinical outcomes. Even though significant heterogeneity in acute stroke care may be present across different national systems and also within institutions from the same country, the SITS-EAST registry reflects ‘real-life’ clinical experience from several countries and thus we consider our results to be independent from particular healthcare system features and thus directly generalizable. Second, it should be noted that neuroimaging parameters that could represent significant confounders (e.g., the presence of hyperdense cerebral artery sign or early ischemic changes on baseline CT scan) were unavailable. Third, CREC was defined by reduction in NIHSS-score at 2 h and not using vascular imaging. Fourth, this was a retrospective analysis of prospectively collected data and potential selection bias cannot be excluded despite the fact that all our analyses were adjusted to the variables that differed in the unmatched and propensity-matched cohorts. Last, the volume of cerebral infarction is not recorded in the SITS registry and we were unable to test the hypothesis that ultra-early delivery of alteplase may be associated with smaller final infarct volumes in comparison to treatment outside the GH time window.
In conclusion, the present report suggests that AIS patients treated with IVT within the GH have substantially higher odds of early neurological improvement, CREC, 3-month FFO and functional improvement. They also outline that the potential ability of MSUs to treat a significantly higher proportion of AIS within the GH window may result in further improvement of early outcomes of AIS patients.
References
Saver JL (2006) Time is brain–quantified. Stroke 37:263–266
Hacke W, Donnan G, Fieschi C et al (2004) ATLANTIS Trials Investigators; ECASS Trials Investigators; NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 363:768–774
Lees KR, Bluhmki E, von Kummer R et al (2010) ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet 375:1695–1703
Emberson J, Lees KR, Lyden P et al (2014) Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384:1929–1935
Muchada M, Rodriguez-Luna D, Pagola J et al (2014) Impact of time to treatment on tissue-type plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 45:2734–2738
Weber J, Ebinger M, Audebert HJ (2015) Prehospital stroke care: telemedicine, thrombolysis and neuroprotection. Expert Rev Neurother 15:753–761
Tsivgoulis G, Alexandrov AV (2014) Does “time is brain” also mean “time is clot”? Time dependency of tissue-type plasminogen activator-induced recanalization in acute ischemic stroke. Stroke 45:2555–2556
Grotta JC (2014) tPA for stroke: important progress in achieving faster treatment. JAMA 311:1615–1617
Tsivgoulis G, Kadlecová P, Kobayashi A et al (2015) Safety of statin pretreatment in intravenous thrombolysis for acute ischemic stroke. Stroke 46:2681–2684
Mikulík R, Kadlecová P, Czlonkowska A et al (2012) Factors influencing in-hospital delay in treatment with intravenous thrombolysis. Stroke 43:1578–1583
Alexandrov AV, Molina CA, Grotta JC et al (2004) Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med 351:2170–2178
Mazya M, Egido JA, Ford GA et al (2012) Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke 43:1524–1531
Mikulik R, Ribo M, Hill MD et al (2007) Accuracy of serial National Institutes of Health Stroke Scale scores to identify artery status in acute ischemic stroke. Circulation 115:2660–2665
Molina CA, Alexandrov AV, Demchuk AM et al (2004) CLOTBUST Investigators. Improving the predictive accuracy of recanalization on stroke outcome in patients treated with tissue plasminogen activator. Stroke 35:151–156
Tsivgoulis G, Katsanos AH, Sharma VK et al (2016) Statin pretreatment is associated with better outcomes in large artery atherosclerotic stroke. Neurology 86:1103–1111
Alexandrov AV, Nguyen HT, Rubiera M et al (2009) Prevalence and risk factors associated with reversed Robin Hood syndrome in acute ischemic stroke. Stroke 40:2738–2742
Tsivgoulis G, Zand R, Katsanos AH et al (2015) Safety and outcomes of intravenous thrombolysis in dissection-related ischemic stroke: an international multicenter study and comprehensive meta-analysis of reported case series. J Neurol 262:2135–2143
Saver JL, Gornbein J (2009) Treatment effects for which shift or binary analyses are advantageous in acute stroke trials. Neurology 72:1310–1315
Strbian D, Engelter S, Michel P et al (2012) Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol 71:634–641
Strbian D, Meretoja A, Ahlhelm FJ et al (2012) Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. Neurology 78:427–432
Kim JT, Fonarow GC, Smith EE et al (2017) Treatment with tissue plasminogen activator in the golden hour and the shape of the 4.5-hour time-benefit curve in the National United States get with the guidelines-stroke population. Circulation 135:128–139
Saver JL, Fonarow GC, Smith EE et al (2013) Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 309:2480–2488
Fonarow GC, Smith EE, Saver JL et al (2011) Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 min. Circulation 123:750–758
Strbian D, Michel P, Ringleb P et al (2013) Relationship between onset-to-door time and door-to-thrombolysis time: a pooled analysis of 10 dedicated stroke centers. Stroke 44:2808–2813
Mullen MT, Branas CC, Kasner SE et al (2015) Optimization modeling to maximize population access to comprehensive stroke centers. Neurology 84:1196–1205
Rajan SS, Baraniuk S, Parker S, Wu TC, Bowry R, Grotta JC (2015) Implementing a mobile stroke unit program in the United States: why, how, and how much? JAMA Neurol 72:229–234
Ebinger M, Winter B, Wendt M et al (2014) Effect of the use of ambulance-based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA 311:1622–1631
Walter S, Kostopoulos P, Haass A, Keller I et al (2012) Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol 11:397–404
Parker SA, Bowry R, Wu TC et al (2015) Establishing the first mobile stroke unit in the United States. Stroke 46:1384–1391
Mokin M, Snyder KV, Siddiqui AH, Levy EI, Hopkins LN (2016) Recent endovascular stroke trials and their impact on stroke systems of care. J Am Coll Cardiol 67:2645–2655
Köhrmann M, Schellinger PD, Breuer L et al (2011) Avoiding in hospital delays and eliminating the three-hour effect in thrombolysis for stroke. Int J Stroke 6:493–497
Casolla B, Bodenant M, Girot M et al (2013) Intra-hospital delays in stroke patients treated with rt-PA: impact of preadmission notification. J Neurol 260:635–639
Vidale S, Arnaboldi M, Bezzi G et al (2016) Reducing time delays in the management of ischemic stroke patients in Northern Italy. Int J Cardiol 215:431–434
Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M (2012) Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology 79:306–313
Sauser K, Levine DA, Nickles AV, Reeves MJ (2014) Hospital variation in thrombolysis times among patients with acute ischemic stroke: the contributions of door-to-imaging time and imaging-to-needle time. JAMA Neurol 71:1155–1161
Sommer P, Seyfang L, Posekany A et al (2017) Prehospital and intra-hospital time delays in posterior circulation stroke: results from the Austrian Stroke Unit Registry. J Neurol 264:131–138
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical standards
The research documented in the submitted manuscript has been carried out in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and has been approved by the appropriate ethics committees of the participating institutions.
Sources of funding
Dr Georgios Tsivgoulis, Pavla Kadlecova and Robert Mikulik have been supported by the project no. LQ1605 from the National Program of Sustainability II (MEYS CR).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsivgoulis, G., Katsanos, A.H., Kadlecová, P. et al. Intravenous thrombolysis for ischemic stroke in the golden hour: propensity-matched analysis from the SITS-EAST registry. J Neurol 264, 912–920 (2017). https://doi.org/10.1007/s00415-017-8461-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8461-8