Abstract
Few studies have evaluated whether the retina is involved in migraine through the evaluation of retinal nerve fiber layer (RNFL) examined with ocular coherence tomography (OCT) with conflicting results. Aim of this case–control study is to evaluate the retina and the choroid in migraine. Patients having migraine with aura (MwA) or without aura (MoA) and chronic migraine (CM) were evaluated. Age- and sex-matched normal subjects were selected as healthy controls (HC). Patients and HC were examined with OCT. RNFL, ganglion cell layer (GCL), foveal thickness (FT), choroidal thickness (CT) and total macular volume (TMV) were calculated for right eyes (RE) and left eyes (LE). Seventy-seven patients (62 women; 80.5%), 21 MoA, 12 MwA, 44 CM and 42 HC were enrolled in the study. Patients compared to HC had a significant reduction of RNFL (RE: 91.2 ± 9.2 vs 99.3 ± 7.5 μm; p < 0.001. LE: 93.3 ± 8.7 vs 100.2 ± 6.5 μm; p < 0.001). GCL (RE: 80.6 ± 6.4 vs 86.9 ± 2.1 μm; p < 0.0001. LE: 81.5 ± 5.7 vs 87.1 ± 2.6 μm; p < 0.0001) and CT (RE: 286.4 ± 31.4 vs 333.2 ± 3.1 μm; p < 0.0001. LE: 287.2 ± 31.6 vs 334.5 ± 4.1 μm; p < 0.0001) were thinner in patients compared to HC. Moreover, CM showed reduction of RNFL and of GCL compared to the other migraineurs. Finally, we found a significant inverse correlation between RNFL thickness and total number of headache attacks per months. Our data suggest the involvement of retina and choroid in migraineurs, especially in the CM group. Although migraine is an episodic and recurrent disease, its chronic nature might cause permanent structural abnormalities involving not only the brain, but also the retina.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Migraine is a common disorder characterized by recurrent attacks of headache associated with vegetative features such as nausea or vomiting and hypersensitivity to sounds, lights and smells. Migraine affects around 15% of general population, representing the third most common disease worldwide in both genders. Episodic migraine is divided into two main subtypes: migraine with aura (MwA) and migraine without aura (MoA) [1]. Besides episodic migraine, chronic migraine (CM), definitely influences the patient’s quality of life and represents a worldwide societal burden [2]. The pathogenesis of migraine is still debated among researchers, but the neurovascular system seems mainly involved. Migraine episodes lead to the activation of the trigeminal vascular system (TGVS) responsible for regulating vascular tone and for the transmission of pain signals [3]. The TGVS is composed of the trigeminal nerve and the fibers which innervate both intra- and extrameningeal blood vessels and the brainstem [4]. Moreover, the TGVS joins the extracranial tissues including the structures of the eyes [5]. Phases of hypoperfusion and hyperperfusion follow each other during headache, involving not only the brain, but also other areas such as the retina [6, 7]. Although the vasospasm of cerebral and retrobulbar blood vessels is a transient phenomenon, the chronic nature of the disease might be a risk factor for structural abnormalities of the brain and also of the retina [6]. Retinal infarctions caused by retinal artery occlusions have been reported in migraine [7, 8]. Several reports suggest that vascular abnormalities such as vasospasm [9] and ischemia, either chronic or intermittent, are possible causes of optic nerve damage [6]. Alterations in quality of perfusion in optic nerve head microcirculation or even in the retina, may also lead to ganglion cell (GC) death in patients with migraine. Whether similar changes take place in the retina in MwA and MoA is debated in the literature and few studies have addressed the issue with conflicting results.
Optical coherence tomography (OCT) is a reliable and reproducible technique allowing the retinal fiber nerve layer (RNFL) thickness measurement [10, 11], used as an index to assess ganglion cells layer (GCL) and RNFL damages [12]. The aim of the present study is to evaluate and to compare RNFL thickness in the prepapillary and macular areas in the eyes of migraineurs’ patients (MwA, MoA and CM) and age- and sex-matched healthy subjects by OCT.
Materials and methods
Subjects
This observational and cross-sectional study screened migraine patients, consecutively admitted to the Headache Center of the University of Catania from September 1st 2014 to January 1st 2015, with diagnosis MwA, MoA or CM according to the criteria of the International Classification of Headache Disorders (ICHD-3 beta) [1]. All CM patients have complained high frequency of headache attacks at least since one year. As usual in our clinic, all patients filled in a headache diary the number of days of headache per month and the main characteristics of headache attacks occurring during the previous 6 months. The control group consisted of 44 age- and sex-matched healthy controls (HC) consecutively recruited. HC experienced fewer than three headaches in the past year and reported no migraine history. All patients gave their consent to participate in the study and local ethical committee approved the study protocol. Migraineurs’ clinical features were collected and symptomatic and prophylactic medications were recorded. Patients treated with topiramate, beta-blockers and calcium-antagonists were excluded for the potential side effects of topiramate on optical nerve and for the vasoactive mechanism of beta-blockers and calcium-antagonists. Medications and migraine overuse headache (MOH), when present, were recorded and the implications were analyzed. All migraineurs were tested interictally. Eyes with retinal and optic disc pathology, glaucoma, dense cataract or corneal opacity, which did not consent to OCT imaging and eyes previously subjected to intraocular surgery or ocular laser treatment were excluded. Patients with diabetes mellitus, hypertension, cardiovascular and renal disease, history of central nervous system disorders including brain tumors, infarction, encephalitis, epilepsy, multiple sclerosis, head or eye trauma and any type of headache except for migraine were not considered for enrollment.
Ophthalmological evaluation
All the participants underwent a complete ocular examination including best corrected visual acuity testing with Snellen charts, slit lamp biomicroscopy, dilated fundus examination and intraocular pressure measurement. The optic disc was considered normal when having a cup/disc area ratio less or equal to 0.35.
OCT imaging
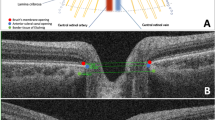
The OCT was performed with the Stratus OCT (model Cyrrus 5000, Carl Zeiss Meditec, Dublin, CA, USA). Average measurements of three sequential circular scans of diameter 3.46 mm centered on the optic disc were recorded. A single operator masked to the subject diagnosis collected all measurements. Scans of the eyes were performed with papillary dilatation under the same intensity of dim room lighting. Only well focused and centered scans with a signal strength of >7 were included. For each eye, we studied the mean RNFL thickness, single quadrant thickness, all automatically calculated by OCT using the existing software. Peripapillary RNFL was acquired with the optic disc cube 200 × 200 protocol that images the optic disc in a 6 mm × 6 mm region. The mean RNFL and those from individual quadrants were obtained. We also evaluated foveal thickness (FT) and macular volume (MV) in all groups. Macular cube 512 × 128 and 5-line raster scans were also performed. The macular cube 512 × 128 scan uses a raster scan mode that scans a 6 × 6-mm macular area into 512 × 128 (length by width) points. Macular GCL was obtained using the protocol that scans a 6 mm × 6 mm area centered at the fovea. The GCL was derived automatically by the machine software over an elliptical annulus (2 mm × 2.4 mm radius), excluding the central foveal region (0.5 mm × 0.6 mm radius). The 5-line raster scan comprises five 6-mm-long lines at 250-μm intervals. In both scans, only those cases with signal intensity greater than or equal to 7 were included in the study results. Layer segmentation of the OCT data was performed using a previously developed and validated algorithm for detecting 8 layers within the macula. The algorithm had three stages: preprocessing, pixel classification and graph-based multilayer segmentation. Additionally, estimates of the inner and outer retinal boundaries (inner limiting membrane and Bruch’s membrane) were used to restrict the region of interest for the algorithm, as well as to flatten the data to the Bruch’s membrane boundary. Constraints were used to limit the minimum and maximum distance between each boundary and to limit the smoothness of the final segmentation. The choroidal thickness (CT) was also measured by Enhanced Depth Imaging using the spectral-domain OCT. Each section, consisting of 30 average scans, was obtained in a 15° × 30° rectangle centered at the macula. CT was determined as the distance from the outer surface of the hyperreflective line, referred to as the “retinal pigment epithelium” layer, to the hyperreflective line of the inner scleral border. CT was measured at the fovea, 1500 μm nasal and 1500 μm temporal to the fovea in a horizontal scan section. Quality control and APOSTEL recommendations according to published criteria were used [13–15].
Statistical analysis
The numerical data sets were tested for normal distribution with the Shapiro–Wilk test. For the purpose of statistical analysis, both eyes retinal parameters were included in the analysis [13, 15]. Two-sample t test was used to compare RNFL thickness differences between the eyes of patients and the eyes of HC. Categorical variables were analyzed using the Chi-square test. The differences between the subgroups were tested using ANOVA (Bonferroni, post hoc analysis) and Kruskal/Wallis when appropriated. At the eye level, each outcome variable (RNFL, retinal sectors analysis, MV, FT, CT of both eye) was examined using generalized estimating equation (GEE) models [16], which account for within-subject correlation. Univariate GEE models between each main variable and covariate were constructed for each outcome. The considered covariates were: age, sex, number of days of headache per month, aura, attack duration and age at onset. Forward and backward methodologies were applied to construct a final multivariate model for each outcome with p < 0.2 at the univariate level being the criterion for being introduced (forward modeling) or retained (backward modeling) in the final multivariate model. The analysis was adjusted for age and sex.
For correlation analysis, the Pearson correlation coefficient (r) was used. A probability value of 0.05 was considered indicative of statistical significance. Statistical analysis was assessed using STATA 11 [17].
Results
Out of 81 subjects, a total of 77 patients who met the inclusive/exclusive criteria were selected for the study. Other four patients did not meet inclusion criteria and were excluded from the study: they were on prophylactic therapy (one patient with beta-blockers, one patient with topiramate and two patients with calcium-antagonists). Patients with migraine had characteristics similar to HC for age (39.0 ± 10.4 vs 37.0 ± 7.8 years), sex distribution (women 80.5 vs 83%) and body mass index (23.9 ± 3.2 vs 22.8 ± 2.3). Out of 77 patients, 33 patients had episodic migraine (12 with MoA, 21 with MwA) and 44 had CM. Demographic features, frequency and the main clinical characteristics of headache attacks, obtained by the headache diaries, are shown in Table 1. CM patients showed an older age at onset, respect to the MwA subgroup. The mean of the days of headache per month, considering the last 6 months, was significantly higher in CM. The intensity of attacks was more severe in the group of patients with episodic migraine (MwA and MoA). Patients with episodic migraine complained more frequently vegetative symptoms respect with CM patients (Table 1). Eleven patients were on prophylactic therapy at OCT evaluation; 8 were treated with amitriptyline and 3 with paroxetine. Forty-four patients were taking symptomatic medication at the moment of headache attack. In the CM group we had ten MOH patients: seven abused of non-steroidal anti inflammatory drugs (NSAIDs) and three of triptans. No differences were found between the three subgroups in terms of ophthalmological examination data and visual acuity.
The overall group of patients showed a thinner RNFL compared to HC (Table 2); in particular nasal, temporal and inferior quadrants showed significant reduction compared to HC. Moreover, CGL and CT were thinner in the eyes of migraineurs compared to HC. No significant differences were found in retinal parameters between MoA and MwA subgroups. The ANOVA analysis showed that CM group had thinner RNFL and GCL compared to MwA and MoA (Table 3).
We found a significant inverse correlation between RNFL thickness in both eyes and number of days of headache per month, considering the diaries of the previous 6 months (Fig. 1). The association still remained significant in the full multivariate model, which adjusted for all explored covariates with GEE analysis (Table 4). No other associations were found even considering symptomatic and prophylactic treatments and retinal profile in migraineurs.
Discussion
The present study showed that RNFL was significantly thinner in migraineurs compared with HC, with an involvement of nasal, temporal and inferior quadrants. Moreover, CM patients presented a reduction of RNFL, particularly evident in the superior and inferior sectors compared to other migraineurs, and a thinner GCL. No differences in retinal parameters between MwA and MoA were found. Although a few studies have recently evaluated whether the retina was involved in patients with migraine using OCT, the results have not always been consistent [18–26].
Some studies had previously claimed the issue of an involvement of retina in migraine. Tan et al. [19] did not report any statistically significant difference in RNFL measurements between migraineurs and HC. In contrast, Martinez et al. [21] reported that the RNFL thickness was significantly reduced in the subjects with migraine when compared to HC, although there were no differences between migraineurs and HC in the RNFL thickness in the superior and inferior areas. In another study, Martinez et al. [22] found that the RNFL thickness in migraineurs was similar to HC, with only a thinner temporal quadrant. The RNFL thickness parameters correlated with the migraine disability assessment score, number of attacks, and length of migraine history, suggesting that the length of migraine history critically influenced the RNFL thickness. Gipponi et al. [23] found a significant thinning in the RNFL of the upper quadrant, but found no differences in the foveal thickness and macular volume in female patients with migraine respect with healthy women. Differently from our results, they found no correlation between reduction in RNFL thickness and frequency of migraine attacks. They proposed that the reduction in RNFL thickness was strictly related to the presence of migraine itself [23]. In a recent study, Yulek et al. [25] found no statistically significant differences between HC and patients with migraine for the retinal thickness in any of the quadrants. In this study, the RNFL was significantly thinner in the migraineurs compared to the HC, but the statistical analysis did not reveal any significant differences among the MoA, MwA and HC. Ekinci et al. found that the thinning of the RNFL and GCL was detected only in the MwA group [27]. In our study we found that GCL was thinner in migraineurs compared to HC. Moreover, our data, differently from previous reports, demonstrated the existence of a correlation between number of days of headache per month and the reduction of the RNFL thickness in both eyes [22, 23]. In the study of Kirbas et al. [24] the OCT was used to investigate the RNFL thickness and macular changes in patients with CM. The RNFL did not result thinner in patients with CM, except for the superior quadrant, which was significantly thinner in the CM patients respect to HC. In our study we found that CM patients showed thinner RNFL compared to MwA and MoA, and the analysis of single sectors showed a reduction of superior and inferior sectors in CM compared to MoA and MwA patients. Moreover, GCL was thinner in CM group compared to MwA and to MoA patients.
It is possible to speculate that the putative vascular mechanism due to the TGVS activation, involved in the migraine pathogenesis, could be linked to the structural retinal changes. Indeed, as for the MRI structural brain white matter lesions common in migraineurs [28–30] whose pathogenetic and clinical meanings are still unclear [28, 31]; the retinal structural changes found in migraine could represent a specific marker of the disease. Different pathophysiological mechanisms have been proposed to explain MRI white matter lesions in migraine [32, 33], but neuropathological data are lacking. Furthermore, several questions remain unclear, including whether white matter lesions accumulate over time, whether their presence constitutes a risk for stroke or whether they have an impact on cognitive functions in migraine patients [34]. These lesions, even if are believed of being of ischemic origin, do not seem to be associated with the frequency, the severity and the therapy of migraine [29, 30].
On the contrary, in our study the frequency of attacks seemed to affect the RNFL thickness in migraineurs and in particular in CM patients, who definitively presented an increased number of days of headache per month. These data consent to speculate that the retinal changes in CM eyes are linked to the number of headache episodes, indicating a possible role of vasospasm and ischemia in the retinal optical nerve damages, as previously suggested [6, 7]. Demircan et al. found that the RNFL thickness for nasal sectors was significantly thinner in the migraineurs’ eyes than in those of HC, as was the choroid thickness at the fovea. However, the macular thickness was not significantly different between the groups. They concluded that migraine led to a reduction in the peripapillary RNFL thickness and to thinning in choroidal structures probably due to the chronic ischemic insult related to migraine pathogenic mechanisms [26]. On these grounds, our results may suggest that in the chronic form of migraine, where the frequency of headache attacks is higher, structural changes in the retina could be related to vasospam leading to retina ischemia. Nevertheless this assumption remains to be demonstrated, because reduced blood supply did not lead to morphological or functional changes of the RNFL [35].
Another possible explanation that should be taken into account is the effects of drugs used for migraine attacks. Even if it is high speculative, it is not possible to rule out a contribute of these drugs to the establishment of the retinal damage, especially in case of medication overuse [36]. Nevertheless, in our study nor the prophylactic neither the symptomatic treatments seemed to have any statistical correlation with RNFL reduction. Moreover, it has been speculated that other treatments could be related to retinal damage. Ewering et al. [37] reported a bilateral temporal RNFL reduction in cluster headache patients, hypothesizing a possible harmful role of frequent oxygen inhalation. However, no definitive conclusions could be drawn on the role of drugs used in treatment of headache.
This study has several limitations: first of all, this is a retrospective single centre study, with a limited number of patients and a possible recall bias due to analysis of headache diaries. Another limitation of the study is the possible influence of prophylactic and symptomatic treatments on the retina, even if it seemed to have no statistical correlation with RNFL reduction. We are aware that excluding patients on prophylactic therapies with beta-blockers, calcium-antagonists and topiramate could represent a bias of selection; however, we considered the potential influence of these drugs on the retina vascularization more consistent than the bias itself.
In conclusion our study, even with these limitations, suggests that migraine may lead to reduction in the peripapillary RNFL thickness, in particular in the chronic form of the disease characterized by high frequency of headache attacks. Based on the theories that the etiology of optic nerve damage in patients with migraine depends on vascular abnormalities, such as vasospasm or focal ischemia [7, 38, 39], our findings support the issue that a structural retinal damage in migraine could be sustained by the chronic nature of the disease based on the recurrence of vasoactive events during the subsequent migraine attacks. Further studies should evaluate the possible correlation between brain white matter lesions at MRI and retinal alterations at OCT in order to define if a common pathogenetic mechanism exists.
References
Headache Classification Committee of the International Headache S (2013) The international classification of headache disorders, 3rd edition (beta version). Ceph Int J Headache 33(9):629–808. doi:10.1177/0333102413485658
Schwedt TJ (2014) Chronic migraine. BMJ 348:g1416. doi:10.1136/bmj.g1416
Russo A, Tessitore A, Tedeschi G (2013) Migraine and trigeminal system-I can feel it coming. Curr Pain Headache Rep 17(10):367. doi:10.1007/s11916-013-0367-2
Aguggia M, Saracco MG, Cavallini M, Bussone G, Cortelli P (2013) Sensitization and pain. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 34(Suppl 1):S37–S40. doi:10.1007/s10072-013-1382-0
Friedman DI (2015) The eye and headache. Continuum 21(4 Headache):1109–1117. doi:10.1212/CON.0000000000000204
Flammer J, Pache M, Resink T (2001) Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retinal Eye Res 20(3):319–349
Killer HE, Forrer A, Flammer J (2003) Retinal vasospasm during an attack of migraine. Retina 23(2):253–254
Banik S, Bhutto HU, Bagga P (2006) Recurrent branch retinal vein occlusion with factor V leiden mutation. Eye (Lond) 20(8):948–949. doi:10.1038/sj.eye.6702060
Wang JJ, Mitchell P, Smith W (1997) Is there an association between migraine headache and open-angle glaucoma? Findings from the Blue Mountains Eye Study. Ophthalmology 104(10):1714–1719
Drexler W, Sattmann H, Hermann B, Ko TH, Stur M, Unterhuber A, Scholda C, Findl O, Wirtitsch M, Fujimoto JG, Fercher AF (2003) Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol 121(5):695–706. doi:10.1001/archopht.121.5.695
Fercher AF (2010) Optical coherence tomography—development, principles, applications. Z Med Phys 20(4):251–276. doi:10.1016/j.zemedi.2009.11.002
Wang J, Gao X, Huang W, Wang W, Chen S, Du S, Li X, Zhang X (2015) Swept-source optical coherence tomography imaging of macular retinal and choroidal structures in healthy eyes. BMC Ophthalmol 15(1):122. doi:10.1186/s12886-015-0110-3
Tewarie P, Balk L, Costello F, Green A, Martin R, Schippling S, Petzold A (2012) The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS One 7(4):e34823. doi:10.1371/journal.pone.0034823
Schippling S, Balk LJ, Costello F, Albrecht P, Balcer L, Calabresi PA, Frederiksen J, Frohman E, Green AJ, Klistorner A, Outteryck O, Paul F, Plant GT, Traber G, Vermersch P, Villoslada P, Wolf S, Petzold A (2015) Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult Scler J 21(2):163–170. doi:10.1177/1352458514538110
Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, Saidha S, Martinez-Lapiscina EH, Lagreze WA, Schuman JS, Villoslada P, Calabresi P, Balcer L, Petzold A, Green AJ, Paul F, Brandt AU, Albrecht P, consortium I (2016) The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 86(24):2303–2309. doi:10.1212/WNL.0000000000002774
Zeger SL, Liang KY (1986) Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42(1):121–130
Boston RC, Sumner AE (2003) STATA: a statistical analysis system for examining biomedical data. Adv Exp Med Biol 537:353–369
Ekinci M, Ceylan E, Cagatay HH, Keles S, Huseyinoglu N, Tanyildiz B, Cakici O, Kartal B (2014) Retinal nerve fibre layer, ganglion cell layer and choroid thinning in migraine with aura. BMC Ophthalmol 14:75. doi:10.1186/1471-2415-14-75
Tan FU, Akarsu C, Gullu R (2005) Retinal nerve fiber layer thickness is unaffected in migraine patients. Acta Neurol Scand 112(1):19–23. doi:10.1111/j.1600-0404.2005.00423.x
Feng YF, Guo H, Huang JH, Yu JG, Yuan F (2015) Retinal nerve fiber layer thickness changes in migraine: a meta-analysis of case–control studies. Curr Eye Res 41(6):814–822. doi: 10.3109/02713683.2015.1056373
Martinez A, Proupim N, Sanchez M (2009) Scanning laser polarimetry with variable corneal compensation in migraine patients. Acta Ophthalmol 87(7):746–753. doi:10.1111/j.1755-3768.2008.01356.x
Martinez A, Proupim N, Sanchez M (2008) Retinal nerve fibre layer thickness measurements using optical coherence tomography in migraine patients. Br J Ophthalmol 92(8):1069–1075. doi:10.1136/bjo.2008.137471
Gipponi S, Scaroni N, Venturelli E, Forbice E, Rao R, Liberini P, Padovani A, Semeraro F (2013) Reduction in retinal nerve fiber layer thickness in migraine patients. Neurol Sci 34(6):841–845. doi:10.1007/s10072-012-1103-0
Kirbas S, Tufekci A, Turkyilmaz K, Kirbas A, Oner V, Durmus M (2013) Evaluation of the retinal changes in patients with chronic migraine. Acta Neurol Belg 113(2):167–172. doi:10.1007/s13760-012-0150-x
Yulek F, Dirik EB, Eren Y, Simavli H, Ugurlu N, Cagil N, Simsek S (2015) Macula and retinal nerve fiber layer in migraine patients: analysis by spectral domain optic coherence tomography. Semin Ophthalmol 30(2):124–128. doi:10.3109/08820538.2013.833270
Demircan S, Atas M, Arik Yuksel S, Ulusoy MD, Yuvaci I, Arifoglu HB, Baskan B, Zararsiz G (2015) The impact of migraine on posterior ocular structures. J Ophthalmol 2015:868967. doi:10.1155/2015/868967
Huseyinoglu N, Ekinci M, Ozben S, Buyukuysal C, Kale MY, Sanivar HS (2014) Optic disc and retinal nerve fiber layer parameters as indicators of neurodegenerative brain changes in patients with obstructive sleep apnea syndrome. Sleep Breath 18(1):95–102. doi:10.1007/s11325-013-0854-z
Kruit MC, van Buchem MA, Hofman PA, Bakkers JT, Terwindt GM, Ferrari MD, Launer LJ (2004) Migraine as a risk factor for subclinical brain lesions. JAMA 291(4):427–434. doi:10.1001/jama.291.4.427
Hougaard A, Amin FM, Ashina M (2014) Migraine and structural abnormalities in the brain. Curr Opin Neurol 27(3):309–314. doi:10.1097/WCO.0000000000000086
Bashir A, Lipton RB, Ashina S, Ashina M (2013) Migraine and structural changes in the brain: a systematic review and meta-analysis. Neurology 81(14):1260–1268. doi:10.1212/WNL.0b013e3182a6cb32
Sacco S, Pistoia F, Degan D, Carolei A (2015) Conventional vascular risk factors: their role in the association between migraine and cardiovascular diseases. Ceph Int J Headache 35(2):146–164. doi:10.1177/0333102414559551
Kruit MC, van Buchem MA, Launer LJ, Terwindt GM, Ferrari MD (2010) Migraine is associated with an increased risk of deep white matter lesions, subclinical posterior circulation infarcts and brain iron accumulation: the population-based MRI CAMERA study. Ceph Int J Headache 30(2):129–136. doi:10.1111/j.1468-2982.2009.01904.x
Speciali JG, Bigal ME (2006) Subcortical lesions in migraine: are they related to mitochondrial dysfunction? Headache 46(9):1461–1462. doi:10.1111/j.1526-4610.2006.00591_1.x
Le Pira F, Reggio E, Quattrocchi G, Sanfilippo C, Maci T, Cavallaro T, Zappia M (2014) Executive dysfunctions in migraine with and without aura: what is the role of white matter lesions? Headache 54(1):125–130. doi:10.1111/head.12158
Hessler H, Zimmermann H, Oberwahrenbrock T, Kadas EM, Mikolajczak J, Brandt AU, Kauert A, Paul F, Schreiber SJ (2015) No evidence for retinal damage evolving from reduced retinal blood flow in carotid artery disease. Biomed Res Int. Artn 604028. doi:10.1155/2015/604028
Biagi C, Poluzzi E, Roberto G, Puccini A, Vaccheri A, D’Alessandro R, Motola D, Montanaro N (2011) Pattern of triptan use and cardiovascular coprescription: a pharmacoepidemiological study in Italy. Eur J Clin Pharmacol 67(12):1283–1289. doi:10.1007/s00228-011-1076-6
Ewering C, Hasal N, Alten F, Clemens CR, Eter N, Oberwahrenbrock T, Kadas EM, Zimmermann H, Brandt AU, Osada N, Paul F, Marziniak M (2015) Temporal retinal nerve fibre layer thinning in cluster headache patients detected by optical coherence tomography. Cephalalgia 35(11):946–958. doi:10.1177/0333102414560632
Abdul-Rahman AM, Gilhotra JS, Selva D (2011) Dynamic focal retinal arteriolar vasospasm in migraine. Indian J Ophthalmol 59(1):51–53
Beversdorf D, Stommel E, Allen C, Stevens R, Lessell S (1997) Recurrent branch retinal infarcts in association with migraine. Headache 37(6):396–399
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no financial or other conflict of interests.
Ethical standard
The study was approved by the institutional review board of the University of Catania, Italy and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Previous written informed consent was obtained from each patient.
Rights and permissions
About this article
Cite this article
Reggio, E., Chisari, C.G., Ferrigno, G. et al. Migraine causes retinal and choroidal structural changes: evaluation with ocular coherence tomography. J Neurol 264, 494–502 (2017). https://doi.org/10.1007/s00415-016-8364-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8364-0