Abstract
Background
It has long been known that the majority of patients with multiple sclerosis (MS) display an intrathecal, polyspecific humoral immune response to a broad panel of neurotropic viruses. This response has measles virus, rubella virus and varicella zoster virus as its most frequent constituents and is thus referred to as the MRZ reaction (MRZR).
Objective
Re-evaluation of the specificity of MRZR as a marker of MS.
Methods
Structured review of the existing English-, German- and Spanish-language literature on MRZR testing, with evaluation of MRZR in a cohort of 43 unselected patients with MS and other neurological diseases as a proof of principle.
Results
A positive MRZ reaction, defined as a positive intrathecal response to at least two of the three viral agents, was found in 78% of MS patients but only in 3% of the controls (p < 0.00001), corresponding to specificity of 97%. Median antibody index values were significantly lower in non-MS patients (measles, p < 0.0001; rubella, p < 0.006; varicella zoster, p < 0.02). The 30 identified original studies on MRZR reported results from 1478 individual MRZR tests. A positive MRZR was reported for 458/724 (63.3%) tests in patients with MS but only for 19/754 (2.5%) tests in control patients (p < 0.000001), corresponding to cumulative specificity of 97.5% (CI 95% 96–98.4), cumulative sensitivity of 63.3% (CI 95% 59.6–66.8) (or 67.4% [CI 95% 63.5–71.1] in the adult MS subgroup), a positive likelihood ratio of 25.1 (CI 95% 16–39.3) and a negative likelihood ratio of 0.38 (CI 95% 0.34–0.41). Of particular note, MRZR was absent in 52/53 (98.1%) patients with neuromyelitis optica or MOG-IgG-positive encephalomyelitis, two important differential diagnoses of MS.
Conclusion
MRZR is the most specific laboratory marker of MS reported to date. If present, MRZR substantially increases the likelihood of the diagnosis of MS. Prospective and systematic studies on the diagnostic and prognostic impact of MRZR testing are highly warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disorder of the central nervous system (CNS) of putative autoimmune aetiology. The diagnosis of MS is hampered by the lack of a specific laboratory marker. Intrathecal production of IgG as detected by calculation of the IgG cerebrospinal fluid (CSF)/serum ratio (QIgG) or by testing for CSF-restricted oligoclonal bands (OCBs) is considered a hallmark of MS. However, OCBs and an elevated IgG ratio are also found in a plethora of other autoimmune and infectious CNS disorders and are thus of low specificity for MS. It has been known for many decades that the intrathecal IgG response in patients with MS comprises antibodies to a broad panel of neurotropic viruses. As antibodies to measles virus (M), rubella virus (R) and varicella zoster virus (Z) are its most frequent constituents, it has been referred to as the MRZ reaction (MRZR) [2, 17, 18, 30, 49, 60]. That anti-viral antibody response is not thought to be directly involved in the pathogenic process but has been suggested to reflect non-specific bystander activation of B cells or, more recently, to be the result of nonsense activity of immortalised B cell clones.
The potential diagnostic relevance of MRZR testing as a ‘rule-in’ marker of MS, as opposed to total IgG OCB or QIgG testing, which are rather ‘rule-out’ markers, was pointed out in a European consensus statement on CSF diagnostics [3]. MRZR testing in patients with suspected MS is also been recommended by the German Society for Cerebrospinal Fluid Diagnostics and Clinical Neurochemistry in its current diagnostic guidelines [53]. Finally, the potential diagnostic relevance of MRZR testing has been stressed by a panel of experts on the occasion of the latest critical revision of the diagnostic criteria for MS [72]. However, no systematic review of the existing literature on the specificity and sensitivity of MRZR testing in MS exists so far.
For this study, we carried out a structured review of the entire existing English-, German-, and Spanish-language literature on MRZR. In addition, we evaluated MRZR in a cohort of patients with MS and other neurological disorders as a proof of principle.
Methods
MRZR testing
Matched serum and CSF samples from 43 unselected patients with relapsing-remitting MS (RRMS) (n = 9) or other CNS disorders (n = 34) were tested for MRZR as described before [30, 32]. None of these patients had been tested for MRZR before. The diagnoses in the control group included, among others, AQP4-IgG-positive neuromyelitis optica spectrum disorders (NMOSD), neuroborreliosis, neuro-lupus, neuro-Behçet, CNS vasculitis, migraine, trigeminal neuralgia and depression. The demographic and clinical features of all patients are summarised in Table 1. Behcet’s disease was diagnosed according to international consensus criteria [1]. All patients diagnosed with neuroborreliosis had an increased Borrelia burgdorferi-specific IgG antibody index (AI), and most had an increased Borrelia burgdorferi-specific IgM AI (Table 1). Systemic lupus erythematosus (SLE) was diagnosed according to the American College of Rheumatology criteria [24, 71]. Virus-specific antibody levels in CSF and serum were determined using a commercially available enzyme-linked immunosorbent assay (Siemens Healthcare/Dade Behring, Germany) according to the manufacturer’s instructions. Total IgG and total albumin concentrations in CSF and serum were determined nephelometrically (BN ProSpec, Siemens Healthcare/Dade Behring, Germany). The intrathecal synthesis of antibodies to M, R and Z was detected by calculation of the corresponding virus-specific AIs: AI = Q IgG[spec]/Q IgG[total], if Q IgG[total] < Q lim, and AI = Q IgG[spec]/Q lim, if Q IgG[total] > Q lim, with Q IgG[spec] = IgGspec[CSF]/IgGspec[serum], and Q IgG[total] = IgGtotal[CSF]/IgGtotal[serum]) [60]. The upper reference range of Q IgG, Q lim, was calculated according to Reiber’s formula [57]:
AI values >1.5 were considered to be indicative of intrathecal IgG production against the respective pathogen [60]. All samples were stored at −80 °C until testing. The study was approved by the institutional review board of each participating centre, and all patients gave written informed consent. If no consent could be obtained retrospectively, samples were tested in a strictly anonymised fashion as requested by the institutional review board of the University of Heidelberg. All samples were tested as part of a larger project on the differential laboratory diagnosis of MS, NMOSD and related disorders.
Review criteria
References were identified by searches of the databases of the National Library of Medicine (http://www.ncbi.nlm.nih.gov/pubmed) and of Thompson Reuters® (http://www.webofknowledge.com) for articles published between 1985 (i.e. the year in which Felgenhauer’s seminal work “Cerebrospinal fluid virus antibodies. A diagnostic indicator for multiple sclerosis?” [18] appeared) and September 2016 using the following search expression: “MRZ reaction” OR (IgG OR antibody OR antibodies OR IgG) AND (CSF OR “cerebrospinal fluid” OR intrathecal) AND ((measles AND rubella AND varicella) OR (“neurotropic viruses” OR “neurotropic virus”)). All studies that investigated the intrathecal production of antibodies to measles virus, rubella virus and varicella zoster virus and which contained information on the proportion of patients with a positive MRZR were considered eligible for this review. A positive MRZR was defined as intrathecal synthesis of antibodies against at least two of the three viral species defining the MRZ spectrum (measles, rubella, varicella zoster). Accordingly, studies that either tested for only one or two of the three reactivities or reported only on the frequency of each single antibody reactivity in the total cohort but not on the proportion of patients with a polyspecific (i.e. bi- or trispecific) reaction were excluded (Fig. 1), as were studies that tested for MRZR exclusively in preselected OCB-negative MS subgroups [5, 69]. To reduce the risk of publication bias, a search of the Thomson Reuters ® Web of Knowledge database of meeting abstracts was performed using the same search expression as stated above.
Statistical analysis
Sensitivity was calculated as true positives/(true positives + false negatives), specificity as true negatives/(true negatives + false positives). The positive likelihood ratio (pLR) was calculated as (true positives/(true positives + false negatives))/(1 − (true negatives/(true negatives + false positives))), the negative likelihood ratio (nLR) as (1 − (true positives/(true positives + false negatives)))/(true negatives/(true negatives + false positives)). Fisher’s exact test (two-tailed) was used to analyse contingency tables. The Mann–Whitney U test was used to test for significant differences in median AI values between groups. Ninety-five per cent confidence intervals were calculated for all test parameters evaluated.
Results
MRZR in the study cohort
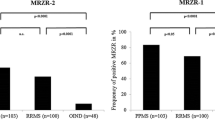
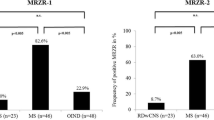
MRZR as defined by a combination of at least two positive AIs was present in 7/9 (78%) patients with RRMS, but only in 1/34 (3%) patients with other CNS disorders (p < 0.00001) (Table 1). The only MRZR-positive control patient had been diagnosed with neuroborreliosis and showed a bispecific intrathecal antibody reaction to measles virus (AI 3.16) and varicella zoster virus (AI 4.22). MRZR was negative in all patients with neuro-Behçet and in all patients with neuro-lupus. One patient with neuro-Behçet and one with neuroborreliosis showed a monospecific intrathecal reaction to varicella zoster virus (2.05 and 4.81, respectively) and one patient with neuro-SLE showed a borderline reaction to rubella virus (1.56). Median AI values for measles differed significantly between patients with MS and those with CNS disorders other than MS [measles virus-specific AI: median 5.45, range (1st–9th percentile) 2.09–8.09, vs. 0.94, range 0.71–1.51, p < 0.0001; rubella virus-specific AI: median 4.1, range 0.78–5.78, vs 0.92, range 0.77–1.4, p < 0.006; varicella zoster virus-specific AI: median 4.0, range 0.68–6.06, vs. 0.82, range 0.72–1.43, p < 0.02] (Table 1; Fig. 2).
Antibody indices for measles (M), rubella (R) and varicella zoster (Z) virus in patients with MS (n = 9) and patients with other neurological disorders (n = 34). The boundary of the box closest to zero indicates the 25th percentile, the line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile
Literature review
A structured literature search of PubMed and of Thompson Reuter’s Web of Knowledge® database retrieved 65 publications (Fig. 1). Among these, 30 studies on MRZR were identified that met the inclusion criteria (see Table 2 for references). In these and the present study, results of 1478 MRZR tests were reported. A positive MRZR was found in 458/724 (63.3%) tests performed in patients with MS but only in 19/754 (2.5%) tests performed in control patients (p < 0.000001) (Tables 2, 3). This corresponds to cumulative specificity of 97.48% (CI 95% 96–98.4) for MS, cumulative sensitivity of 63.3% (CI 95% 59.6–66.8), pLR of 25.1 (CI 95% 16–39.3) and nLR of 0.38 (CI 95% 0.34–0.41). The difference between MS patients and non-MS patients remained highly significant (p < 0.000001) after exclusion of all healthy and non-inflammatory controls.
The frequency of positive MRZR was higher in adults with MS (67.4%, CI 95% 63.5–71.1; 14 studies; 617 tests) than in children with MS (39.3%, CI 95% 30.1–49.2; 3 studies; 107 tests) (p < 0.000001) (Table 4), and was highest in adult MS patients from Western Europe (69.4%, CI 95% 65.3–73.2; 11 studies; 546 tests) (Table 2).
Notably, the cumulative sensitivity in adult patients (67.4%; n = 617; 14 studies) was in close agreement with the sensitivity found in the largest single study performed in adult MS (67.2%; n = 177) [60].
Apart from MS, MRZR positivity was mainly reported in patients with rheumatic disorders and CNS symptoms (RD/CNS), which included cases of SLE, Sjögren’s syndrome, and Wegener’s granulomatosis (Table 2). If patients with MS and patients with RD/CNS were considered together, only 1.8% of the remaining controls were positive for MRZR (p < 0.000001), corresponding to specificity of 98.2% (CI 95% 96.9–99) and pLR of 35.1 (CI 95% 20.4–60.3) (Table 3). If patients with MS or RD/CNS were compared with patients with other inflammatory neurological diseases of the CNS (see Table 2 for details), cumulative specificity of 96.21% (CI 95% 93.3–97.9) and pLR of 16.2 (CI 95% 9.3–28.3) resulted.
Only 1 of the 305 patients with non-inflammatory diseases of the CNS or inflammatory diseases of the peripheral nervous system and none of the 99 healthy controls had a positive MRZR.
Of particular note, MRZR was absent in 52/53 patients with neuromyelitis optica or MOG-IgG-positive encephalomyelitis [5, 32, 35, 37, 54] (Table 2), two conditions that were considered variants of MS in the past and have only relatively recently been recognised as disease entities distinct from MS based on immunopathophysiological and neuropathological grounds.
Discussion
MRZR testing is currently performed at most CSF laboratories in Germany as a complementary test to OCB and QIgG testing in patients with suspected MS, and has recently been proposed in the current guidelines of the German Society for Cerebrospinal Fluid Diagnostics and Clinical Neurochemistry as a ‘rule-in’ test for the diagnosis of MS. Our results indicate that MRZR may in fact be a highly specific laboratory marker, which if present substantially increases the likelihood for a diagnosis of MS.
High specificity makes MRZR a typical ‘rule-in’ test
Currently, there is no other laboratory marker with a similarly high specificity for MS (97.5%; CI 95% 96–98.4). While testing for total IgG OCBs (≥95%, probably with a latitudinal gradient [16]) or QIgG is highly sensitive in MS, total IgG OCBs are present in a plethora of other autoimmune and infectious conditions and are thus not very specific for MS [7, 10, 46]. Conversely, MRZR testing is highly specific for MS but only moderately sensitive (67.4%, CI 95% 63.5–71.1, in adults). This translates into a very high pLR and a relatively weak nLR (Table 3), which makes MRZR a ‘rule-in’ test rather than a ‘rule-out’ test. Conversely, the high sensitivity and low specificity of total IgG OCB or QIgG testing results in a weak pLR but strong nLR, making OCBs a better ‘rule-out’ than ‘rule-in’ test for MS. Parallel testing for OCBs and MRZR might therefore substantially strengthen the diagnostic relevance of CSF analysis in patients with suspected MS.
MRZR is present at disease onset and may predict conversion from CIS to MS
Given that early treatment is thought to be of high prognostic impact in MS [8, 9, 29, 40–42], it is of clinical relevance that MRZR was demonstrated to be present early in the disease course and to predict later conversion to MS in patients with a clinically isolated syndrome (CIS) suggestive of MS, including in patients with acute monosymptomatic optic neuritis [6, 32, 73]. MRZR could thus assist physicians in making early treatment decisions. The combination of MRZR testing with OCB testing and brain MRI was suggested to further increase the predictive value of MRZR seropositivity for a diagnosis of MS in patients with CIS [6, 32, 73].
Repeat testing increases sensitivity of MRZR for MS
In MRZR-negative patients, repeat lumbar puncture was reported to increase the sensitivity of MRZR for MS. Petereit and Reske (2005) found a frequency of MRZR positivity of 63% in a cohort of 70 MS patients if only the first lumbar puncture (LP) was considered, but in 79% if follow-up samples (median 1, range 1–6) were also taken into account (unpublished data from Ref. [52]). This is in accordance with the fact that most patients with MS who do not show a positive MRZ reaction as defined by two or three positive AIs showed at least a monospecific, so-called incomplete response to one of the three viruses at first LP (89% [60] and 94% [17] in the two largest studies using enzyme-linked immunosorbent assay [ELISA] for determining antibodies to M, R and Z and 100% in a study using affinity-mediated capillary immunoblotting, an alternative method for studying the intrathecal production of pathogen-specific antibodies [67]).
MRZR is detectable also in a subset of OCB-negative patients with MS
Determination of antigen-specific IgG by AI calculation or affinity blotting has been repeatedly reported to be more sensitive than total IgG OCB and QIgG determination in patients with infectious conditions [17, 20, 31, 38, 39, 68]. In accordance with these observations, two recent studies independently found a positive MRZ reaction also in a subset of patients with RRMS, secondary progressive MS and primary progressive MS negative for total IgG OCBs [5, 67, 70]. Stich et al. recently demonstrated a positive MRZ reaction as defined by a response to at least two of the three antigens in 4/17 (18%) OCB-negative patients with MS according to McDonald et al. (2001/2005) (with six additional OCB-negative patients displaying a monospecific reaction) and in 2/17 (12%) by means of affinity blotting employing recombinant viral antigens and a highly sensitive chemiluminescence detection technique, but in none of 11 controls [69, 70]. Similarly, Brecht et al. found a positive MRZ reaction in 11/46 (24%) OCB-negative patients with MS according to McDonald et al. (2005) using the same standardised ELISA test used in the present study, but in none of 37 controls [5]. The authors did not report if any distinctive clinical features were present in these patients.
MRZR is part of the polyspecific intrathecal humoral immune response in MS
The intrathecal humoral immune response in MS comprises antibodies to a broad panel of viral and bacterial agents such as herpes simplex virus (HSV), Epstein Barr virus (EBV), human herpes virus 6, mumps virus and Chlamydia pneumoniae [14, 15, 50, 54, 63, 65]. However, antibodies to measles virus, rubella virus and varicella zoster virus are considered its most common constituents and are thus best evaluated. Whether inclusion of antibody reactivities other than those against M, R and Z would improve the sensitivity or specificity has not been studied systematically. However, Reiber et al. (1998) found no significant increase in sensitivity when antibodies to HSV were tested in addition to antibodies to M, R and Z [60]. Instead, inclusion of HSV in the diagnostic panel could lower the specificity because of a possible cross-reactivity between varicella zoster virus and HSV antibodies [13, 66, 67]. In children with MS, Rostasy et al. (2003) found slightly higher sensitivity after inclusion of Chlamydia pneumoniae (52 versus 44%) without loss of specificity; however, the control group was relatively small (n = 10).
Conversely, it seems inadvisable to limit the analysis to two of the three parameters (e.g. for economic reasons), since this would result in substantial loss of sensitivity. Although some studies have found intrathecal antibodies to Z to be slightly less frequent in MS than those to M and R, the proportion of patients with a bispecific reaction that included Z (i.e. either M + Z or R + Z) was still 16% in two large cohorts [52, 60]. Instead of changing the test panel, weighting of the three antibody specificities (M, R and Z) might be useful: In a recent study, Brettschneider et al. (2009) found that the use of a scoring system (with different scores for M, R and Z), established by means of logistic regression analysis, may possibly further increase the predictive value of MRZR for conversion to MS within 2 years in patients with CIS [6].
Pathophysiological implications of MRZR positivity
The exact reason for the presence of the polyspecific humoral immune in the CSF of patients with MS, which is detectable by OCB, QIgG and MRZR testing, is still not well understood. As simultaneous infection with several neurotropic viruses is highly unlikely, and the polymerase chain reaction (PCR) for measles virus, rubella virus and varicella zoster virus has been shown to be negative in MRZR-positive patients with MS [22], MRZR is thought to represent non-specific, so-called bystander activation of B cells (e.g. long-lived plasma cells) within the CNS in the absence of viral replication. This is further corroborated by recent data demonstrating that the virus-specific fraction of total intrathecally synthesised IgG is significantly (20- to 60-fold) lower in MS than typically found during acute viral infection [28]. The total intrathecally synthesised M + R + Z antibody concentration in the CSF was shown to represent less than 2% of the total intrathecally synthesised IgG [60].
The polyspecific intrathecal IgG response in MS may indicate an enhanced B cell-promoting environment in the CNS of patients with MS, which is also suggested by the life-long persistence of OCBs and the recent observation of B cell follicles in the meninges of patients with MS [47]. Whether the presence of EBV-infected B cells in these intrameningeal follicles (as well in white matter lesions) provides an explanation for the continuous B cell activation in MS is currently a matter of debate [19]. EBV can efficiently immortalise B cells, and thus establish lymphoblastoid cell lines in vitro [47].
Of note, the presence of intrathecally produced polyspecific anti-viral antibodies in MS as quantitatively evidenced by ELISA has been confirmed qualitatively by the discovery of CSF-restricted anti-viral OCBs (as opposed to total-IgG OCBs) [20]. Sindic (1998) found OCBs to measles, rubella, varicella zoster, and mumps virus in 18/18 patients with MS using an antigen-driven capillary blot technique, 15 of whom (83%) showed a polyspecific reaction [20, 67]. Interestingly, these bands did not correspond to the main OCBs present in the same patients, indicating that M, R and Z are not the main targets of the intrathecal IgG response in MS [20, 67].
The fact that MRZR was shown to be mostly absent in patients with other well-established autoimmune conditions of the CNS, such as paraneoplastic neurological disorders, neuromyelitis optica and MOG-IgG-associated encephalomyelitis, or chronic infectious diseases, including neuro-borreliosis, neuro-syphilis and neuro-tuberculosis (Table 2), suggests that MRZR is not generally associated with CNS autoimmunity or a general result of chronic CNS inflammation, but may be more specifically linked to the immunopathophysiology of MS.
Of interest, antibodies to measles, rubella and varicella zoster have been demonstrated not only in the CSF but also in extracts of brain tissue from patients with MS [64].
MRZR-negative multiple sclerosis
It is not fully understood why some patients with MS lack a positive MRZ reaction. This could simply reflect variations—or an increase over time—in the amount of intrathecally produced IgG or in IgG affinity between patients and, thus, limited sensitivity of the immunoassays used [59, 60]. This view is supported by the findings that repeat lumbar puncture was found to result in increased sensitivity of MRZR testing [52]. Alternatively, the lack of positive MRZR could reflect interindividual differences in history of previous infections or immunisations, as suggested by differences between populations from countries with different rubella prevalences and/or vaccination schemes [47]. Similarly, regional differences (with a latitudinal gradient) in the frequency of OCBs in MS have been reported [16]. Finally, the lack of positive MRZR could be due to real pathophysiological differences among patients with MS, a condition considered by some to be histopathologically heterogeneous [43]; studies of MRZR in histopathologically characterised patients with MS are currently in progress. Moreover, patients with MOG-IgG-positive encephalomyelitis (MOG-EM) [34–36, 44, 51] or NMOSD [37, 74], which are now recognised as disease entities in their own right immunopathophysiologically distinct from MS, sometimes meet the current clinicoradiological criteria for MS and, in consequence, were frequently misdiagnosed with MS in the past. Of interest, MRZR was negative in virtually all (52/53) patients with MOG-EM or NMOSD analysed so far [5, 32, 35, 37, 54] (Table 2). Systematic studies on the frequency of MOG-IgG and AQP4-IgG in MRZR-negative patients diagnosed with MS seem warranted.
Differential diagnostic considerations
Apart from MS, MRZR positivity has been observed mainly in patients with rheumatic diseases and CNS involvement (RD/CNS) (Table 2). RD/CNS has thus to be considered as a potential differential diagnosis of MS in MRZR-positive patients. However, the number of cases detected in previous studies was low (n = 6), confirmatory data from large and well-defined cohorts is missing so far, and the possibility of co-existing MS was not excluded in some of those cases. Moreover, well-established standardised serological tests as well as diagnostic criteria for RD exist, which can be applied in patients with suspected MS to exclude RD/CNS. Finally, it should be taken into account that CNS involvement due to RD is very rare compared with MS.
Besides its diagnostic implications, the fact that a positive MRZR is present also in a subset of patients with RD/CNS is interesting from an immunological point of view, as it could indicate possible similarities in the pathophysiology of MS and RD/CNS (e.g. presence of an enhanced B cell-promoting environment [47]).
A positive MRZ reaction has also been reported in a few patients with autoantibody-associated CNS disorders (3/113), neuroborreliosis (2/54) or neuro-sarcoidosis (2/26) (Table 2; Fig. 3). As a limitation, however, the latter two conditions are difficult to diagnose and, in addition, may well co-exist with classical MS in some patients. Unfortunately, it is unknown whether the few MRZR-positive control patients reported in the literature met the current diagnostic criteria for MS.
Stacked bar graph showing the proportion of MRZ-positive (grey) and MRZ-negative (white) test results in various disease groups as reported in the literature (see Table 2 for a list of references). MS multiple sclerosis, RD/CNS rheumatic diseases (including vasculitis) with CNS involvement, NMO neuromyelitis optica, MOG-EM myelin oligodendrocyte glycoprotein-associated encephalomyelitis, Other Ab/CNS other autoantibody-associated disorders of the CNS (including paraneoplastic neurological disorders), ADEM acute disseminated encephalomyelitis, NB neuroborreliosis, Sy/Tb/Sa neuro-syphilis/neuro-tuberculosis/neuro-sarcoidosis, CJD Creutzfeldt–Jakob disease, FNP peripheral facial nerve palsy, others other neurological disorders (see the footnotes to Table 2 for a list of diagnoses); HC healthy controls. P values were corrected for multiple testing according to Bonferroni
Diagnostic caveats
Some caveats need to be considered when dealing with MRZR results. First, given that a positive MRZR has also been observed in a few patients with diseases other than MS, it must be kept in mind that a positive MRZR alone does not prove the presence of MS in a given patient. Rather, it increases the pre-test odds for a diagnosis of MS by a factor indicated by the test-specific pLR (e.g. by a factor of 27 in adults, see Table 3; conventionally, laboratory tests with a pLR of 10 are considered useful [11]), with the pre-test odds depending mainly on the reliability of the diagnostic criteria used.
Second, only an intrathecal reaction against at least two of the three viral agents (M + R, M + Z, R + Z or M + R + Z) may be taken into account. Monospecific reactions against M, R or Z are present in many patients with MS who lack the typical bi- or trispecific pattern (likely representing a forme fruste of the complete MRZR) but are non-specific, since they are frequently found in conditions other than MS and may indeed indicate acute infection with the respective virus. While absolute AI levels do not permit discrimination between a microorganism-driven process and the bystander or ‘nonsense’ activation found in MS, calculation of the virus-specific intrathecal IgG fraction, F(s), can be helpful in selected cases [28, 50], alongside PCR testing of paired CSF and serum samples.
Third, the rate of MRZR positivity in a given population may depend on the natural prevalence of measles, rubella and varicella zoster virus as well as on the local vaccination coverage. The frequency of MRZR in MS has been suggested to be lower in patients from tropical or subtropical regions than in Western European patients [61].
Finally, it should be kept in mind that the frequency of bispecific MRZR seropositivity is lower in children and adolescents (Table 4), particularly before puberty. This possibly reflects the pre- vs. postpubertal prevalence of rubella virus antibodies [59].
Limitations
This study has obvious limitations, some of which are inherent to the study design. First, patient ethnicity, median age, pretreatment and diagnostic criteria, and laboratory methods differed among the various studies analysed here. This may in part explain the interstudy variations in MRZR positivity rates (Fig. 4). Notably, however, the cumulative sensitivity of all studies in adult MS patients found in the present literature review (67.4%) was in almost perfect accordance with the sensitivity found in the largest single study (67.2%), suggesting that this analysis may be robust despite those differences, owing to the high number of studies and patients included. Second, negative studies are generally less likely to be published, which could hypothetically have introduced a bias towards studies that found a positive MRZ reaction in MS. However, the fact that a positive MRZ reaction in MS was consistently observed in so many studies over a period of three decades, by independent groups and by independent methods, argues against a strong influence of such bias. Moreover, we also screened congress proceedings to reduce the risk of publication bias. Finally, despite the high total number of non-MS controls tested for MRZR (n = 754), patient numbers in individual control groups were rather small. Studies that systematically evaluate the rate of MRZR positivity in large, homogeneous control cohorts are warranted.
Forest plot showing the frequency of MRZR in adult patients with MS (centre value and upper and lower limits of the 95% confidence interval) as observed in the previous literature. Studies with fewer than seven patients are not shown. The uppermost red box and whiskers refer to the cumulative sensitivity (red box and red dotted line) and 95% confidence interval (red whiskers) of the total cohort (N = 617 tests). The black dotted vertical line indicates the frequency of MRZR reported in adult patients with diseases other than MS (2.5%; N = 754 tests). *Studies from non-Western European countries (MRZR frequency in a given population depends on local vaccination schemes and history [40])
Conclusion and outlook
There is a need for a highly specific laboratory marker of MS. Established CSF markers such as OCBs and QIgG are sensitive but rather unspecific. Accordingly, a huge number of differential diagnoses have to be excluded before MS can formally be diagnosed according to the current criteria [48, 55]. MRZR is the most specific routine laboratory marker of MS available so far and substantially increases the likelihood of that diagnosis. Our results provide a strong rationale for prospective and systematic studies on the diagnostic and prognostic impact of MRZR testing in MS and CIS.
Abbreviations
- ADEM:
-
Acute disseminated encephalomyelitis
- AI:
-
Antibody index
- APL:
-
Antiphospholipid syndrome
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- EBV:
-
Epstein–Barr virus
- HIV:
-
Human immunodeficiency virus
- HSV:
-
Herpes simplex virus
- HTLV-1:
-
Human T-lymphotropic virus 1
- IgG:
-
Immunoglobulin G
- M:
-
Measles virus
- MRZR:
-
Measles virus, rubella virus, and varicella zoster virus reaction
- MS:
-
Multiple sclerosis
- NB:
-
Neuroborreliosis
- NDT:
-
Not detectable
- NIND:
-
Non-inflammatory neurological disorders
- nLR:
-
Negative likelihood ratio
- NMO:
-
Neuromyelitis optica
- OIND/CNS:
-
Other inflammatory neurological disorders of the CNS
- OND:
-
Other neurological disorders
- RD/CNS:
-
Rheumatic disorders with CNS involvement
- pLR:
-
Positive likelihood ratio
- PND:
-
Paraneoplastic neurological disorders
- Q:
-
Quotient
- R:
-
Rubella virus
- SLE:
-
Systemic lupus erythematosus
- Z or VZV:
-
Varicella zoster virus
References
Weichsler B, Davatchi F, Mizushima Y, Hamza M, Dilsen N, Kansu E, Yazici H et al (1990) Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet 335:1078–1080
Adams JM, Imagawa DT (1962) Measles antibodies in multiple sclerosis. Proc Soc Exp Biol Med 111:562–566
Andersson M, Alvarez-Cermeno J, Bernardi G, Cogato I, Fredman P, Frederiksen J, Fredrikson S, Gallo P, Grimaldi LM, Gronning M et al (1994) Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry 57:897–902
Bednarova J, Stourac P, Adam P (2005) Relevance of immunological variables in neuroborreliosis and multiple sclerosis. Acta Neurol Scand 112:97–102
Brecht I, Weissbrich B, Braun J, Toyka KV, Weishaupt A, Buttmann M (2012) Intrathecal, polyspecific antiviral immune response in oligoclonal band negative multiple sclerosis. PLoS One 7:e40431
Brettschneider J, Tumani H, Kiechle U, Muche R, Richards G, Lehmensiek V, Ludolph AC, Otto M (2009) IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS One 4:e7638
Chu AB, Sever JL, Madden DL, Iivanainen M, Leon M, Wallen W, Brooks BR, Lee YJ, Houff S (1983) Oligoclonal IgG bands in cerebrospinal fluid in various neurological diseases. Ann Neurol 13:434–439
Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernandez O, Hartung H, Seeldrayers P, Sorensen PS, Rovaris M, Martinelli V, Hommes OR (2001) Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet 357:1576–1582
Comi G, Martinelli V, Rodegher M, Moiola L, Bajenaru O, Carra A, Elovaara I, Fazekas F, Hartung HP, Hillert J, King J, Komoly S, Lubetzki C, Montalban X, Myhr KM, Ravnborg M, Rieckmann P, Wynn D, Young C, Filippi M (2009) Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 374:1503–1511
Correale J, de los Milagros Bassani Molinas M (2002) Oligoclonal bands and antibody responses in multiple sclerosis. J Neurol 249:375–389
Deeks JJ, Altman DG (2004) Diagnostic tests 4: likelihood ratios. BMJ 329:168–169
Denne C, Kleines M, Dieckhofer A, Ritter K, Scheithauer S, Merz U, Hausler M (2007) Intrathecal synthesis of anti-viral antibodies in pediatric patients. Eur J Paediatr Neurol 11:29–34
Dennin RH, Herb E (1989) Immunological diagnosis in viral infections of the central nervous system: course of antibody titres against homo- and heterologous viruses. Med Microbiol Immunol 178:255–268
Derfuss T, Gurkov R, Then Bergh F, Goebels N, Hartmann M, Barz C, Wilske B, Autenrieth I, Wick M, Hohlfeld R, Meinl E (2001) Intrathecal antibody production against Chlamydia pneumoniae in multiple sclerosis is part of a polyspecific immune response. Brain 124:1325–1335
Derfuss T, Hohlfeld R, Meinl E (2005) Intrathecal antibody (IgG) production against human herpesvirus type 6 occurs in about 20% of multiple sclerosis patients and might be linked to a polyspecific B-cell response. J Neurol 252:968–971
Dobson R, Ramagopalan S, Davis A, Giovannoni G (2013) Cerebrospinal fluid oligoclonal bands in multiple sclerosis and clinically isolated syndromes: a meta-analysis of prevalence, prognosis and effect of latitude. J Neurol Neurosurg Psychiatry 84:909–914
Felgenhauer K, Reiber H (1992) The diagnostic significance of antibody specificity indices in multiple sclerosis and herpes virus induced diseases of the nervous system. Clin Investig 70:28–37
Felgenhauer K, Schadlich HJ, Nekic M, Ackermann R (1985) Cerebrospinal fluid virus antibodies. A diagnostic indicator for multiple sclerosis? J Neurol Sci 71:291–299
Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F (2008) B cells and multiple sclerosis. Lancet Neurol 7:852–858
Frederiksen JL, Sindic CJ (1998) Intrathecal synthesis of virus-specific oligoclonal IgG, and of free kappa and free lambda oligoclonal bands in acute monosymptomatic optic neuritis. Comparison with brain MRI. Mult Scler 4:22–26
Gahr M, Lauda F, Wigand ME, Connemann BJ, Rosenbohm A, Tumani H, Reindl M, Uzelac Z, Lewerenz J (2015) Periventricular white matter lesion and incomplete MRZ reaction in a male patient with anti-N-methyl-d-aspartate receptor encephalitis presenting with dysphoric mania. BMJ Case Rep. doi:10.1136/bcr-2014-209075
Godec MS, Asher DM, Murray RS, Shin ML, Greenham LW, Gibbs CJ Jr, Gajdusek DC (1992) Absence of measles, mumps, and rubella viral genomic sequences from multiple sclerosis brain tissue by polymerase chain reaction. Ann Neurol 32:401–404
Graef IT, Henze T, Reiber H (1994) Polyspecific immune reaction in the central nervous system in autoimmune diseases with CNS involvement. Z Arztl Fortbild 88:587–591
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Hottenrott T, Dersch R, Berger B, Rauer S, Eckenweiler M, Huzly D, Stich O (2015) The intrathecal, polyspecific antiviral immune response in neurosarcoidosis, acute disseminated encephalomyelitis and autoimmune encephalitis compared to multiple sclerosis in a tertiary hospital cohort. Fluids Barriers CNS 12:27
Hyden D, Roberg M, Forsberg P, Fridell E, Fryden A, Linde A, Odkvist L (1993) Acute “idiopathic” peripheral facial palsy: clinical, serological, and cerebrospinal fluid findings and effects of corticosteroids. Am J Otolaryngol 14:179–186
Jacobi C, Arlt S, Reiber H, Westner I, Kretzschmar HA, Poser S, Zerr I (2005) Immunoglobulins and virus-specific antibodies in patients with Creutzfeldt-Jakob disease. Acta Neurol Scand 111:185–190
Jacobi C, Lange P, Reiber H (2007) Quantitation of intrathecal antibodies in cerebrospinal fluid of subacute sclerosing panencephalitis, herpes simplex encephalitis and multiple sclerosis: discrimination between microorganism-driven and polyspecific immune response. J Neuroimmunol 187:139–146
Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, Simonian NA, Slasor PJ, Sandrock AW (2000) Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 343:898–904
Jarius S, Eichhorn P, Jacobi C, Wildemann B, Wick M, Voltz R (2009) The intrathecal, polyspecific antiviral immune response: specific for MS or a general marker of CNS autoimmunity? J Neurol Sci 280:98–100
Jarius S, Eichhorn P, Wildemann B, Wick M (2012) Usefulness of antibody index assessment in cerebrospinal fluid from patients negative for total-IgG oligoclonal bands. Fluids Barriers CNS 9:14
Jarius S, Franciotta D, Bergamaschi R, Rauer S, Wandinger KP, Petereit HF, Maurer M, Tumani H, Vincent A, Eichhorn P, Wildemann B, Wick M, Voltz R (2008) Polyspecific, antiviral immune response distinguishes multiple sclerosis and neuromyelitis optica. J Neurol Neurosurg Psychiatry 79:1134–1136
Jarius S, Franciotta D, Marchioni E, Hohlfeld R, Wildemann B, Voltz R (2006) Intrathecal polyspecific immune response against neurotropic viruses discriminates between multiple sclerosis and acute demyelinating encephalomyelitis. J Neurol 253:486
Jarius S, Kleiter I, Ruprecht K, Asgari N, Pitarokoili K, Borisow N, Hummert MW, Trebst C, Pache F, Winkelmann A, Beume LA, Ringelstein M, Stich O, Aktas O, Korporal-Kuhnke M, Schwarz A, Lukas C, Haas J, Fechner K, Buttmann M, Bellmann-Strobl J, Zimmermann H, Brandt AU, Franciotta D, Schanda K, Paul F, Reindl M, Wildemann B (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 3: Brainstem involvement - frequency, presentation and outcome. J Neuroinflammation 13:281
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hummert MW, Ringelstein M, Trebst C, Winkelmann A, Schwarz A, Buttmann M, Zimmermann H, Kuchling J, Franciotta D, Capobianco M, Siebert E, Lukas C, Korporal-Kuhnke M, Haas J, Fechner K, Brandt AU, Schanda K, Aktas O, Paul F, Reindl M, Wildemann B (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 13:280
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hummert MW, Trebst C, Ringelstein M, Aktas O, Winkelmann A, Buttmann M, Schwarz A, Zimmermann H, Brandt AU, Franciotta D, Capobianco M, Kuchling J, Haas J, Korporal-Kuhnke M, Lillevang ST, Fechner K, Schanda K, Paul F, Wildemann B, Reindl M (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 1: Frequency, syndrome specificity, influence of disease activity, long-term course, association with AQP4-IgG, and origin. J Neuroinflammation 13:279
Jarius S, Ruprecht K, Wildemann B, Kuempfel T, Ringelstein M, Geis C, Kleiter I, Kleinschnitz C, Berthele A, Brettschneider J, Hellwig K, Hemmer B, Linker RA, Lauda F, Mayer CA, Tumani H, Melms A, Trebst C, Stangel M, Marziniak M, Hoffmann F, Schippling S, Faiss JH, Neuhaus O, Ettrich B, Zentner C, Guthke K, Hofstadt-van Oy U, Reuss R, Pellkofer H, Ziemann U, Kern P, Wandinger KP, Then Bergh F, Boettcher T, Langel S, Liebetrau M, Rommer PS, Niehaus S, Munch C, Winkelmann A, Zettl UK, Metz I, Veauthier C, Sieb JP, Wilke C, Hartung HP, Aktas O, Paul F (2012) Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: A multicentre study of 175 patients. J Neuroinflammation 9:14
Jarius S, Stich O, Rasiah C, Voltz R, Rauer S (2008) Qualitative evidence of Ri specific IgG-synthesis in the cerebrospinal fluid from patients with paraneoplastic neurological syndromes. J Neurol Sci 268:65–68
Jarius S, Stich O, Speck J, Rasiah C, Wildemann B, Meinck HM, Rauer S (2010) Qualitative and quantitative evidence of anti-glutamic acid decarboxylase-specific intrathecal antibody synthesis in patients with stiff person syndrome. J Neuroimmunol 229:219–224
Kappos L, Comi G, De Stefano N, Freedman M, Barkhof F, Polman C, Uitdehaag B, Casset-Semanaz F, Hennessy B, Rocak S, Stubinski B (2011) Efficacy of two dosing frequencies of subcutaneous interferon beta-1a on risk of conversion from a first demyelinating event to multiple sclerosis: results of a phase III, randomised, double-blind, placebo-controlled, multi-centre trial (REFLEX). J Neurol 258:264
Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Radu EW, Bauer L, Dahms S, Lanius V, Pohl C, Sandbrink R (2007) Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet 370:389–397
Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Bauer L, Jakobs P, Pohl C, Sandbrink R (2006) Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 67:1242–1249
Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, Trebst C, Weinshenker B, Wingerchuk D, Parisi JE, Lassmann H (2002) A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 125:1450–1461
Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, Lutterotti A, Jarius S, Di Pauli F, Kuenz B, Ehling R, Hegen H, Deisenhammer F, Aboul-Enein F, Storch MK, Koson P, Drulovic J, Kristoferitsch W, Berger T, Reindl M (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 8:184
Mathiesen T, von Holst H, Fredrikson S, Wirsen G, Hederstedt B, Norrby E, Sundqvist VA, Wahren B (1989) Total, anti-viral, and anti-myelin IgG subclass reactivity in inflammatory diseases of the central nervous system. J Neurol 236:238–242
McLean BN, Luxton RW, Thompson EJ (1990) A study of immunoglobulin G in the cerebrospinal fluid of 1007 patients with suspected neurological disease using isoelectric focusing and the Log IgG-Index. A comparison and diagnostic applications. Brain 113(Pt 5):1269–1289
Meinl E, Krumbholz M, Hohlfeld R (2006) B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol 59:880–892
Miller DH, Weinshenker BG, Filippi M, Banwell BL, Cohen JA, Freedman MS, Galetta SL, Hutchinson M, Johnson RT, Kappos L, Kira J, Lublin FD, McFarland HF, Montalban X, Panitch H, Richert JR, Reingold SC, Polman CH (2008) Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler 14:1157–1174
Norrby E, Link H, Olsson JE, Panelius M, Salmi A, Vandvik B (1974) Comparison of antibodies against different viruses in cerebrospinal fluid and serum samples from patients with multiple sclerosis. Infect Immun 10:688–694
Otto C, Oltmann A, Stein A, Frenzel K, Schroeter J, Habbel P, Gartner B, Hofmann J, Ruprecht K (2011) Intrathecal EBV antibodies are part of the polyspecific immune response in multiple sclerosis. Neurology 76:1316–1321
Pache F, Zimmermann H, Mikolajczak J, Schumacher S, Lacheta A, Oertel FC, Bellmann-Strobl J, Jarius S, Wildemann B, Reindl M, Waldman A, Soelberg K, Asgari N, Ringelstein M, Aktas O, Gross N, Buttmann M, Ach T, Ruprecht K, Paul F, Brandt AU (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 4: Afferent visual system damage after optic neuritis in MOG-IgG-seropositive versus AQP4-IgG-seropositive patients. J Neuroinflammation 13:282
Petereit HF, Reske D (2005) Expansion of antibody reactivity in the cerebrospinal fluid of multiple sclerosis patients - follow-up and clinical implications. Cerebrospinal Fluid Res 2:3
Petereit HF, Sindern E, Wick M (2007) [CSF diagnostics. Guidelines and catalogue of methods of the German Society for Cerebrospinal Fluid Diagnostics and Clinical Neurochemistry]. Springer, Heidelberg
Pohl D, Rostasy K, Jacobi C, Lange P, Nau R, Krone B, Hanefeld F (2010) Intrathecal antibody production against Epstein-Barr and other neurotropic viruses in pediatric and adult onset multiple sclerosis. J Neurol 257:212–216
Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O’Connor PW, Sandberg-Wollheim M, Thompson AJ, Weinshenker BG, Wolinsky JS (2005) Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol 58:840–846
Puccioni-Sohler M, Kitze B, Felgenhauer K, Graef IT, Lange P, Novis S, Reiber H, Vaz B (1995) The value of CSF analysis for the differential diagnosis of HTLV-I associated myelopathy and multiple sclerosis. Arq Neuropsiquiatr 53:760–765
Reiber H (1998) Cerebrospinal fluid–physiology, analysis and interpretation of protein patterns for diagnosis of neurological diseases. Mult Scler 4:99–107
Reiber H, Lange P (1991) Quantification of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem 37:1153–1160
Reiber H, Teut M, Pohl D, Rostasy KM, Hanefeld F (2009) Paediatric and adult multiple sclerosis: age-related differences and time course of the neuroimmunological response in cerebrospinal fluid. Mult Scler 15:1466–1480
Reiber H, Ungefehr S, Jacobi C (1998) The intrathecal, polyspecific and oligoclonal immune response in multiple sclerosis. Mult Scler 4:111–117
Robinson-Agramonte M, Reiber H, Cabrera-Gomez JA, Galvizu R (2007) Intrathecal polyspecific immune response to neurotropic viruses in multiple sclerosis: a comparative report from Cuban patients. Acta Neurol Scand 115:312–318
Rosche B, Laurent S, Conradi S, Hofmann J, Ruprecht K, Harms L (2012) Measles IgG antibody index correlates with t2 lesion load on MRI in patients with early multiple sclerosis. PLoS One 7:e28094
Rostasy K, Reiber H, Pohl D, Lange P, Ohlenbusch A, Eiffert H, Maass M, Hanefeld F (2003) Chlamydia pneumoniae in children with MS: frequency and quantity of intrathecal antibodies. Neurology 61:125–128
Rostrom B (1982) Antibodies against viruses and structural brain components in oligoclonal IgG obtained from multiple sclerosis brain. J Neurol 226:255–263
Schubert J, Weissbrich B (2007) Detection of virus-specific intrathecally synthesised immunoglobulin G with a fully automated enzyme immunoassay system. BMC Neurol 7:12
Schultze D, Weder B, Cassinotti P, Vitek L, Krausse K, Fierz W (2004) Diagnostic significance of intrathecally produced herpes simplex and varizella-zoster virus-specific antibodies in central nervous system infections. Swiss Med Wkly 134:700–704
Sindic CJ, Monteyne P, Laterre EC (1994) The intrathecal synthesis of virus-specific oligoclonal IgG in multiple sclerosis. J Neuroimmunol 54:75–80
Stich O, Graus F, Rasiah C, Rauer S (2003) Qualitative evidence of anti-Yo-specific intrathecal antibody synthesis in patients with paraneoplastic cerebellar degeneration. J Neuroimmunol 141:165–169
Stich O, Kluge J, Speck J, Rauer S (2009) Detection of virus-specific (measles, rubella, zoster) oligoclonal IgG-bands in CSF from multiple sclerosis patients without oligoclonal bands of total IgG. Multiple Scler 15:S86
Stich O, Kluge J, Speck J, Rauer S (2015) Oligoclonal restriction of antiviral immunoreaction in oligoclonal band-negative MS patients. Acta Neurol Scand 131:381–388
Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ (1982) The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25:1271–1277
Tumani H, Deisenhammer F, Giovannoni G, Gold R, Hartung HP, Hemmer B, Hohlfeld R, Otto M, Stangel M, Wildemann B, Zettl UK (2011) Revised McDonald criteria: the persisting importance of cerebrospinal fluid analysis. Ann Neurol 70:520 (author reply 521)
Tumani H, Tourtellotte WW, Peter JB, Felgenhauer K (1998) Acute optic neuritis: combined immunological markers and magnetic resonance imaging predict subsequent development of multiple sclerosis. The Optic Neuritis Study Group. J Neurol Sci 155:44–49
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, de Seze J, Fujihara K, Greenberg B, Jacob A, Jarius S, Lana-Peixoto M, Levy M, Simon JH, Tenembaum S, Traboulsee AL, Waters P, Wellik KE, Weinshenker BG, International Panel for NMOD (2015) International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 85:177–189
Wurster U, Stachan R, Windhagen A, Petereit HF, Leweke FM (2006) Reference values for standard cerebrospinal fluid examinations in multiple sclerosis. Results from 99 healthy volunteers. Mult Scler 12:P248
Acknowledgements
This work was supported by a research fellowship from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) to SJ, by a research fellowship from the European Neurological Society (ENS) to SJ, and by research grants from Bayer Healthcare, from the Dietmar Hopp Foundation, and from MerckSerono to BW. We are grateful to Mrs D. Menzel, Mrs R. Herbst, Mrs M. Hoehne and Mrs H. Pahl, Department of Clinical Chemistry, Ludwig Maximilian University, Munich, Germany, and to Mrs Annemarie Eschlbeck, Department of Neurology, University of Heidelberg, Germany, for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflicts of interest.
Ethical standards
The study was approved by the institutional review board of the University of Heidelberg.
Rights and permissions
About this article
Cite this article
Jarius, S., Eichhorn, P., Franciotta, D. et al. The MRZ reaction as a highly specific marker of multiple sclerosis: re-evaluation and structured review of the literature. J Neurol 264, 453–466 (2017). https://doi.org/10.1007/s00415-016-8360-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8360-4