Abstract
Natalizumab discontinuation is associated with a disease reactivation in multiple sclerosis (MS) patients. Whether this reactivation involves also cognitive functions is not known to date. To assess the persistence of the effect of natalizumab on cognitive functions 1 year after its discontinuation, we compared the longitudinal changes of cognitive performances in two groups of patients. The interrupters, 30 MS patients, have stopped natalizumab due to PML concern, and the continuers, 28 MS patients, continued the treatment. The cognitive impairment index (CII) was used as main outcome measure. As expected, during the natalizumab treatment, we observed a significant reduction of the relapse rate and the number of gadolinium-enhancing lesions along with a reduction of the CII. After 1 year of discontinuation, the beneficial effect on cognitive functions was lost in the interrupters group, as the mean CII increased in comparison with the mean at the end of natalizumab treatment (12.2 ± 7.9 vs 9.3 ± 8.1, p < 0.0001). As opposite, in the continuers group, the CII further decreased after an additional year of treatment (8.4 ± 5.1 vs 9.8 ± 4.6, p = 0.007). A multivariate logistic regression model revealed as predictors of cognitive worsening male sex, disease duration, and the treatment discontinuation. The worsening of cognitive functions after natalizumab discontinuation goes in parallel with the clinical/radiological disease reactivation. Our data reinforce the hypothesis that, in the short-term, natalizumab exerts its positive impact on cognitive functions by means of its anti-inflammatory properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cognitive impairment is a common feature of multiple sclerosis (MS), affecting 40–65 % of patients [1]. The most common affected cognitive domains in MS are information processing speed, abstract reasoning, executive functioning, sustained attention, and long-term memory [1]. Cognitive impairment adversely affects the ability to participate fully in society and to maintain employment with a consequent negative impact on the overall patient’ quality of life [2]. Once developed, cognitive impairment worsens during the disease course [3]. To date, no effective specific treatment for cognitive impairment has been developed [4]. Available disease modifying drugs (DMDs) have shown a little to moderate effect on some aspects of cognitive deficits [5–9]. Unlike other DMDs, natalizumab (NTZ) treatment has shown to be able to improve cognitive impairment in relapsing MS (RRMS) patients, over a three-year period [10–13]. In particular, it has been shown a significant improvement of performances in cognitive tests exploring the sustained attention and information processing speed [11].
Several studies have demonstrated that NTZ discontinuation is associated with a high risk of clinical and radiological disease reactivation [14–26]. The risk correlates with the disease activity before and during NTZ treatment [22, 25, 26], and it is higher in patients discontinuing NTZ due to lack of efficacy, patient’s choice, and adverse events and in those with a wash-out (WO) duration of more than 3 months [22, 24, 26].
Switching to interferon beta (INFβ) or to glatiramer acetate (GA), was found to be ineffective to control this phenomenon [21, 23, 27, 28], whereas a switch to fingolimod (FIN), preferably within 1–2 months after NTZ suspension, was recently demonstrated to be more effective than injectable drugs by an Italian study [26].
Whether the disease reactivation seen after NTZ discontinuation involves also cognitive functions is not known to date. Also, it should be considered that some MS relapses due to the NTZ discontinuation may also involve cognitive functions [29]. Moreover, no studies have been carried out to evaluate the persistence of the overall positive impact on cognitive deficits exerted by the NTZ treatment when this drug is withdrawn. Therefore, the main aim of our study was to assess the persistence of the effect of NTZ treatment on cognitive functions 1 year after NTZ discontinuation.
Methods
Patients
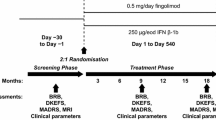
As routine clinical practice at our MS center, all the RRMS patients scheduled for NTZ treatment, and underwent a neuropsychological evaluation before the start of NTZ therapy (time point 1—T1) and every 12 months during the treatment. Thirty RRMS patients stopping NTZ (time point 2—T2) due to PML concern [30] were evaluated during the first year (time point 3—T3) after NTZ discontinuation (interrupters). Twenty-eight RRMS patients with a low risk of PML based on the negative anti-JCV status and who have continued the treatment over the 24th infusion have been also evaluated at the same time point (continuers). The two groups of patients (interrupters and continuers) have been evaluated at the same time points; therefore, they are matched at the time point 2. Patients with a visual function impairment interfering with the performances in the cognitive tests were not included in the study. This study was approved by our institution’s research ethics committee and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Neuropsychological evaluation
The neuropsychological evaluation consisted of the brief repeatable battery (BRB), and the Stroop test (ST). BRB included tests of verbal memory acquisition and delayed recall (selective reminding test—SRT), visual memory acquisition and delayed recall (10/36 spatial recall test—SPART), attention, concentration, and speed of information processing (PASAT 3; PASAT 2; symbol digit modalities test—SDMT), and verbal fluency on semantic stimulus (word list generation—WLG). Frontal lobe executive functions were assessed by the Stroop color-word task (ST). Versions A and B of the BRB were used alternatively at each examination. Cognitive impairment was defined as the failure in at least 3 tests on BRB and ST using a cutoff value of 2 standard deviation (SD) below the mean Italian normative values for each test [31]. A global score, defined cognitive impairment index (CII), allowing the evaluation of changes in cognitive performances independently by the number of cognitive tests failed at the BRB and the ST, was obtained using the mean and SD from the normative sample of Rao’s battery and the ST [9, 11, 32]. For each patient, a grading system was applied to individual cognitive tests, based on the number of SDs below the control mean (i.e., grade 0 was given if the patient scored at or above the control mean, 1 if he/she scored below the control mean, but at or above 1 SD below the control mean, and so on, until all patient scores were accommodated). Finally, all the patient’s scores were summed to give one overall measure of cognitive function. Moreover, an individual cognitive change index (CCI) was calculated to provide more precise indication of change in cognition over the follow-up period (T3–T2) [32, 33].
The Beck Depression Inventory (BDI-II) was used to assess the presence of depressive symptoms at each time point. If a relapse occurred at the time of scheduled neuropsychological assessments, cognitive testing was delayed until 30 days after the last steroid administration.
Clinical and MRI measures
The neurological disability status as evaluated by the Expanded disability status scale (EDSS) score, the annualized relapse rate (ARR), and the number of gadolinium enhancing lesions (GD) was recorded for each patient, before, during, and after the NTZ treatment.
Statistical analyses
In descriptive analyses, continuous variables were summarized as mean, SD, and median and range, and categorical variables were expressed as percentages. Patients’ characteristics according to outcome were compared using the Mann–Withney U test for continuous variables and the χ2 test for categorical variables.
The longitudinal changes of the CII, ARR, and number of GD lesions were compared at three time points using the Friedman two-way test for repeated measures, followed by pairwise comparisons performed by applying the Wilcoxon signed—rank test for paired samples in both groups. The proportions of patients free from any measure of disease activity (relapse, GD lesions, and cognitive worsening) were compared using the χ2 test.
A variation of at least 2 points on the CCI identified patients with improving or worsening cognitive performances. Demographic and clinical predictors of cognitive worsening at T3 were assessed through a multivariate stepwise logistic regression model, including as covariates age, sex, education, disease duration, EDSS score, BDI-II score, CII score at T2, number of relapses in the year prior natalizumab, number of relapse during the natalizumab treatment, number of GD enhancing lesions at the last scan performed prior natalizumab, and number of GD enhancing lesions during the natalizumab treatment and treatment group (interrupters vs continuers). Risks were reported as odds ratio (OR) with 95 % confidence interval (CI). Data analyses were performed by SPSS 22.0. The p values <0.05 were considered significant.
Results
Demographic and clinical characteristics at the time point 2, which correspond to the last NTZ infusion for the interrupters, and the time of coeval evaluation for the continuers, are shown in Table 1. All the interrupters received a new DMDs treatment after a mean WO time of 4.7 ± 4.2 months (18 patients received different formulations of INFβ, 9 received GA, and 3 switched to FIN). The neuropsychological evaluation was postponed 30 days after last steroid pulse administration in 3 patients who were experienced a relapse at the time of the scheduled visit.
The mean ARR significantly increased during the first year after NTZ discontinuation (T3 vs T2: 0.9 ± 0.8 vs 0.5 ± 0.6, p = 0.04), but it was also significantly lower than the ARR in the year prior NTZ (T3 vs T1: 1.5 ± 0.8, p = 0.003) in the interrupters group, whereas in the patients still in NTZ treatment, the ARR did not change between T2 and T3 (T2 vs T3: 0.3 ± 0.5 vs 0.1 ± 0.3, p = 0.13) (Table 2).
During the NTZ treatment, the mean number of GD lesions was close to zero (T2; interrupters: 0.1 ± 0.2; continuers: 0.1 ± 0.2) in both groups. At T3 evaluation, the mean number of GD lesions significantly increased compared with the number recorded during the NTZ treatment (T3 vs T2: 0.9 ± 1.9, p = 0.002) in the interrupters patients, whereas it was unchanged in patients continuing the NTZ treatment (p = 1.0) (Table 2).
During the NTZ treatment, the mean CII significantly decreased in both groups (T1 vs T2; interrupters: 12.6 ± 7.9 vs 9.3 ± 8.1, p < 0.0001; continuers: 15.6 ± 5.9 vs 9.7 ± 4.6, p < 0.0001). After 1 year of NTZ suspension, the beneficial effect on cognitive functions was completely lost in the interrupters group, as the mean CII increased in comparison with the mean at the end of NTZ treatment (T3 vs T2; 12.2 ± 7.9 vs 9.3 ± 8.1, p < 0.0001), and returned at the same level registered before the NTZ treatment. As opposite, in the continuers group, the CII further decreased after an additional year of treatment (T3 vs T2; 8.4 ± 5.1, p = 0.007) (Table 2).
At T3, 19 patients (63.3 %) discontinuing NTZ presented a cognitive worsening as defined by the CCI, whereas the proportion of patients with a cognitive worsening in the continuers group was 7.1 % (2/28 patients).
During the year post NTZ withdrawal, 16 patients reported no MRI activity, 9 patients reported no clinical activity, and 7 patients were free from combined relapses and MRI activity. In patients continuing NTZ, no MRI activity was reported in 27 patients, no clinical activity was reported in 26 patients, and 25 patients were free from combined relapses and MRI activity. The proportion of patients free from any measures of disease activity, including cognitive worsening, was significantly higher in the continuers group (82.1 vs 13.3 %, p < 0.0001).
The multivariate stepwise logistic regression model performed to estimate the predictors of cognitive worsening at T3 retained the following factors: sex, disease duration, and treatment group. More in details, male sex (OR 8.932, 95 % CI 1.241–64.270, p = 0.03) and a longer disease duration (OR 1.161, 95 % CI 1.007–1.339, p = 0.04) were predictors of cognitive worsening at the last neuropsychological evaluation. Whereas continuing the NTZ treatment was found to be a significant protective factor against cognitive worsening (OR 0.027, 95 % CI 0.004–250.1870.7, p < 0.0001) (Table 3).
Discussion
In our cohort of RRMS patients discontinuing NTZ due to PML concern, we have observed, as expected, a clinical and radiological disease reactivation, and this is in line with most of previous reports on the same topic [13–27].
The main result of our observational study is the demonstration that the beneficial effect of NTZ on cognitive functions is completely lost after the discontinuation of the drug. This is the first study in which patients have been re-evaluated after the NTZ suspension; therefore, there are no data to compare our results.
We have showed a significant reduction of the CII during the NTZ treatment, and subsequently a significant increase of this measure in patients discontinuing the drug at 1 year. The CII [9, 11, 29], as opposed to the number of tests failed, may be considered a more suitable tool to evaluate changes in cognitive performances. This is because the CII is independent by the number of cognitive tests failed at the BRB, and the ST (i.e., if an individual patient was impaired in six tests at baseline and failed four tests at follow-up, that patient would be still considered cognitively impaired, despite the fact that his or her performances were objectively improved), and therefore, its use strengthens the results of this study. Moreover, we used alternate versions of cognitive tests [34], and adequate time intervals between them to reduce the major methodological issue when neuropsychological tests are repeated over time that is the practice effect.
The reduction of the CII during the NTZ treatment is in line with previous reports showing a positive impact of NTZ on cognitive functioning [10–13]. Moreover, we have also evaluated the longitudinal changes of the CII by means of the CCI, and consequently, we have defined patients as cognitively worsened or stable/ameliorated. The proportion of cognitively worsened patients was higher in patients discontinuing NTZ in comparison with those patients who have continued the treatment. The multivariate logistic regression model, which included also the BDI-II score as covariates to account for the presence of depressive symptoms, revealed that the major risk factors of cognitive worsening were the disease duration and male sex, whereas the only protective factors were the persistence on NTZ treatment.
Disease duration was already found to be related to the clinical and MRI reactivation after NTZ suspension in two large cohorts of MS patients [23, 25].
In our cohort, the reintroduction of a first line immunomodulatory treatment did not affect the recurrence of the cognitive deficits after the NTZ suspension as well as it did not affect the clinical and MRI disease reactivation.
This could be explained by a null effect, or at most a modest effect of these therapies on cognitive deficits [5–9].
The degree and the time-trend of the improvement induced by NTZ and the subsequent worsening of the cognitive functions after its suspension could be explained by the anti-inflammatory properties of the drug. Several reports suggest that the presence and the degree of cognitive deficits, at least in mild disabled RRMS patients, could be more related to inflammation than to neurodegenerative mechanisms. In the rodent model of MS, the experimental autoimmune encephalomyelitis, it was demonstrated that the inflammatory cytokines released from infiltrating lymphocytes, especially TNF-a, and the activation of microglia, are able to alter synaptic transmission [35, 36], leading to a synaptopathy which is related to cognitive dysfunction in this animal model of MS [35, 36].
In humans, a significant reduction of cerebrospinal fluid (CSF) and plasma levels of pro-inflammatory cytokines and of CSF levels of the light-chain neurofilament, a marker of axonal loss, during the NTZ treatment have been reported [37–40]. In particular, our group recently reported that the improvement of cognitive functions in NTZ treated RRMS patients could be associated with a decrease in plasma osteopontin levels [40].
The main limitation of this study is the small sample size of our cohort, and of course, a larger sample size is needed to confirm our preliminary results.
In conclusion, our data reinforce the hypothesis that, in the short-term, NTZ exerts its positive impact on cognitive functions likely by means of its anti-inflammatory properties. Our data also indicate that this effect disappear at the withdrawal of the drug when an inflammatory reactivation occurs in most of patients. These results emphasize the importance to test cognitive functions of our RRMS patients before, during, and after every DMDs treatment.
References
Chiaravalloti ND, DeLuca J (2008) Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151
Rao SM (1995) Neuropsychology of multiple sclerosis. Curr Opin Neurol 8:216–220
Amato MP, Zipoli V, Portaccio E (2006) Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. J Neurol Sci 245:41–46
He D, Zhang Y, Dong S et al (2013) Pharmacological treatment for memory disorder in multiple sclerosis. Cochrane Database Syst Rev 17(12):CD008876
Pliskin NH, Hamer DP, Goldstein DS, Towle VL, Reder AT et al (1996) Improved delayed visual reproduction test performance in multiple sclerosis patients receiving interferon b-1b. Neurology 47:1463–1468
Fischer JS, Priore RL, Jacobs LD, Cookfair DL, Rudick RA et al (2000) Neuropsychological effects of interferon b-1a in relapsing multiple sclerosis. Ann Neurol 48:885–892
Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR et al (2002) Benefit of interferon b-1a on MSFC progression in secondary progressive MS. Neurology 59:679–687
Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP et al (2009) Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 8:987–997
Patti F, Amato MP, Bastianello S, Caniatti L, Di Monte E et al (2010) Effects of immunomodulatory treatment with subcutaneous interferon beta-1a on cognitive decline in mildly disable patients with relapsing remitting multiple sclerosis. Mult Scler 16:68–77
Mattioli F, Stampatori C, Capra R (2011) The effect of Natalizumab on cognitive function in patients with relapsing-remitting multiple sclerosis: preliminary results of a 1-year follow-up study. Neurol Sci 32:83–88
Iaffaldano P, Viterbo RG, Paolicelli D et al (2012) Impact of Natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: a prospective, open-label. Two years observational study. PLoS One 7(4):e35843. doi:10.1371/journal.pone.0035843
Kunkel A, Fischer M, Faiss J et al (2015) Impact of Natalizumab treatment on fatigue, mood, and aspects of cognition in relapsing-remitting multiple sclerosis. Front Neurol 11(6):97
Mattioli F, Stampatori C, Bellomi F, Scarpazza C, Capra R (2015) Natalizumab significantly improves cognitive impairment over 3 years in MS: pattern of disability progression and preliminary MRI findings. PLoS One 10(7):e0131803
Killestein J, Vennegoor A, Strijbis EM et al (2010) Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 68:392–395
West TW, Cree BA (2010) Natalizumab dosage suspension: are we helping or hurting? Ann Neurol 68:395–399
Borriello G, Prosperini L, Marinelli F, Fubelli F, Pozzilli C (2011) Observations during an elective interruption of Natalizumab treatment: a post-marketing study. Mult Scler 17:372–375
Borriello G, Prosperini L, Mancinelli C, Gianni C, Fubelli F, Pozzilli C (2012) Pulse monthly steroids during an elective interruption of Natalizumab: a post-marketing study. Eur J Neurol 19:783–787
Havla J, Gerdes LA, Meinl I et al (2011) De-escalation from Natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 258:1665–1669
Kaufman MD, Lee R, Norton HJ (2011) Course of relapsing remitting multiple sclerosis before, during and after Natalizumab. Mult Scler 17:490–494
Kerbrat A, Le Page E, Leray E et al (2011) Natalizumab and drug holiday in clinical practice: an observational study in very active relapsing remitting multiple sclerosis patients. J Neurol Sci 308:98–102
Magraner MJ, Coret F, Navarre A et al (2011) Pulsed steroids followed by glatiramer acetate to prevent inflammatory activity after cessation of Natalizumab therapy: a prospective, 6-month observational study. J Neurol 258:1805–1811
O’Connor PW, Goodman A, Kappos L, Lublin FD, Miller DH, Polman C et al (2011) Disease activity return during Natalizumab treatment interruption in patients with multiple sclerosis. Neurology 76(22):1858–1865
Rossi S, Motta C, Studer V et al (2013) Effect of glatiramer acetate on disease reactivation in MS patients discontinuing Natalizumab. Eur J Neurol 20:87–94
Cohen M, Maillart E, Tourbah A, De Sèze J, Vukusic S, Brassat D et al (2014) Switching from Natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol. doi:10.1001/jamaneurol.2013.6240
Jokubaitis VG, Li V, Kalincik T, Izquierdo G, Hodgkinson S, Alroughani R et al (2014) Fingolimod after Natalizumab and the risk of short-term relapse. Neurology 82(14):1204–1211. doi:10.1212/WNL.0000000000000283
Iaffaldano P, Lucisano G, Pozzilli C, et al. (2015) Fingolimod versus interferon beta/glatiramer acetate after Natalizumab suspension in multiple sclerosis. Brain 138(11):3275–3286. doi:10.1093/brain/awv260
Clerico M, Schiavetti I, De Mercanti SF, Piazza F, Gned D, Brescia Morra V et al (2014) Treatment of relapsing-remitting multiple sclerosis after 24 doses of Natalizumab: evidence from an Italian spontaneous, prospective, and observational study (the TY-STOP Study). JAMA Neurol. doi:10.1001/jamaneurol.2014.1200
Fox RJ, Cree BA, De Sèze J, Gold R, Hartung HP, Jeffery D et al (2014) MS disease activity in RESTORE: a randomized 24-week Natalizumab treatment interruption study. Neurology 82(17):1491–1498
Morrow SA, Jurgensen S, Forrestal F, Munchauer FE, Benedict RH (2011) Effects of acute relapses on neuropsychological status in multiple sclerosis patients. J Neurol 258(9):1603–1608
McGuigan C, Craner M, Guadagno J, Kapoor R, Mazibrada G, Molyneux P et al (2016) Stratification and monitoring of Natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 87(2):117–125. doi:10.1136/jnnp-2015-311100 (Epub 2015 Oct 22)
Amato MP, Portaccio E, Goretti B, Zipoli V, Ricchiuti L et al (2006) The Rao’s brief repeatable battery and stroop test: normative values with age, education and gender corrections in an Italian population. Mult Scler 12:787–793
Camp SJ, Stevenson VL, Thompson AJ, Miller DH, Borras C et al (1999) Cognitive function in primary progressive and transitional progressive multiple sclerosis. A controlled study with MRI correlates. Brain 122:1341–1348
Amato MP, Goretti B, Ghezzi A, Hakiki B, Niccolai C, Lori S et al (2014) Neuropsychological features in childhood and juvenile multiple sclerosis: five-year follow-up. Neurology 83(16):1432–1438. doi:10.1212/WNL.0000000000000885
Goretti B, Patti F, Cilia S, Mattioli F, Stampatori C et al (2014) The Rao’s Brief Repeatable Battery version B: normative values with age, education and gender corrections in an Italian population. Neurol Sci 35(1):79–82. doi:10.1007/s10072-013-1558-7 Epub 2013 Oct 8
Mandolesi G, Grasselli G, Musumesi G, Centonze D (2010) Cognitive deficits in experimental autoimmune encephalomyelitis: neuroinflammation and synaptic degeration. Neurol Sci 31(Suppl 2):S255–S259
Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V et al (2009) Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29:3442–3452
Gunnarsson M, Malmestro¨m C, Axelsson M, Sundstro¨m P, Dahle C et al (2010) Axonal damage in relapsing multiple sclerosis is markedly reduced by Natalizumab. Ann Neurol 69:83–89
Khademi M, Bornsen L, Rafatnia F, Andersson M, Brundin L et al (2009) The effects of Natalizumab on inflammatory mediators in multiple sclerosis: prospects for treatment-sensitive biomarkers. Eur J Neurol 16:528–536
Mellergard J, Edstrom M, Vrethem M, Erneurdh J, Dahle C (2010) Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler 16:208–217
Iaffaldano P, Ruggieri M, Viterbo RG, Mastrapasqua M, Trojano M (2014) The improvement of cognitive functions is associated with a decrease of plasma osteopontin levels in Natalizumab treated relapsing multiple sclerosis. Brain Behav Immun 35:176–181
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Iaffaldano P. has served on scientific advisory boards for Biogen Idec and Bayer, and has received funding for travel and/or speaker honoraria from Sanofi-Aventis, Biogen Idec, Teva and Novartis. Viterbo R.G. has served on scientific advisory boards for Biogen Idec and has received speaker honoraria from Teva and Novartis. Trojano M. has received honoraria for consultancy or speaking from Biogen, Sanofi-Aventis, Merck Serono and Bayer-Schering and research grants from Merck Serono, Biogen and Novartis.
Rights and permissions
About this article
Cite this article
Iaffaldano, P., Viterbo, R. & Trojano, M. Natalizumab discontinuation is associated with a rebound of cognitive impairment in multiple sclerosis patients. J Neurol 263, 1620–1625 (2016). https://doi.org/10.1007/s00415-016-8177-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8177-1