Abstract
Brain gray matter volume (GMV) reduction has been reported in Parkinson’s disease (PD) with mild cognitive impairment (PD-MCI) and in PD patients with dementia (PDD) with cumulative evidence using voxel-based morphometry (VBM). However, the findings of these studies have not been entirely concordant. Whole-brain VBM studies comparing PD-MCI with PD patients without cognitive impairment (PD-NCI) and comparing PDD with PD patients without dementia (PDND) were systematically searched in PubMed and EMBASE databases from January 1995 to December 2015. Coordinates with significant differences were extracted from each cluster. Meta-analysis was performed using AES-SDM to quantitatively evaluate the GMV changes. Five studies comparing 92 PD-MCI with 192 PD-NCI patients were included in the PD-MCI vs. PD-NCI meta-analysis. Ten studies with 168 PDD and 233 PDND patients were included in the PDD vs. PDND meta-analysis. Compared with PD-NCI, GMV reductions were observed in left superior temporal lobe, left insula and left superior frontal lobe in PD-MCI patients. Significant GMV reduction were found in bilateral superior temporal lobe extending to hippocampus, and left superior frontal lobe in PDD patients comparing with PDND. Meta-regression of PDD studies showed that disease duration was negatively correlated with GMV in the left superior frontal lobe. GMV reductions in the frontal-limbic-temporal regions were main features of cognitive decline in PD. Unilateral-to-bilateral development of GMV reduction in the frontal-limbic-temporal regions is a possible indicator for PD-MCI to PDD progression, whereas significant hippocampal GMV reduction may not be a marker for early cognitive decline in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative illness marked by typical motor features as well as non-motor symptoms. Cognitive decline, ranging from mild cognitive impairment (MCI) to dementia, is frequently observed in PD patients [1]. Parkinson’s disease with mild cognitive impairment (PD-MCI) was reported in more than 60 % of PD patients and Parkinson’s disease dementia (PDD) was found in 22–31.3 % of PD patients [2–4]. Further, a prospective longitudinal study reported that 36 % of PD patients had mild cognitive impairment and an additional 48 % met the diagnostic criteria for PDD within 15 years [5]. It has been suggested that PDD patients have increased health care burden, declined quality of life and increased mortality, which highlight the importance of PDD prediction [6]. Since PD-MCI is a widely accepted risk factor for PDD [7], methods quantifiably evaluating small changes in PD-MCI and PDD are needed to better understand the underlying neuropathological process.
Various neuroimaging techniques have been proposed to study the neuropathological basis of cognitive impairments in PD patients [2, 8–10]. Voxel-based morphometry (VBM), a processing method that can identify subtle morphological changes in the whole brain, has been commonly used to evaluate gray matter volume (GMV) abnormalities in PD patients with cognitive impairments. Previous VBM studies on GMV of PD-MCI patients have illustrated gray matter (GM) atrophy in bilateral temporal lobes and caudate nucleus, left frontal lobe, left insula, left parahippocampal gyrus and left precentral gyrus relative to PD patients without cognitive impairment (PD-NCI), defined as PD patients who did not meet diagnostic criteria for PD-MCI [11–13], whereas in VBM studies comparing with PD patients without dementia (PDND), defined as PD patients who did not meet PDD diagnostic criteria, GMV abnormalities were found in more widespread regions in PDD [2, 14–16]. However, the results of the studies were inconsistent in comparisons of PD-MCI vs. PD-NCI as well as PDD vs. PDND. In PD-MCI and PDD patients, GMV reduction in temporal lobe was frequently reported, but the side and regions involved differed between studies [11, 15–19]. Some PDD studies demonstrated GM atrophy in hippocampus [14, 15, 20], whereas some other studies did not recognize such changes [11, 18]. Moreover, the differences in GMV abnormalities between PD-MCI and PDD were rarely discussed. Therefore, a meta-analysis is essential to identify consistent GMV changes in PD-MCI and PDD relative to PD-NCI and PDND patients, respectively.
Anisotropic effect size-based signed differential mapping (AES-SDM) is an updated quantitative voxel-based meta-analytic technique for meta-analyzing studies on differences in brain structures that used various neuroimaging methods [21, 22]. This signed differential mapping (SDM) technique has been successfully applied in VBM studies on GMV of PD and other neurological disorders [23, 24]. One previous voxel-wise meta-analysis on GMV reduction in PDD relative to healthy controls (HC) found significant GMV reduction in bilateral medial temporal lobe and right caudate [25]. However, this study did not specifically differentiate the possible influence of background PD pathologies. We conducted the current meta-analysis using AES-SDM technique by comparing PD-MCI vs. PD-NCI and PDD vs. PDND separately to minimize the effect of predisposed PD-related GMV changes.
Methods
Data source
We conducted systematic searches of the PubMed and EMBASE database from January 1995 to December 2015 using the combination of the following keywords: (“Parkinson’s disease dementia” OR “PDD” OR “Parkinson’s disease with mild cognitive impairment” OR “PD-MCI”) and (“voxel*” OR “VBM” OR “morphometry”). Reference lists of relevant articles were searched for additional studies.
Study selection and data extraction
Studies were considered for inclusion if they (1) reported the VBM results of GMV from a comparison either between PD-MCI and PD-NCI patients or between PDD and PDND patients; (2) reported whole-brain changes of GMV in a standard stereotactic space [Talairach or Montreal Neurological Institute (MNI)] with three-dimensional coordinates (x, y, z); (3) used significance thresholds that were corrected for multiple comparisons or uncorrected with spatial extent thresholds; (4) published in English or Chinese; and (5) within one comparison pair, of the studies from one research population, the study with the largest sample size and the most comprehensive data was included. Studies were excluded if (1) the PD-MCI or PDD group was only compared with HC group; (2) the PD-MCI or PDD group was compared with AD or DLB patients; (3) the stereotactic coordinates of the reported GMV changes were not obtained even after corresponding with the authors by email. Study selection and data extraction were performed in a standardized form by two authors (Yaqian Xu and Jing Yang) independently. In cases of disagreements, a third opinion (Huifang Shang) was obtained.
Voxel-based meta-analysis of VBM studies
The voxel-based meta-analysis was performed on the included studies using the AES-SDM software (version 4.31, http://www.sdmproject.com) to compare GMV alterations between two comparison pairs: PD-MCI vs. PD-NCI and PDD vs. PDND. The analytical processes were referred to the AES-SDM tutorial and guidelines [22]. To preprocess the input data, all coordinates were first converted into MNI space and were convolved with a Gaussian kernel to account for spatial error using a relatively wide full-width at half-maximum (FWHM = 20 mm) [22]. After effect size signed maps of the differences in GMV were created, the mean map was calculated representing the weighted mean regional difference in GM. The statistical threshold was set to P < 0.005 (cluster >30 voxels). Additional jackknife sensitivity analysis was carried out to assess the robustness of the results across studies.
Further meta-regression analyses were conducted in the PDD-PDND comparison, to explore the correlation between clinical variables (such as age, disease duration, education, MMSE and UPDRS III) and GMV changes. We used a voxel threshold of P < 0.0005 and a cluster-level threshold of 10 voxels for the meta-regression analyses. Meta-regression was not performed in the PD-MCI comparison due to limited number of studies.
Results
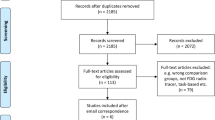
The search strategy identified a total of 997 studies. Five studies met the inclusion criteria and were included in the PD-MCI vs. PDNCI comparison analysis. Ten studies met the inclusion criteria for the PDD vs. PDND comparison analysis. Two studies were included in both of the comparisons [11, 26]. No additional articles were found in the reference list of those studies. In total, coordinates from 13 studies were used in our study. One of the selected studies was written in Chinese, and others were in English. A flow diagram of the study selection process is provided in Fig. 1.
PD-MCI < PD-NCI
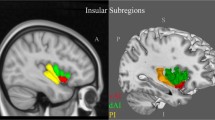
In the PD-MCI vs. PD-NCI comparison, a total of 92 PD-MCI patients and 192 PD-NCI patients from five studies were involved. The technical details of these studies and demographic characteristics of the participants are provided in Table 1. One study reported no significant GMV reduction [26], while the rest of the included studies reported significant GMV reduction. As illustrated in Fig. 2a and Table 3, significant GMV reduction were found in the left superior temporal lobe extending to the left insula, and the left superior frontal lobe. The results remained largely unchanged after examined by whole brain jackknife sensitivity analysis.
Gray matter atrophy in PD-MCI relative to PD-NCI patients (a) and PDD relative to PDND patients (b); PD-MCI Parkinson’s disease with mild cognitive impairment, PD-NCI Parkinson’s disease with no cognitive impairment, PDD Parkinson’s disease dementia, PDND Parkinson’s disease patients who did not meet PDD diagnostic criteria
PDD < PDND
In the PDD vs. PDND comparison, a total of 168 PDD patients and 233 PDND patients from 10 studies were involved. The characteristics of the participants and the technical details of the studies are summarized in Table 2. All of the included studies reported GM atrophy in PDD patients relative to PDND patients. As illustrated in Fig. 2b and Table 3, significant GMV reduction were found in the following regions: bilateral superior temporal lobes extending to hippocampus, insula and inferior frontal lobe, and the left superior frontal lobe. Two of the included studies compared PDD patients with PD patients with normal cognition [12, 26], but the results remained largely unchanged in all combinations of the jackknife sensitivity analyses.
Meta-regression
In the PDD-PDND comparison, meta-regression analysis was conducted in nine studies because demographic data of PDD patients was not available in one study [20]. We found in the PDD-PDND comparison that disease duration is negatively associated with GMV in the left superior fontal lobe (Fig. 3). Meta-regression analysis showed that there were no significant associations between GMV reduction and age or MMSE scores in PDD. The association between GMV reduction and education, UPDRS III scores and Hoehn and Yahr stages were not performed by meta-regression analysis because less than nine of the included studies reported original data.
Discussion
By conducting this meta-analysis, we found significant GMV alterations in the frontal-limbic-temporal regions in the PD-MCI vs. PD-NCI comparison and the PDD vs. PDND comparison. GMV reduction in PDD patients involved a broader region including bilateral temporal lobe, insula, hippocampus and left frontal lobe, whereas, in PD-MCI patients, only the left side of temporal lobe, insula and frontal lobe were involved. These findings illustrated a strong association between frontal-limbic-temporal regional GM atrophy and PD cognitive decline. In addition, the differences between PD-MCI and PDD may represent a pathophysiological progression pattern in PD.
GMV reduction in superior temporal lobe, inferior frontal lobe and insula was observed in the PDD vs. PDND comparison as well as in the PD-MCI vs. PD-NCI comparison. These regions are functionally related with human auditory process, facial recognition, social cognitive process, emotional feelings and linguistic integration [27–30]. GMV alteration in these regions could contribute to the lexical and sematic function impairment as well as depression and apathy that were often present in PD patients with cognitive impairment [31].
In the current meta-analysis, GM atrophy in the left superior frontal lobe was identified in both PD-MCI vs. PD-NCI and PDD vs. PDND comparisons. Frontal GMV reduction has also been observed in both PDD and DLB patients in a study comparing PDD with DLB, indicating a shared Lewy body pathology in this region [32]. Moreover, in the PDD vs. PDND comparison, GMV decreased significantly in the left anterior cingulate gyrus, where the greatest concentration of Lewy bodies was found in PD patients [33, 34]. Since we found a significant association between PD disease duration and GMV reduction in the left superior frontal lobe in PDD patients, PD duration-related Lewy body accumulation in this region was indicated. In addition, Nagano-Saito et al. suggested that frontal volumetric reduction was associated with deficits in executive function, which were common features of early cognitive decline in PD [14]. Therefore, GMV reduction in the left superior frontal lobe, related with Lewy body pathology, could serve as an early marker to PD cognitive decline.
An interesting progression pattern, from left (PD-MCI) to both sides (PDD), was indicated by our results. GMV reductions in the left temporal lobe, inferior frontal lobe and insula were significant in PD-MCI vs. PD-NCI comparison, whereas bilateral reductions were found in PDD vs. PDND comparison. In addition, only GM atrophy in the left superior frontal lobe was observed in both PD-MCI vs. PD-NCI and PDD vs. PDND comparisons. However, according to the jackknife sensitivity analysis of the PDD vs. PDND comparison, in the combination where the Melzer study was not included [26], GMV reduction in the right superior frontal lobe was also significant. Thus, this indicated a similar left to bilateral progression from PD-MCI to PDD in the superior fontal lobe. This transformation from left to both sides and our result that all regions of GMV reduction reported in PD-MCI were also present in PDD further supported the hypothesis that PD-MCI is a prototype of PDD [35, 36]. One possible explanation to this pattern is that it resembled a similar pattern in the MCI to AD process. However, while one study suggested left side involvement in MCI and more general atrophy in AD [37], other AD studies did not recognize a similar pattern [38, 39]. Another assumption regarding this progression pattern is related with PD pathology. The unilateral-to-bilateral development of GM atrophy in PD cognitive decline might be a unique feature related to the unilateral-to-bilateral progression of PD motor symptoms and dopaminergic pathology [40]. However, since side of motor symptoms onset or dominant side was not provided in the included studies, further investigation is required.
Similar with prior VBM meta-analysis comparing PDD patients with HC [25], we found hippocampal GMV reduced significantly in the PDD vs. PDND comparison. This finding is consistent with studies using other neuroimaging methods including ROI studies [41, 42]. Also, Zarei et al. used a relatively more sensitive cortical thinning approach and identified hippocampus volume as having 80 % accuracy for the identification of PDD patients [43]. Comparing with AD studies, hippocampal atrophy in PDD patients closely resembles the typical neuroanatomical changes in AD [38, 44] and was commonly interpreted as AD type pathology that coexisted in PDD patients [45]. Interestingly, hippocampal GMV reduction was not significant when comparing PD-MCI with PD-NCI, which was different from AD as they often present with significant hippocampal atrophy in the MCI stage [46]. This may be interpreted as hippocampal atrophy being a gradual progressive process in the cognitive decline of PD. A 25-month longitudinal study on PD cognitive decline showed that PDD patients had more significant hippocampal GM atrophy than PDND patients when compared with baseline correspondingly, suggesting a progressive volume reduction of hippocampus associated with PDD [47]. In addition, Ibarretxe-Bilbao et al. found that the neurodegeneration process in PD patients starts in hippocampal head and then spread to tail [48]. Combined with our finding of meta-regression analysis, hippocampal GMV reduction is not an early marker for predicting cognitive decline in PD.
Contrary to our expectation, GMV reduction was not significant in the occipital lobe, which was suggested to be related with PD cognitive decline in two of the included studies [16, 49]. In these two studies, significant decrease in occipital GMV was reported on the left and on the right side, respectively, which might lead to the insignificance in results. In addition, occipital GM atrophy in PDD was explained by visual hallucination that presented in many PDD patients [16, 50]. When the study results were adjusted for hallucination as a confounding factor, like in the Goldman study [20], our findings could also be affected. However, in our sensitivity analysis, occipital GMV reduction was still not significant when the Goldman study was not included in the PDD vs. PDND analysis. Similarly, we did not find significant GMV reduction in the parietal lobe in the PDD vs. PDND analysis or in the sensitivity analysis. GMV reduction was reported in four of the included studies, but the side and regions reported varied between studies [11, 12, 16, 17]. Parietal lobe abnormality was also reported to be associated with visual hallucination in PD patients, which may increase the risk for PDD [20, 50]. Future studies focusing on the association between specific brain region GMV reduction in PDD patients and impairment in functional domain are needed to better predict cognitive decline by neuroimaging methods.
Limitation
It is important to note that our meta-analysis has several limitations. First, all of the included studies were cross-sectional studies. Longitudinal cohort studies are required to further understand the neuropathological progression of cognitive decline in PD. Second, the present meta-analysis is based on publications that reported coordinates and the data acquired by contacting the corresponding authors, unpublished studies and studies in languages other than English or Chinese were not included. Third, the methodological differences of VBM studies, including differences in pre-processing protocols, smoothing kernels, and statistical thresholding methods, cannot be ruled out entirely. Also, the diagnostic criteria for PDD and PD-MCI were not consistent in the included studies, which could affect the results. However, the results remained largely unchanged when the study with different diagnostic criteria was excluded in the sensitivity analysis. The number of studies included in both comparisons was limited, but our findings are considered robust because almost all of the studies contributed to the results. We found a unilateral-to-bilateral progression pattern of GMV reduction in the frontal-limbic-temporal region, but were unable to analyze its association with side of motor symptom onset in PD due to limited data from the included studies. In addition, because most of the included studies did not provide sub-scores for individual domains of cognitive decline, we were unable to analyze the association between functional domains with regional GMV reductions in PDD.
Conclusion
In summary, the present meta-analysis not only identified consistent GMV reductions in PD-MCI and PDD patients, mainly located in the frontal-limbic-temporal regions, but also revealed morphological predictor for the progression of cognitive decline in PD. Unilateral-to-bilateral development of GMV reduction in the frontal-limbic-temporal regions can be considered as a quantitative morphological predictor for PD-MCI to PDD progression and further advocate therapeutic interventions. Significant hippocampal GMV reduction may also be a sign for the progression from PD-MCI to PDD, but was not a marker for early cognitive decline in PD. More longitudinal studies looking into the pattern of morphological changes in PD cognitive decline are necessary to reveal the underlying neuropathological process of PD cognitive decline.
References
Schapira AH (2015) The measurement and importance of non-motor symptoms in Parkinson disease. Eur J Neurol 22(1):2–3. doi:10.1111/ene.12523
Lee JE, Cho KH, Song SK, Kim HJ, Lee HS, Sohn YH, Lee PH (2014) Exploratory analysis of neuropsychological and neuroanatomical correlates of progressive mild cognitive impairment in Parkinson's disease. Journal of neurology, neurosurgery, and psychiatry 85 (1):7-16. doi:10.1136/jnnp-2013-305062
Petrou M, Kotagal V, Bohnen NI (2012) An update on brain imaging in parkinsonian dementia. Imaging Med 4(2):201–213. doi:10.2217/iim.12.10
Hu XF, Zhang JQ, Zhang YL, Wu Y, Chen LH, Wang J (2013) Mild cognitive impairment in patients with Parkinson's disease: A voxel-based morphometry analysis of gray matter. [Chinese]. Chinese Journal of Medical Imaging Technology 29 (1):24-29
Green J, McDonald WM, Vitek JL, Evatt M, Freeman A, Haber M, Bakay RA, Triche S, Sirockman B, DeLong MR (2002) Cognitive impairments in advanced PD without dementia. Neurology 59(9):1320–1324
Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 289(1–2):18–22. doi:10.1016/j.jns.2009.08.034
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-l-dopa-responsive problems dominate at 15 years. Mov Disord 20(2):190–199. doi:10.1002/mds.20324
Leroi I, McDonald K, Pantula H, Harbishettar V (2012) Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 25(4):208–214. doi:10.1177/0891988712464823
Pedersen KF, Larsen JP, Tysnes OB, Alves G (2013) Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 70(5):580–586. doi:10.1001/jamaneurol.2013.2110
Carbon M, Marie RM (2003) Functional imaging of cognition in Parkinson’s disease. Curr Opin Neurol 16(4):475–480. doi:10.1097/01.wco.0000084225.82329.3c
Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D (2007) Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage 34(2):714–723. doi:10.1016/j.neuroimage.2006.09.003
Peraza LR, Colloby SJ, Firbank MJ, Greasy GS, McKeith IG, Kaiser M, O’Brien J, Taylor JP (2015) Resting state in Parkinson’s disease dementia and dementia with Lewy bodies: commonalities and differences. Int J Geriatr Psychiatry 30(11):1135–1146. doi:10.1002/gps.4342
Beyer MK, Janvin CC, Larsen JP, Aarsland D (2007) A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry 78(3):254–259. doi:10.1136/jnnp.2006.093849
Song SK, Lee JE, Park HJ, Sohn YH, Lee JD, Lee PH (2011) The pattern of cortical atrophy in patients with Parkinson’s disease according to cognitive status. Mov Disord 26(2):289–296. doi:10.1002/mds.23477
Mak E, Zhou J, Tan LC, Au WL, Sitoh YY, Kandiah N (2014) Cognitive deficits in mild Parkinson’s disease are associated with distinct areas of grey matter atrophy. J Neurol Neurosurg Psychiatry 85(5):576–580. doi:10.1136/jnnp-2013-305805
Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, Dagher A, Ito K (2005) Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64(2):224–229. doi:10.1212/01.wnl.0000149510.41793.50
Summerfield C, Junque C, Tolosa E, Salgado-Pineda P, Gomez-Anson B, Marti MJ, Pastor P, Ramirez-Ruiz B, Mercader J (2005) Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol 62(2):281–285. doi:10.1001/archneur.62.2.281
Xia J, Miu J, Ding H, Wang X, Chen H, Wang J, Wu J, Zhao J, Huang H, Tian W (2013) Changes of brain gray matter structure in Parkinson’s disease patients with dementia. Neural Regen Res 8(14):1276–1285. doi:10.3969/j.issn.1673-5374.2013.14.004
Compta Y, Ibarretxe-Bilbao N, Pereira JB, Junque C, Bargallo N, Tolosa E, Valldeoriola F, Munoz E, Camara A, Buongiorno M, Marti MJ (2012) Grey matter volume correlates of cerebrospinal markers of Alzheimer-pathology in Parkinson’s disease and related dementia. Parkinsonism Relat Disord 18(8):941–947. doi:10.1016/j.parkreldis.2012.04.028
Lee SH, Kim SS, Tae WS, Lee SY, Lee KU, Jhoo J (2013) Brain volumetry in Parkinson’s disease with and without dementia: where are the differences? Acta Radiol 54(5):581–586. doi:10.1177/0284185113476029
Mak E, Su L, Williams GB, O’Brien JT (2015) Neuroimaging correlates of cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord 21(8):862–870. doi:10.1016/j.parkreldis.2015.05.013
Goldman JG, Stebbins GT, Dinh V, Bernard B, Merkitch D, deToledo-Morrell L, Goetz CG (2014) Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain 137(Pt 3):849–859. doi:10.1093/brain/awt360
Radua J, Mataix-Cols D, Phillips ML, El-Hage W, Kronhaus DM, Cardoner N, Surguladze S (2012) A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry 27(8):605–611. doi:10.1016/j.eurpsy.2011.04.001
Radua J, Rubia K, Canales-Rodriguez EJ, Pomarol-Clotet E, Fusar-Poli P, Mataix-Cols D (2014) Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry 5:13. doi:10.3389/fpsyt.2014.00013
Sheng L, Ma H, Zhong J, Shang H, Shi H, Pan P (2015) Motor and extra-motor gray matter atrophy in amyotrophic lateral sclerosis: quantitative meta-analyses of voxel-based morphometry studies. Neurobiol Aging 36(12):3288–3299. doi:10.1016/j.neurobiolaging.2015.08.018
Zhong J, Pan P, Dai Z, Shi H (2014) Voxelwise meta-analysis of gray matter abnormalities in dementia with Lewy bodies. Eur J Radiol 83(10):1870–1874. doi:10.1016/j.ejrad.2014.06.014
Pan PL, Shi HC, Zhong JG, Xiao PR, Shen Y, Wu LJ, Song YY, He GX, Li HL (2013) Gray matter atrophy in Parkinson’s disease with dementia: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci 34(5):613–619. doi:10.1007/s10072-012-1250-3
Melzer TR, Watts R, MacAskill MR, Pitcher TL, Livingston L, Keenan RJ, Dalrymple-Alford JC, Anderson TJ (2012) Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry 83(2):188–194. doi:10.1136/jnnp-2011-300828
Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, Price CJ (2009) The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain 132(Pt 12):3401–3410. doi:10.1093/brain/awp273
Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE (2007) Superior temporal gyrus, language function, and autism. Dev Neuropsychol 31(2):217–238. doi:10.1080/87565640701190841
Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16(2):331–348. doi:10.1006/nimg.2002.1087
Dupont S (2002) Investigating temporal pole function by functional imaging. Epileptic Disord Int Epilepsy J Videotape 4(Suppl 1):S17–S22
Henry JD, Crawford JR (2004) Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc JINS 10(4):608–622. doi:10.1017/s1355617704104141
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, Calopa M, Jauma S, Juncadella M, Junque C (2009) Correlations between gray matter reductions and cognitive deficits in dementia with Lewy Bodies and Parkinson’s disease with dementia. Mov Disord 24(12):1740–1746. doi:10.1002/mds.22488
Braak H, Braak E (2000) Pathoanatomy of Parkinson’s disease. J Neurol 247(Suppl 2):ii3–ii10
Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M (2000) Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson’s disease. Acta Neuropathol 100(3):285–290
Ibarretxe-Bilbao N, Junque C, Segura B, Baggio HC, Marti MJ, Valldeoriola F, Bargallo N, Tolosa E (2012) Progression of cortical thinning in early Parkinson’s disease. Mov Disord 27(14):1746–1753. doi:10.1002/mds.25240
Compta Y, Pereira JB, Rios J, Ibarretxe-Bilbao N, Junque C, Bargallo N, Camara A, Buongiorno M, Fernandez M, Pont-Sunyer C, Marti MJ (2013) Combined dementia-risk biomarkers in Parkinson’s disease: a prospective longitudinal study. Parkinsonism Relat Disord 19(8):717–724. doi:10.1016/j.parkreldis.2013.03.009
Greene SJ, Killiany RJ (2010) Subregions of the inferior parietal lobule are affected in the progression to Alzheimer’s disease. Neurobiol Aging 31(8):1304–1311. doi:10.1016/j.neurobiolaging.2010.04.026
Pennanen C, Testa C, Laakso MP, Hallikainen M, Helkala EL, Hanninen T, Kivipelto M, Kononen M, Nissinen A, Tervo S, Vanhanen M, Vanninen R, Frisoni GB, Soininen H (2005) A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry 76(1):11–14. doi:10.1136/jnnp.2004.035600
Apostolova LG, Morra JH, Green AE, Hwang KS, Avedissian C, Woo E, Cummings JL, Toga AW, Jack CR Jr, Weiner MW, Thompson PM (2010) Automated 3D mapping of baseline and 12-month associations between three verbal memory measures and hippocampal atrophy in 490 ADNI subjects. Neuroimage 51(1):488–499. doi:10.1016/j.neuroimage.2009.12.125
Rousseaux MW, Marcogliese PC, Qu D, Hewitt SJ, Seang S, Kim RH, Slack RS, Schlossmacher MG, Lagace DC, Mak TW, Park DS (2012) Progressive dopaminergic cell loss with unilateral-to-bilateral progression in a genetic model of Parkinson disease. Proc Natl Acad Sci USA 109(39):15918–15923. doi:10.1073/pnas.1205102109
Junque C, Ramirez-Ruiz B, Tolosa E, Summerfield C, Marti MJ, Pastor P, Gomez-Anson B, Mercader JM (2005) Amygdalar and hippocampal MRI volumetric reductions in Parkinson’s disease with dementia. Mov Disord 20(5):540–544. doi:10.1002/mds.20371
Apostolova LG, Beyer M, Green AE, Hwang KS, Morra JH, Chou YY, Avedissian C, Aarsland D, Janvin CC, Larsen JP, Cummings JL, Thompson PM (2010) Hippocampal, caudate, and ventricular changes in Parkinson’s disease with and without dementia. Mov Disord 25(6):687–695. doi:10.1002/mds.22799
Zarei M, Ibarretxe-Bilbao N, Compta Y, Hough M, Junque C, Bargallo N, Tolosa E, Marti MJ (2013) Cortical thinning is associated with disease stages and dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 84(8):875–881. doi:10.1136/jnnp-2012-304126
Wang WY, Yu JT, Liu Y, Yin RH, Wang HF, Wang J, Tan L, Radua J, Tan L (2015) Voxel-based meta-analysis of grey matter changes in Alzheimer’s disease. Transl Neurodegener 4:6. doi:10.1186/s40035-015-0027-z
Jellinger KA (2005) Alpha-synuclein lesions in normal aging, Parkinson disease, and Alzheimer disease: evidence from the Baltimore Longitudinal Study of Aging (BLSA). J Neuropathol Exp Neurol 64(6):554
Du AT, Schuff N, Amend D, Laakso MP, Hsu YY, Jagust WJ, Yaffe K, Kramer JH, Reed B, Norman D, Chui HC, Weiner MW (2001) Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 71(4):441–447
Ramirez-Ruiz B, Marti MJ, Tolosa E, Bartres-Faz D, Summerfield C, Salgado-Pineda P, Gomez-Anson B, Junque C (2005) Longitudinal evaluation of cerebral morphological changes in Parkinson’s disease with and without dementia. J Neurol 252(11):1345–1352. doi:10.1007/s00415-005-0864-2
Ibarretxe-Bilbao N, Ramirez-Ruiz B, Tolosa E, Marti MJ, Valldeoriola F, Bargallo N, Junque C (2008) Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J Neurol 255(9):1324–1331. doi:10.1007/s00415-008-0885-8
Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT (2004) Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127(Pt 4):791–800. doi:10.1093/brain/awh088
Ramirez-Ruiz B, Marti MJ, Tolosa E, Gimenez M, Bargallo N, Valldeoriola F, Junque C (2007) Cerebral atrophy in Parkinson’s disease patients with visual hallucinations. Eur J Neurol 14(7):750–756. doi:10.1111/j.1468-1331.2007.01768.x
Acknowledgments
We thank all the authors of the included studies. We especially thank Dr. Tracy Melzer for his kind help and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest concerning this study.
Rights and permissions
About this article
Cite this article
Xu, Y., Yang, J., Hu, X. et al. Voxel-based meta-analysis of gray matter volume reductions associated with cognitive impairment in Parkinson’s disease. J Neurol 263, 1178–1187 (2016). https://doi.org/10.1007/s00415-016-8122-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8122-3