Abstract
Despite the potential immediate access to diagnosis and care, in-hospital stroke (IHS) is associated with delay in diagnosis, lower rates of reperfusion treatment, and unfavorable outcome. Endovascular reperfusion therapy has shown promising results in recent trials for community-onset strokes (COS) and is limited by less contraindications than systemic thrombolysis. Thus, endovascular approaches may offer additional acute treatment options for IHS. We performed a retrospective, observational monocentric analysis of patients with acute ischemic stroke between January 2010 and December 2014. Out of 3506 acute ischemic strokes, 331 (9.4 %) were IHS. In-hospital mortality (31.4 vs. 8.0 %) and duration of stay after stroke (19.5 vs. 12.1 days) were higher in IHS than in COS. Most IHS occurred in cardiologic and cardiosurgical patients after catheterization or surgery. In 111 cases (33.5 %) the time of onset could not be established as a result of sedation or delayed referral resulting in delayed symptom recognition. 52 IHS (15.7 %) and 828 COS (26.0 %, p < 0.001) patients received any kind of reperfusion therapy, of which 59.6 % (IHS) and 12.1 % (COS) comprised isolated endovascular interventions (p < 0.001). Intra-hospital delays (time to brain imaging, systemic thrombolysis, and angiography) were longer and outcome parameters (mRS d90, in-hospital mortality, length of stay) were worse in IHS, whereas rates of procedural complications and intracranial hemorrhages were similar in both groups. The overall rate of reperfusion treatment is lower in IHS compared to COS, as IHS patients are less likely to be eligible for systemic thrombolysis. Interventional stroke treatment is a safe and feasible therapeutic option for patients who are not eligible for systemic thrombolysis and should be anticipated whenever IHS is diagnosed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute ischemic stroke (AIS) which occurs in hospitalized patients [“In-hospital stroke” (IHS)] is reported to have a proportion between 2.2 and 17 % of all strokes [1, 2]. It is associated with an overall unfavorable clinical outcome, a higher mortality, and a lower adherence-to-guidelines treatment compared to community-onset stroke (COS) [1]. It has been shown that essential time intervals, such as onset of symptoms to imaging and initiation of therapy, often last longer in IHS than in COS despite omission of the pre-hospital phase [3]. This is problematic because of the general time dependency of treatment benefit in AIS [4, 5],.
Patients suffering from IHS are more prone to contraindications for the application of systemic rtPA [6], for example due to prior major surgery [2]. However, increasing experiences with the application of systemic thrombolysis and endovascular revascularization approaches also show that the presence of contraindications does not always equal treatment inaccessibility [7]. Compared with systemic thrombolysis, mechanical endovascular revascularization approaches for the treatment of large vessel occluding (LVO) AIS are not limited by relevant contraindications [8]. This holds especially true for stent-assisted thrombectomy procedures which lately showed promising results regarding revascularization rates and clinical outcome in randomized clinical trials (RTCs) of COS (MR CLEAN [9], ESCAPE [10], EXTEND-IA [11], SWIFT PRIME [12], REVASCAT [13]). We hypothesized that a significant fraction of IHS patients would be eligible for endovascular treatment, if symptoms were recognized in time. The objective of this retrospective study was to identify patients at risk and reasons for possible delays in IHS patients to improve quality of care for IHS.
Methods
Data acquisition
We performed a retrospective analysis of IHS, which occurred to patients at the University Hospital RWTH Aachen/Germany (number of beds (2013): 1400; number of annual fulltime in-hospital treatments (2013): approx. 45,000) between January 2010 and December 2014. The reference group of all acute ischemic strokes within the study period was identified by enquiry of the International Classification of Disease 10 German Modification (ICD-10-GM). Following ICD10 codes were applied: I63.0, I63.1, I63.2, I63.3, I63.4, I63.5, I63.6, I63.8, I63.9, I64. All stroke patients with these codes as main diagnosis were regarded to be COS patients. The remaining cases were evaluated individually and identified as IHS via patients’ medical records. To identify the population with high risk for IHS, the underlying condition, admitting department and hospital ward, preceding interventions and surgeries, vascular risk factors, and transfer to neurological ward were collected. Data of patients who received any kind of reperfusion treatment were also retrieved from a local database, which was initiated as part of the ENDOSTROKE registry trial (ClinicalTrails.gov NCT1399762). These included the National Institute of Health Stroke Scale (NIHSS) [14] and modified Rankin Scale (mRS) [15] at admission, mRS at discharge and after 90 days (mRS d90), procedural complications, time intervals (onset of symptoms to emergency department, door-to-imaging, door-to-needle, door-to-puncture), stroke etiology (modified classification of the Trial of Org 10172, TOAST [16]) as well as vascular risk factors. Follow-up (mRS d90 ± d10) was performed via a standardized, structured telephone interview [17], if informed consent was given. Ethics approval for the retrospective analyses was granted by the ethics board of the medical faculty of RWTH Aachen University.
Primary end-points of our analyses were the rate of reperfusion therapy and the choice of treatment method. In-hospital mortality and functional outcome after 90 days (mRS d90) were defined as secondary end-points.
Procedures
At the University Hospital RWTH Aachen Tertiary Stroke Centre COS patients were treated according to national and international guidelines on a certified Stroke Unit (SU) or neurological Intensive Care Unit (ICU). For the evaluation of IHS patients, the neurologist on call or SU neurologist was contacted as an emergency consultant. Acute reperfusion therapy consisted of systemic thrombolysis with recombinant tissue Plasminogen Activator (rtPA), isolated endovascular therapy or combined (“bridging”) approaches. Acute endovascular therapy with stent-assisted mechanical thrombectomy (using Solitaire™ FR Revascularization Device, Covidien, Dublin, Ireland; Trevo© XP ProVue Retriever 4 mm, Stryker neurovascular, Fremont, California and others) has been established as a routine procedure in our clinic since the beginning of the study period. A consensual decision of a vascular neurologist and an interventional neuroradiologist determined the method of treatment. All patients received brain imaging before and within 24 h after acute reperfusion therapy. Post-interventional intracranial hemorrhages (parenchymal hemorrhages according to ECASS classification [18] and subarachnoid hemorrhages) were registered from the beginning of reperfusion treatment to discharge. Procedural complications and intracranial hemorrhages were regarded as clinically relevant if they made further medical intervention necessary (e.g., transfusion after hemorrhage) or if there was a neurological deterioration of equal or more than 4 points on the NIHSS.
Statistical analysis
Patient characteristics were compared between IHS and COS groups applying Pearson-Chi2-tests for nominal parameters and Wilcoxon rank-sum tests for metric parameters for univariate analysis. Logistic regression was used for multivariate analysis. p values were two sided with p < 0.05 being considered statistically relevant. Values are expressed as means if not stated differently. The completeness of documentation of the mentioned parameters reached over 80 % with the exception of mRS d90, which was reported in 39 (75 %) of IHS and 647 (78.1 %) of COS. If a parameter was missing, the patient was excluded from the corresponding analysis. If the remaining data was available, the patient was still included in further tests. Analyses were performed using IBM SPSS Statistics 22 (IBM, Armonk, USA).
Results
Baseline characteristics
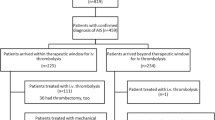
Between January 2010 and December 2014, 3506 patients with AIS were treated in our institution. 331 were identified as IHS (9.4 %). IHS occurred at a significantly younger age than COS (Table 1). However, patients after IHS remained hospitalized longer than after COS (mean 19.5 vs. 12.1 days, p < 0.001, Table 1), had a significantly higher in-house mortality (31.4 % vs. 8.0 %) and were less likely to be discharged home (29.6 vs. 63.4 %, p < 0.001, Table 1).
In-house demographics
More than half of the IHS patients had a cardiac (51.1 %) or vascular disease (9.1 %) as underlying conditions. Among them coronary artery disease (13.6 %) and myocardial infarction (16.6 %) were the most common ones (Table 2). At the time of stroke onset 59.5 % of patients were treated on intermediate care wards (IMC) or intensive care units (ICU) with 52.3 % of the latter being ventilated. Consequently, most IHS occurred within the departments of cardiology (32.0 %) and cardiothoracic surgery (25.7 %). Other frequently affected departments were neurosurgery (10.0 %), vascular surgery (5.7 %), general surgery (4.5 %), and other departments of internal medicine (gastroenterology 5.4 %, hematology/oncology 3.9 %, nephrology 2.4 %). A majority of patients had a history of surgery (49.2 %), or endovascular or endoscopic intervention (22.7 %) within 4 weeks prior to the IHS. Mean time between surgery and stroke was 4.6 days (median 2, IQR 1-6, SD 6.1 days). Mean time between intervention and stroke was 2.1 days (median 1, IQR 0-3, SD 2.7 days).
Reperfusion treatment
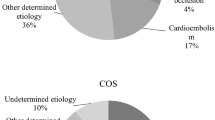
Following IHS, a significantly smaller percentage of patients received reperfusion treatment compared to patients suffering from COS (Table 3). Endovascular interventions (n = 348) were performed in 71.2 % (n = 37/52) of IHS and in 37.6 % (n = 311/828) of COS patients who received reperfusion therapy (p < 0.001). Modern stent retrievers were used in 93.4 % of these cases (n = 325/348). In IHS endovascular interventions without IV rtPA made up for 59.6 % (n = 31/37) of reperfusions compared to 12.1 % (n = 100/311) in COS (p < 0.001). Etiology of stroke (TOAST) was more likely to be cardioembolic and undetermined in the IHS group (Table 3). Angiographic data were available in 94.2 % (n = 49/52) of IHS and 94.8 % (n = 785/828) of COS that received reperfusion therapies. Large vessel occlusion of the anterior circulation was significantly more frequent in IHS (Table 3). Available data from patients who received reperfusion therapy suggest that time intervals from symptom-onset to brain imaging, IV rtPA and angiography were shorter after IHS (Table 3) due to the omission of the pre-hospital interval [symptom-to-arrival time in COS (min), mean ± SD: 93.1 ± 63.3, median (IQR):73 (48–125)]. However, in 111 cases of IHS (33.5 %) the time of stroke onset was indefinable due to sedation, delayed report on symptoms by the patient or atypical presentation. Furthermore, intra-hospital delays from symptom-onset (IHS), respectively, arrival to the clinic (COS) to the different end-points were significantly longer in the IHS group: time to brain imaging (73.3 vs. 30.9 min p < 0.001), to IV rtPA (84.9 vs. 37.9 min, p < 0.001), and to angiography (124.2 vs. 91.8 min, p = 0.008). Since intervals from CT to thrombolysis (IHS/COS 20.1 ± 15.9 vs. 18.7 ± 23.9 min, p = 0.444) and CT to angiography (IHS/COS 65.8 ± 31.2 vs. 61.75 ± 37.0 min, p = 0.235) were comparable in both groups, delays in the IHS rescue chain can be attributed to prolonged symptom recognition and transportation to imaging. There was greater stroke severity determined by NIHSS at onset (mean 14.9 vs. 11.9, p = 0.005, Table 3) and a statistically non-significant trend towards higher functional impairment determined by mRS in IHS on admission/symptom-onset (mean 4.27 vs. 3.94, p = 0.056, Table 3). After 90 days, mRS of 75 % (IHS) and 78.1 % (COS) could be determined. There was no significant difference in stroke severity at onset in this subgroup. A higher percentage of patients with IHS was currently treated with antiplatelet therapy (APT) (52.1 vs. 33.5 %, dual APT: 12.5 vs. 1.7 %, p < 0.001) and therapeutic anticoagulation (27.1 vs. 7.2 %, p < 0.001) prior to reperfusion therapy.
Outcome
35.7 % patients with IHS (n = 117) were transferred to a Stroke Unit (SU) or Neurological Intensive Care Unit (ICU). After reperfusion treatment, 80.8 % of IHS patients (n = 42) were transferred. Following IHS, patients remained hospitalized significantly longer and were less likely to be discharged home (Table 1). In-hospital mortality was three times higher for patients with IHS (Table 1). In 44.2 % of deceased IHS patients, death was a direct consequence of the additional cerebrovascular event (n = 46). Good functional outcome at follow-up (mRS d90 ≤ 2) was achieved less common in IHS than in COS patients without reaching statistical significance (IHS/COS 25.6 vs. 40.7 %, p = 0.063). After adjusting for patient demographics, vascular risk factors, and stroke severity, mortality was still higher in the IHS group (OR 3.11; CI 1.09–8.88; p = 0.034). There were no significant differences in occurrence of intracranial hemorrhages (ICH) during and after treatment between the IHS and COS groups (Table 4). In total, in 36 cases procedural complications or ICH were the cause of clinical worsening or death (Table 4).
Discussion
The presented analyses showed that substantial numbers of ischemic strokes occur in hospitalized patients and that despite younger age they are associated with greater stroke severity, lower rates of reperfusion therapies, higher in-hospital mortality, and worse functional outcome compared to patients with COS.
Our finding of 9.4 % IHS of all strokes is in line with previous publications, which reported rates between 2.2 and 17 % depending on hospital characteristics and inclusion criteria. Multicentre studies including regional hospitals seem to report lower [1, 3], and university hospitals as well as tertiary centers relatively report higher percentages of IHS [2, 19]. This is most likely attributed to differences in frequencies of high-risk procedures and multi-morbidity of admitted patients. The RWTH University Hospital Aachen is part of the latter category, performing approx. 1400 percutaneous coronary interventions, 700 coronary artery bypass grafts, and 90 carotid endarterectomies per year (2012). The higher prevalence of (cardio-)vascular diseases in men (especially at younger age) [20, 21], and the high percentage of the above-mentioned procedures in patients with IHS most likely explains the male preponderance.
In our cohort, IHS was associated with a high rate of in-hospital mortality. To some extent this is explained by the higher incidence of LVO in IHS (as derived by the prevailing cardioembolic stroke mechanism), which results in more severe strokes. The second relevant factor is the original underlying disease, which contributed to over 50 % of deaths in our IHS cohort.
There were several reasons which contributed to the relatively low rate of reperfusion therapies in patients with IHS compared to patients with COS. One major constraint for IHS treatment was that stroke symptoms often were not recognized early enough to start reperfusion therapies within the recommended time frame (<4.5 h for IV rtPA [22]; <6 h for endovascular therapy [9]). In our cohort, more than one-third of IHS was diagnosed with a significant delay. In most cases, symptom-onset could not be determined due to sedation and insufficient neurological assessment by non-neurological staff. The latter point also contributes to considerable delays in diagnostic and therapeutic intra-hospital procedural intervals. Another important reason for non-treatment was that nearly half of the patients underwent major surgery within 4 weeks before the occurrence of IHS and therefore in many cases application of systemic rtPA was contraindicated. Other frequent and relevant contraindications were prior ST-segment elevation myocardial infarction, therapeutic and sufficient anticoagulant as well as rejection of therapy due to terminal illness or overall unfavorable prognosis. Most of the mentioned medical contraindications for systemic thrombolysis play a tangential role for interventional treatment [9]. Accordingly, IHS patients received isolated stent-assisted thrombectomy more frequently than COS patients.
The high proportion of IHS patients who receive interventional stroke treatment has important implications for in-house protocols. Strategies to improve quality of care of IHS usually comprise education of medical staff about signs and treatment options of stroke and standardization of in-house procedures such as alert processes [23–26]. Our algorithm (Fig. 1) suggests a specific training of nursing and medical personnel treating patients with high risk of IHS, such as cardiologic and cardiosurgical IMCs and ICUs, highlighting basic and regular neurologic examination, and knowledge of standardized diagnostic pathway if stroke is suspected. A further improvement of this concept is an immediate and pragmatic reunion of all involved parties. When acute IHS is suspected, the currently supervising medical team should immediately alert the neurologist on call and perform transportation to the neuroradiology department while checking potential contraindications for reperfusion treatment. The neurologist (specialized in stroke therapy) examines the patient during preparation for imaging (“CT rendezvous”). A plain CT, CT-angiography, and CT-perfusion should be performed routinely. The treatment decision is made consensually with the neuroradiologist and treatment is started immediately. Given that IHS patients are likely to receive interventional treatment, an angiography team should be in standby to anticipate interventional stroke treatment. Technical advances may offer additional options for early IHS recognition. During surgeries with a high stroke risk, such as carotid endarterectomy and aortic reconstructions, neuromonitoring via electroencephalography (EEG) [27], transcranial Doppler-Ultrasound (TCD) of the middle cerebral artery, and near-infrared spectroscopy of cerebral oxygen saturation (NIRS) [28] proved to facilitate early detection of changes in cerebral blood flow and of ischemia. In the future, continuous neuromonitoring may likewise be established on ICU [29] or other locations with patients at risk and allow for reduction in delays in recognition of IHS.
Algorithm for the management of in-hospital stroke. IHS in-hospital stroke, IV rtPA intavenous recombinant tissue Plasminogen Activator (Alteplase); ICB intracranial bleeding, IS ischemic stroke, CA contrast agent, cCT cranial computed tomography, ccCTA cervico-cranial computed tomography angiography, cCTP cranial computed tomography perfusion, AIS acute ischemic stroke, cMRT cranial magnetic resonance tomography, EEG electroencephalography, SU stroke unit, ICU intensive care unit. Asterisk, i.e., continuous EEG, transcranial Doppler-Ultrasound (TCD) of the middle cerebral artery or near-infrared spectroscopy of cerebral oxygen saturation (NIRS)
Limitations
Limitations to this study were the relatively small sample size and its monocentric data acquisition.
Conclusion
IHS is a severe complication of in-patients and is associated with an unfavorable prognosis. Early symptom recognition is hampered by difficulties in evaluation of critically ill or postoperative patients on ICU or IMC. IHS patients often suffer from LVO and treatment is constrained by contraindications for systemic thrombolysis. In these cases, endovascular interventions offer safe and feasible therapeutic treatment options and should be anticipated whenever IHS patients are encountered.
References
Cumbler E, Wald H, Bhatt DL et al (2014) Quality of care and outcomes for in-hospital ischemic stroke:findings from the National Get With The Guidelines-Stroke. Stroke 45(1):231–238. doi:10.1161/STROKEAHA.113.003617
Dulli D, Samaniego EA (2007) Inpatient and community ischemic strokes in a university hospital. Neuroepidemiology 28(2):86–92. doi:10.1159/000098551
Saltman AP, Silver FL, Fang J et al (2015) Care and outcomes of patients with in-hospital stroke. JAMA Neurol. doi:10.1001/jamaneurol.2015.0284
The ATLANTIS, ECASS, and NINDS rt-PA Study Group Investigators (2004) Association of outcome with early stroke treatment:pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. The Lancet 363(9411):768–774. doi:10.1016/S0140-6736(04)15692-4
Manawadu D, Choyi J, Kalra L (2014) The impact of early specialist management on outcomes of patients with in-hospital stroke. PLoS ONE 9(8):e104758. doi:10.1371/journal.pone.0104758
Alvaro LC, Timiraos J, Sadaba F (2008) In-hospital stroke:clinical profile and expectations for treatment (Accidentes cerebrovasculares intrahospitalarios:perfil clinico y expectativas terapeuticas). Neurologia 23(1):4–9
Frank B, Grotta JC, Alexandrov AV et al (2013) Thrombolysis in stroke despite contraindications or warnings? Stroke 44(3):727–733. doi:10.1161/STROKEAHA.112.674622
Sacks D, Black CM, Cognard C et al (2013) Multisociety Consensus Quality Improvement Guidelines for Intraarterial Catheter-directed Treatment of Acute Ischemic Stroke, from the American Society of Neuroradiology, Canadian Interventional Radiology Association, Cardiovascular and Interventional Radiological Society of Europe, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, European Society of Minimally Invasive Neurological Therapy, and Society of Vascular and Interventional Neurology. AJNR Am J Neuroradiol 34(4):E0
Berkhemer OA, Fransen, Puck SS, Beumer D et al (2015) A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372(1):11–20. doi:10.1056/NEJMoa1411587
Goyal M, Demchuk AM, Menon BK et al (2015) Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 372(11):1019–1030. doi:10.1056/NEJMoa1414905
Campbell Bruce C V, Mitchell PJ, Kleinig TJ et al (2015) Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372(11):1009–1018. doi:10.1056/NEJMoa1414792
Saver JL, Goyal M, Bonafe A et al (2015) Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 372(24):2285–2295. doi:10.1056/NEJMoa1415061
Jovin TG, Chamorro A, Cobo E et al (2015) Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 372(24):2296–2306. doi:10.1056/NEJMoa1503780
Brott T, Adams HP, Olinger CP et al (1989) Measurements of acute cerebral infarction:a clinical examination scale. Stroke 20(7):864–870. doi:10.1161/01.STR.20.7.864
van Swieten JC, Koudstaal PJ, Visser MC et al (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19(5):604–607. doi:10.1161/01.STR.19.5.604
Adams HP, Bendixen BH, Kappelle LJ et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24(1):35–41. doi:10.1161/01.STR.24.1.35
Bruno A, Akinwuntan AE, Lin C et al (2011) Simplified modified rankin scale questionnaire:reproducibility over the telephone and validation with quality of life. Stroke 42(8):2276–2279. doi:10.1161/STROKEAHA.111.613273
Larrue V, von Kummer R, Muller A et al (2001) Risk Factors for Severe Hemorrhagic Transformation in Ischemic Stroke Patients Treated With Recombinant Tissue Plasminogen Activator: a Secondary Analysis of the European-Australasian Acute Stroke Study (ECASS II). Stroke 32(2):438–441. doi:10.1161/01.STR.32.2.438
Kelley RE, Kovacs AG (1986) Mechanism of in-hospital cerebral ischemia. Stroke 17(3):430–433. doi:10.1161/01.STR.17.3.430
Lerner DJ, Kannel WB (1986) Patterns of coronary heart disease morbidity and mortality in the sexes:a 26-year follow-up of the Framingham population. Am Heart J 111(2):383–390
Towfighi A, Zheng L, Ovbiagele B (2009) Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch Intern Med 169(19):1762–1766. doi:10.1001/archinternmed.2009.318
Jauch EC, Saver JL, Adams HP et al (2013) Guidelines for the early management of patients with acute ischemic stroke:a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 44(3):870–947
Rodríguez Campello A, Cuadrado Godia E, Giralt Steinhauer E et al (2014) Detección de ictus intrahospitalario:evaluación de resultados de un programa de formación y entrenamiento a personal médico y de enfermería (Detecting in-hospital stroke: Assessment of results from a training programme for medical personnel). Neurologia. doi:10.1016/j.nrl.2014.06.003
Cumbler E, Zaemisch R, Graves A et al (2012) Improving stroke alert response time:applying quality improvement methodology to the inpatient neurologic emergency. J Hosp Med 7(2):137–141
Cumbler E, Anderson T, Neumann R et al (2010) Stroke alert program improves recognition and evaluation time of in-hospital ischemic stroke. J Stroke Cerebrovasc Dis 19(6):494–496. doi:10.1016/j.jstrokecerebrovasdis.2009.09.007
Hamon M, Baron J, Viader F et al (2008) Periprocedural stroke and cardiac catheterization. Circulation 118(6):678–683. doi:10.1161/CIRCULATIONAHA.108.784504
Ballotta E, Saladini M, Gruppo M et al (2010) Predictors of electroencephalographic changes needing shunting during carotid endarterectomy. Ann Vasc Surg 24(8):1045–1052. doi:10.1016/j.avsg.2010.06.005
Wang X, Ji B, Yang B et al (2012) Real-time continuous neuromonitoring combines transcranial cerebral Doppler with near-infrared spectroscopy cerebral oxygen saturation during total aortic arch replacement procedure:a pilot study. ASAIO J 58(2):122–126. doi:10.1097/MAT.0b013e318241abd3
Herman ST, Abend NS, Bleck TP et al (2015) Consensus statement on continuous EEG in critically ill adults and children, part I:indications. J Clin Neurophysiol 32(2):87–95. doi:10.1097/WNP.0000000000000166
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. M. Wiesmann reports grants and personal fees from Stryker Neurovascular, grants and personal fees from SilkRoad Medical, Grants from Covidien, grants from Microvention, grants and personal fees from Bracco, grants and personal fees from Siemens, grants from AB Medica, grants from Acandis, grants from Codman Neurovascular, grants from Penumbra, Grants from Phenox, grants from Abbott, grants from St. Jude, from B. Braun, outside the submitted work. All other authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Schürmann, K., Nikoubashman, O., Falkenburger, B. et al. Risk profile and treatment options of acute ischemic in-hospital stroke. J Neurol 263, 550–557 (2016). https://doi.org/10.1007/s00415-015-8010-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-8010-2