Abstract
This study elucidates the genotypic and phenotypic spectrum and histopathological findings related to cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) in Japan. For this single-center retrospective observational study, we enrolled 215 patients who were clinically suspected of having CADASIL and were examined at Kumamoto University from 1997 to 2014, and we diagnosed CADASIL in 70 patients. We found 19 different NOTCH3 mutations in the patients, with the NOTCH3 Arg133Cys mutation being found most frequently. We also found the Arg75Pro mutation, a cysteine-sparing NOTCH3 mutation. CADASIL patients with this Arg75Pro mutation were frequently found throughout Japan, and fewer patients with the Arg75Pro mutation showed MRI hyperintensity in the anterior temporal pole compared with patients with other NOTCH3 mutations. Significantly more CADASIL patients with the NOTCH3 Arg133Cys mutation had hyperintensity in the external capsule compared with CADASIL patients with the other mutations not including the NOTCH3 Arg75Pro mutation. We also showed postmortem pathological findings of the first Japanese CADASIL case with the NOTCH3 Arg133Cys mutation, and histopathological findings of fresh frozen skin biopsy specimens of CADASIL patients. In conclusions, the spectrum of NOTCH3 mutations in Japanese CADASIL patients may be partially explained by founder effects. Genotype–phenotype correlations may exist in CADASIL, which should be considered so as to make an accurate diagnosis of CADASIL in each population. Fresh frozen skin biopsy specimens may aid detection of Notch3 deposits on vascular walls for an improved diagnosis of CADASIL.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common type of inherited cerebral small vessel disease [1]. Mutations in the NOTCH3 gene cause deposition in small arteries of granular osmiophilic material (GOM), which is thought to be derived from the extracellular domain of Notch3, and result in progressive degeneration of vascular smooth muscle cells [2–4]. Clinically, CADASIL is associated with migraine, recurrent transient ischemic attacks (TIAs), relapsing stroke, mood disorders, and cognitive decline, which leads eventually to dementia [1, 4–6]. To date, more than 200 mutations of the NOTCH3 gene have been reported, but genotype–phenotype correlations in CADASIL remain poorly understood [1, 4].
CADASIL occurs in many populations throughout the world, and the clinicopathological features and the spectrum of NOTCH3 mutations reportedly vary among different populations [1, 4–8]. In Japan, where people with a relatively uniform genetic background live in an isolated island country, a number of CADASIL kindreds were reported with different NOTCH3 gene mutations [9–13]. However, in these Japanese CADASIL patients, the spectrum of NOTCH3 mutations and the clinicopathological features, which are quite important for the diagnosis of CADASIL, have not as yet been clarified.
In this single-center retrospective observational study which was the first large case series of CADASIL in Japan, we reported data from the first Japanese CADASIL case to present cases. We showed the spectrum of NOTCH3 mutations, genotype–phenotype correlations, and histopathological findings in Japanese CADASIL patients.
Methods
Patients
For this single-center retrospective observational study, we enrolled 215 patients who were clinically suspected of having CADASIL and were examined at Kumamoto University from April 1997 to March 2014. Seventy patients were diagnosed as having CADASIL either by skin biopsy or by genetic testing and were evaluated further (Supplementary Fig. 1).
Definition of clinical features and MRI findings
Clinical and MRI data were available for 51 of 70 CADASIL patients [27 men and 24 women, with a mean age of 50.3 years (SD 10.7)]. Migraine was defined according to the International Headache Society [14]. TIA and stroke were defined according to current criteria of the National Institute of Neurological Disorders and Stroke (NINDS II). Cognitive disorders and psychiatric disorders were defined on the basis of a previous report [15]. We analyzed MRI findings at diagnosis. Severe periventricular hyperintensities (PVHs) were defined as diffuse white matter hyperintensities, corresponding to Fazekas grade 3 [16]. External capsular lesions were defined as demonstrating category 5 or 6 external capsular hyperintensities (the Scheltens scale 5 or 6, corresponding to the presence of at least one lesion of at least 11 mm) [17]. Anterior temporal pole hyperintensities were defined as hyperintensities with a Scheltens score 5 or 6 in temporal white matter, which were confined to the anterior part of the temporal lobe [17].
NOTCH3 gene analysis
We performed PCR amplification followed by direct DNA sequencing with the use of ABI BigDye Terminator reagents (Applied Biosystems, Foster City, California, USA) on the 3130 Genetic Analyzer (Applied Biosystems). We utilized specific primers to evaluate the sequences of exons 3, 4, 5, 6, 7, 8, 11, 12, 17, and 18 of the NOTCH3 gene, in which 136 of 178 (76 %) CADASIL-causing mutations were reported in the Human Gene Mutation Database (HGMD) (http://www.hgmd.cf.ac.uk/ac/index.php) (Supplementary Table 1) [18], using the following primers: exon 3 forward: ATCTTTGTGTCTGGGGCCAT; exon 3 reverse: ACCAGGACAGGGTGAGTTTA; exon 4 forward: GTGGTGTCTGCCAGAGTTCAGT; exon 4 reverse: ATTGAGACATCGGTGTCCTGGA; exons 5–6 forward: GAGCCCTACTCAGGAGAGTCA; exons 5–6 reverse: TCAGACTTCTTATTTGCCCTCA; exons 7–8 forward: GTACTCCAGCTTGGGCAACA; exons 7–8 reverse: CCCACTTACACCCCATTCTG; exons 11–12 forward: ATTGGTCCGAGGCCTCACT; exons 11–12 reverse: CGTTGGACAAGAGTCTGCAA; exons 17–18 forward: TCCTGAATTCTTCTCAATCCAA; and exons 17–18 reverse: GACTTGGCTTCTCCCTCCTA.
Histopathological staining
For immunohistochemistry, we used 10-µm frozen tissue specimens. Endogenous peroxidase activity was eliminated by treatment with 0.5 % periodic acid solution for 10 min. Sections were incubated in blocking buffer (1 % bovine serum albumin and 5 % rabbit serum in phosphate-buffered saline). A monoclonal antibody against the extracellular domain of Notch3, clone 1G5 (Abnova, Taipei, Taiwan), diluted 1:100 in blocking buffer, served as the primary antibody. A horseradish peroxidase-conjugated rabbit anti-mouse IgG antibody, diluted 1:100 in blocking buffer, was used as the secondary antibody. Reactivity was visualized with the DAB Liquid System (DAKO, Glostrup, Denmark), as described by the manufacturer. Sections were counterstained with Victoria Blue. For parallel control sections, primary antibody was replaced with a control antibody, a monoclonal mouse antibody against Amyloid A (clone mc1, DAKO, Glostrup, Denmark), which is expressed predominantly by the liver in response to inflammatory stimuli (Supplementary Fig. 2).
The right side of the formalin-fixed autopsied brain of CADASIL patients with the Arg133Cys mutation was cut into horizontal sections and stained with Klüver-Barrera and Holzer stains. To observe the internal elastic lamina of vascular walls, we utilized Victoria Blue–hematoxylin and eosin staining.
Electron microscopy
Tissue specimens were fixed first in 4 % paraformaldehyde and 1 % glutaraldehyde solution and then in 1 % OsO4, after which they were embedded in Epon according to a previous study [19]. Ultrathin sections were stained with 4 % uranyl acetate and lead citrate and were examined by means of a transmission electron microscope (H-7500; Hitachi, Tokyo, Japan).
Statistical analysis
Data were evaluated via t test or Fisher’s exact test. All analyses were performed with JMP version 5.1 (SAS Institute Japan, Tokyo, Japan). We regarded p values less than 0.05 as statistically significant.
Ethics
The Human Ethics Review Committee of Kumamoto University approved the study protocol. All patients or their family members provided signed consent forms.
Results
Diagnosis of CADASIL and the spectrum of NOTCH3 mutations in Japanese CADASIL patients
We examined 215 patients who were clinically suspected of having CADASIL at Kumamoto University from April 1, 1997 to March 31, 2014. We diagnosed CADASIL in 70 patients, with the diagnosis being confirmed by either skin biopsy or genetic testing (Supplementary Fig. 1). Our genetic analyses were focused on several exons which reportedly contained 136 of 178 (76 %) CADASIL-causing NOTCH3 mutations, based on data reported in HGMD (Supplementary Table 1) [18]. We found 19 different NOTCH3 mutations in these patients (Fig. 1). Thirteen patients had mutations in exon 3; 43 patients, exon 4; 3 patients, exon 6; and 3 patients, exon 8. Three different NOTCH3 mutations—Arg133Cys (23 %), Arg75Pro, an unusual cysteine-sparing NOTCH3 mutation (13 %), and Arg182Cys (13 %)—were found frequently in Japan: 10 of 14 (71 %) CADASIL patients with the Arg133Cys mutation were originally from Kyushu, the southwest island of Japan (Fig. 1). In contrast, the other mutations occurred throughout Japan. Based on two kinds of in silico analyses, polymorphism phenotyping-2 (PolyPhen-2) (http://genetics.bwh.harvard.edu/pph2/index.shtml) [20] and Align GVGD (http://agvgd.iarc.fr/agvgd_input.php) [21], those amino acid substitutions were predicted to cause adverse effects on the structure and function of the Notch3 protein (Supplementary Table 2).
Different clinical and MRI findings in CADASIL patients with the Arg75Pro mutation and in CADASIL patients with other mutations
In Japanese CADASIL patients, TIA or brain infarction was the most frequent finding, and the onset of migraine occurred at a younger age than that of other symptoms (Table 1).
We compared clinical and MRI findings in CADASIL patients with the Arg75Pro mutation, an unusual cysteine-sparing NOTCH3 mutation, with those in CADASIL patients with other mutations (Table 2). The ages at onset and at diagnosis in CADASIL patients with the Arg75Pro mutation tended to be higher than those in CADASIL patients with other mutations, although the differences were not statistically significant. A significant difference between CADASIL patients with the Arg75Pro mutation and those with other mutations was found for hyperintensity in the anterior temporal pole; however, CADASIL patients with the Arg75Pro mutation were not significantly different from CADASIL patients with other mutations with regard to other MRI findings and clinical symptoms (Fig. 2; Table 2). In this study, we found hyperintensity in the anterior temporal pole in 36 of 51 (71 %) CADASIL patients. CADASIL patients without hyperintensity in the anterior temporal pole had a significant association with the genotype Arg75Pro, while the hyperintensity in the anterior temporal pole was not significantly associated with age, gender, and clinical symptoms (Supplementary Table 3).
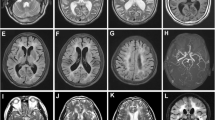
Characteristic MRI findings in CADASIL. a–d FLAIR images for a 63-year-old man with the NOTCH3 Arg141Cys mutation. e–h FLAIR images for a 57-year-old woman with the NOTCH3 Arg75Pro mutation. a Arrows indicate hyperintensities in the anterior temporal pole. b, f Arrows indicate hyperintensities in the external capsule. c, d, g, h Arrows indicate PVH. Arrowheads indicate multiple WMIs. CADASIL cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, PVH periventricular hyperintensity, WMIs white matter injuries
Different clinical and MRI findings in CADASIL patients with the NOTCH3 Arg133Cys mutation and in CADASIL patients with other mutations not including the NOTCH3 Arg75Pro mutation
As shown in Table 3, CADASIL patients with the NOTCH3 Arg133Cys mutation, who were often found in the Kyushu area of Japan (Fig. 1), showed clinical symptoms similar to those of CADASIL patients with other mutations not including the Arg75Pro mutation. Although the frequency of three MRI findings—PVH, hyperintensity in the anterior temporal pole, and multiple WMIs—did not differ in these two groups of patients, significantly more CADASIL patients with the NOTCH3 Arg133Cys mutation had hyperintensity in the external capsule compared with CADASIL patients with the other mutations not including the NOTCH3 Arg75Pro mutation (Table 3). In this study, we found hyperintensity in the external capsule in 33 of 44 (75 %) CADASIL patients not including the patients having the Arg75Pro mutation. CADASIL patients with hyperintensity in the external capsule had a significant association with the genotype Arg133Cys and clinical symptoms of TIA or brain infarction (Supplementary Table 4).
Postmortem findings in the first Japanese CADASIL patient with the NOTCH3 Arg133Cys mutation
In this study, we also provide MRI and postmortem findings for the first Japanese CADASIL patient with the NOTCH3 Arg133Cys mutation (Fig. 3a–i). At age 44 years, his intelligence and physical movement were impaired. He developed dementia at age 51 years. He had severe pseudobulbar palsy, bilateral spastic paresis, recurrent generalized convulsions, and akinetic mutism at age 53 years. Genetic testing revealed that he had the NOTCH3 Arg133Cys mutation. His symptoms worsened in a stepwise progression, and he died at age 62 years. Postmortem analyses revealed microbleeds, multiple WMIs, myelin loss, gliosis, structural changes in the cerebral vessel walls, microaneurysms, enlarged extracellular spaces, and Notch3 and GOM deposits on the walls of the small arteries of the brain (Fig. 3c–i).
Histopathological findings in CADASIL. a–i MRI and postmortem findings of the first Japanese CADASIL patient with the NOTCH3 Arg133Cys mutation. j–l Histopathological findings in the walls of the skin’s blood vessels in CADASIL patients. a, b FLAIR images of the patient with an advanced stage of CADASIL at the age of 62. c T2* image showing microbleeds in the autopsied brain section. d Macroscopic image of the autopsied brain section. e Myelin loss in the autopsied brain section as shown by the Klüver-Barrera stain. f Gliosis in the autopsied brain section as shown by the Holzer stain. g Structural changes in cerebral vessel walls in the globus pallidus as shown by Victoria Blue-hematoxylin and eosin staining. h Granular deposits of the extracellular domain of Notch3 in cerebral vessel walls as detected in fresh frozen specimens via immunohistochemical staining using an anti-extracellular domain of Notch3 antibody (clone 1G5) counterstained with hematoxylin. i Electron microscopic images of meningeal blood vessel walls of the brain. Arrowheads indicate GOM and GOM clusters. j, k Granular deposits of the extracellular domain of Notch3 on the walls of the skin’s blood vessels in fresh frozen biopsy specimens from two CADASIL patients (j, a 62-year-old man with the NOTCH3 Arg133Cys mutation, and k, a 51-year-old man with the NOTCH3 Arg75Pro mutation), as detected by immunohistochemical staining using the anti-extracellular domain of Notch3 antibody (clone 1G5) counterstained with Victoria Blue. l Electron microscopic image of the walls of the skin’s blood vessels from a 51-year-old CADASIL patient with the NOTCH3 Arg75Pro mutation. Arrowheads indicate GOM and GOM clusters. CADASIL cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, GOM granular osmiophilic material
Highly sensitive detection, in fresh frozen skin biopsy specimens, of granular deposits of the extracellular domain of Notch3 on vascular walls in CADASIL patients.
GOM and Notch3 deposition on vascular walls is a hallmark of CADASIL. In this study, we investigated deposition of the extracellular domain of Notch3 on the walls of the skin’s small blood vessels in 57 patients who were clinically suspected of having CADASIL. We found granular Notch3 deposits in fresh frozen skin biopsy specimens from all 16 CADASIL patients (10 patients with NOTCH3 mutations in exon 3 or 4, and 6 patients with rare NOTCH3 mutations in exon 6 or 8) (Supplementary Fig. 1, Fig. 3j, k). We also clearly found Notch3 deposits and GOM on the walls of the skin’s blood vessels in CADASIL patients with a cysteine-sparing NOTCH3 mutation, Arg75Pro (Fig. 3k, l). In addition, we detected Notch3 deposits on the walls of the skin’s blood vessels in 8 patients who did not have NOTCH3 mutations in exons examined by limited genetic analyses of the NOTCH3 gene (Supplementary Fig. 1). To confirm specificity of the immunohistochemistry using an antibody against the extracellular domain of Notch3, we used a control primary antibody and negative control sections (Supplementary Fig. 2).
Discussion
In this study, the first large case series of CADASIL in Japan, we reported data from our referral center experiment, from the first Japanese CADASIL case to present cases. Although our genetic analyses were limited to several exons which reportedly contained the mutation hot spot of NOTCH3 in CADASIL, NOTCH3 mutations in our CADASIL patients were most frequently detected in exon 4 followed by exon 3, findings that are consistent with those for most other populations [1, 6, 22, 23]. CADASIL patients with the Arg133Cys mutation, the mutation most frequently found at our referral center, were usually from the Kyushu district of Japan, which suggests a founder effect of the mutation. Mykkänen et al. [24] reported that this Arg133Cys mutation was also most frequently detected in Finnish CADASIL families and observed a similar haplotype linked to the NOTCH3 Arg133Cys mutation in all of the Finnish pedigrees, which suggests a single common ancestor.
A cysteine-sparing NOTCH3 mutation, Arg75Pro, was also frequently found throughout Japan. Although whether patients with several cysteine-sparing NOTCH3 mutations develop CADASIL remains to be clarified [1, 10, 13, 25–29], we clearly showed patients with this Arg75Pro mutation had deposits of the extracellular domain of Notch3 on vascular walls, which indicates that those patients are developing CADASIL. The Arg75Pro mutation was reported in Korean and Japanese CADASIL patients but was not found in Chinese and Caucasian patients [13, 23]. These results suggest that another founder effect of this mutation may exist in Far East Asia.
As one interesting result, we observed genotype–phenotype correlations in Japanese CADASIL patients, although genotype–phenotype correlations were reportedly unclear in Caucasian CADASIL patients [1, 4, 6]. CADASIL patients with the NOTCH3 Arg75Pro mutation had relatively late-onset disease and showed less hyperintensity in MRI studies of the anterior temporal pole. This genotype-related MRI feature is consistent with previous case series studies of Korean and Japanese CADASIL patients with the NOTCH3 Arg75Pro mutation [13, 23]. Although most NOTCH3 mutations causing CADASIL were reportedly associated with the gain or loss of a cysteine residue, which led to an odd number of cysteine residues [1], the Arg75Pro mutation does not change the number of cysteine residues. The Arg75Pro substitution is thought to affect the position of just the next cysteine residue at position 76, which may induce the disease-causing conformational change in Notch3 [13]. The milder phenotypes of CADASIL patients with the Arg75Pro mutation suggest that a conformational change in Notch3 induced by the Arg75Pro mutation may be smaller than that induced by other substitutions that lead to an odd number of cysteine residues in Notch3. To clarify detailed clinicopathological features of CADASIL patients with the Arg75Pro mutation, we need further investigation with a large number of patients.
Also, more CADASIL patients with the Arg133Cys mutation had hyperintensity in the external capsule than did CADASIL patients with the other mutations not including the Arg75Pro mutation. Although the mechanisms of these genotype–phenotype correlations in Japanese CADASIL patients remain to be fully understood, genotype–phenotype correlations should be considered so as to make an accurate diagnosis of CADASIL in each population. In addition, we should take into account that those MR alterations in CADASIL may progress over time.
Limitation of this study is that our genetic analyses for NOTCH3 were incomprehensive. Our genetic analyses were focused on several exons which reportedly contained the mutation hot spot of NOTCH3 in CADASIL. Based on the list of CADASIL-causing NOTCH3 mutations (Supplementary Table 1), 42 of 178 (24 %) mutations could be missed by our limited genetic analyses. We cannot exclude completely the possibility of sampling bias.
In this study, we used fresh frozen skin biopsy specimens for improved detection of granular deposits of the extracellular domain of Notch3 on vessel walls by immunohistochemical staining. These deposits are thought to be a diagnostic hallmark of CADASIL, as are GOM deposits [1, 4, 30, 31]. Although the few studies of CADASIL patients that utilized immunohistochemical staining of Notch3 demonstrated higher sensitivity of that technique compared with electron microscopic examination of GOM, ranging from 45 to 90 % [23, 32, 33], Lesnik Oberstein et al. [31] reported almost 10 % false-negative results for the immunohistochemical staining of Notch3 with formalin-fixed, paraffin-embedded skin biopsy specimens. In the present study, we observed clear granular deposits of Notch3 on the walls of the skin’s blood vessels in all 16 CADASIL patients with confirmed NOTCH3 mutations. Fresh frozen skin biopsy specimens may thus be better suited than formalin-fixed, paraffin-embedded specimens for detecting deposition of the extracellular domain of Notch3 on vessel walls. To elucidate the actual sensitivity and specificity of this method, prospective studies should be performed in CADASIL and non-CADASIL patients who have confirmed genetic mutations of NOTCH3 and GOM deposits as determined via electron microscopy.
In conclusion, we reported data from our referral center experience in Japan, from the first Japanese CADASIL case to present cases. The spectrum of NOTCH3 mutations in Japanese CADASIL patients may be partially explained by founder effects. Genotype–phenotype correlations may exist in CADASIL, which should be considered so as to make an accurate diagnosis of CADASIL in each population. Fresh frozen skin biopsy specimens may aid detection of Notch3 deposits on vascular walls for an improved diagnosis of CADASIL.
References
Chabriat H, Joutel A, Dichgans M et al (2009) CADASIL. Lancet Neurol 8:643–653
Joutel A, Corpechot C, Ducros A et al (1996) Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature 383:707–710
Joutel A, Andreux F, Gaulis S et al (2000) The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest 105:597–605
Tikka S, Baumann M, Siitonen M et al (2014) CADASIL and CARASIL. Brain Pathol 24:525–544
Pescini F, Nannucci S, Bertaccini B et al (2012) The cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) scale: a screening tool to select patients for NOTCH3 gene analysis. Stroke 43:2871–2876
Opherk C, Peters N, Herzog J et al (2004) Long-term prognosis and causes of death in CADASIL: a retrospective study in 411 patients. Brain 127:2533–2539
Kim YE, Yoon CW, Seo SW et al (2014) Spectrum of NOTCH3 mutations in Korean patients with clinically suspicious cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Neurobiol Aging 35:726.e1–726.e6
Lee YC, Liu CS, Chang MH et al (2009) Population-specific spectrum of NOTCH3 mutations, MRI features and founder effect of CADASIL in Chinese. J Neurol 256:249–255
Uyama E, Tokunaga M, Suenaga A et al (2000) Arg133Cys mutation of Notch3 in two unrelated Japanese families with CADASIL. Intern Med 39:732–737
Uchino M, Hirano T, Uyama E et al (2002) Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) and CADASIL-like disorders in Japan. Ann N Y Acad Sci 977:273–278
Abe K, Murakami T, Matsubara E et al (2002) Clinical Features of CADASIL. Ann N Y Acad Sci 977:266–272
Ishida C, Sakajiri K, Yoshita M et al (2006) CADASIL with a novel mutation in exon 7 of NOTCH3 (C388Y). Intern Med 45:981–985
Mizuno T, Muranishi M, Torugun T et al (2008) Two Japanese CADASIL families exhibiting Notch3 mutation R75P not involving cysteine residue. Intern Med 47:2067–2072
Headache Classification Committee of the International Headache Society (IHS) (2013) The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33:629–808
Pantoni L, Pescini F, Nannucci S et al (2010) Comparison of clinical, familial, and MRI features of CADASIL and NOTCH3-negative patients. Neurology 74:57–63
Fazekas F, Chawluk JB, Alavi A et al (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Neuroradiol 8:421–426
Scheltens P, Barkhof F, Valk J et al (1992) White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease. Evidence for heterogeneity. Brain 115:735–748
Stenson PD, Mort M, Ball EV et al (2014) The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet 133:1–9
Ueda A, Hirano T, Takahashi K et al (2009) Detection of granular osmiophilic material of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy by light microscopy in frozen sections. Neuropathol Appl Neurobiol 35:618–622
Adzhubei IA, Schmidt S, Peshkin L et al (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249
Tavtigian SV, Deffenbaugh AM, Yin L et al (2006) Comprehensive statistical study of 452 BRCA1 missense substitutions with classification of eight recurrent substitutions as neutral. J Med Genet 43:295–305
Peters N, Opherk C, Bergmann T et al (2005) Spectrum of mutations in biopsy-proven CADASIL: implications for diagnostic strategies. Arch Neurol 62:1091–1094
Tikka S, Mykkänen K, Ruchoux MM et al (2009) Congruence between NOTCH3 mutations and GOM in 131 CADASIL patients. Brain 132:933–939
Mykkänen K, Savontaus ML, Juvonen V et al (2004) Detection of the founder effect in Finnish CADASIL families. Eur J Hum Genet 12:813–819
Kim Y, Choi EJ, Choi CG et al (2006) Characteristics of CADASIL in Korea: a novel cysteine-sparing Notch3 mutation. Neurology 66:1511–1516
Scheid R, Heinritz W, Leyhe T et al (2008) Cysteine-sparing NOTCH3 mutations: CADASIL or CADASIL variants? Neurology 71:774–776
Bersano A, Ranieri M, Ciammola A et al (2012) Considerations on a mutation in the NOTCH3 gene sparing a cysteine residue: a rare polymorphism rather than a CADASIL variant. Funct Neurol 27:247–252
Ge W, Kuang H, Wei B et al (2014) A novel cysteine-sparing NOTCH3 mutation in a Chinese family with CADASIL. PLoS One 9:e104533
Brass SD, Smith EE, Arboleda-Velasquez JF et al (2009) Case records of the Massachusetts General Hospital. Case 12-2009. A 46-year-old man with migraine, aphasia, and hemiparesis and similarly affected family members. N Engl J Med 360:1656–1665
Joutel A, Favrole P, Labauge P et al (2001) Skin biopsy immunostaining with a Notch3 monoclonal antibody for CADASIL diagnosis. Lancet 358:2049–2051
Lesnik Oberstein SA, van Duinen SG, van den Boom R et al (2003) Evaluation of diagnostic NOTCH3 immunostaining in CADASIL. Acta Neuropathol 106:107–111
Malandrini A, Gaudiano C, Gambelli S et al (2007) Diagnostic value of ultrastructural skin biopsy studies in CADASIL. Neurology 68:1430–1432
Markus HS, Martin RJ, Simpson MA et al (2002) Diagnostic strategies in CADASIL. Neurology 59:1134–1138
Acknowledgments
We are indebted to Dr. Ikuko Mizuta and Prof. Toshiki Mizuno for kind assistance in the NOTCH3 gene analysis, Ms. Hiroko Katsura and Ms. Mika Oka for excellent technical assistance, and Ms. Judith B. Gandy for providing professional English editing of the manuscript. This research was supported by Grants-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (numbers 24249036 and 25860717).
Conflicts of interest
The authors declare that they have no financial or other conflicts of interest.
Ethical standard
The Human Ethics Review Committee of Kumamoto University approved the study protocol. All patients or their family members provided signed consent forms.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ueda, A., Ueda, M., Nagatoshi, A. et al. Genotypic and phenotypic spectrum of CADASIL in Japan: the experience at a referral center in Kumamoto University from 1997 to 2014. J Neurol 262, 1828–1836 (2015). https://doi.org/10.1007/s00415-015-7782-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7782-8