Abstract
Freezing of gait (FOG) is a disabling feature of Parkinson’s disease. Emerging evidence suggests that dysfunction of the pedunculopontine nucleus (PPN) and pontomedullary reticular formation (pmRF) plays a role in the causation of FOG. These brainstem structures can be examined by the StartReact paradigm, which utilizes a startling stimulus to accelerate reaction times (StartReact). Here, we examined gait initiation in PD patients with and without FOG using this paradigm. Twenty-six patients with Parkinson’s disease (12 freezers and 14 non-freezers) and 15 controls performed two tasks: rapid gait initiation in response to an imperative ‘go’ signal; and a control condition, involving a simple reaction-time task involving ankle dorsiflexion. During both tasks, a startling acoustic stimulus was combined with the imperative signal in 25 % of trials. In controls, the startle accelerated gait initiation and shortened the onset latency of tibialis anterior responses during ankle dorsiflexion. This acceleration was intact in non-freezers, but was significantly attenuated in the freezers. Independent of the occurrence of a startle, freezers showed a reduced length of the first step compared to non-freezers and controls. The diminished StartReact effect in freezers probably reflects deficient representation or release of motor programs at the brainstem reticular level due to dysfunction of the PPN, the pmRF, or both. These brainstem structures are presumably involved in integrating anticipatory postural adjustments with subsequent stepping movements. We suggest that with time-varying demands, these structures may no longer be able to coordinate the integration of anticipatory postural adjustments with steps, leading to FOG episodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freezing of gait (FOG) is a disabling feature of Parkinson’s disease (PD) [1]. The underlying pathophysiology is still poorly understood. There is emerging evidence that dysfunction of the pedunculopontine nucleus (PPN) and pontomedullary reticular formation (pmRF) plays a role in causing FOG [23]. Dysfunction of these brainstem circuits in PD patients with FOG has recently been suggested by a study that evaluated the so-called ‘StartReact’ paradigm [32]. In the StartReact paradigm, a startling auditory stimulus (SAS) accelerates the latencies of movement responses to an imperative ‘go’ signal. The accelerated movement onsets during StartReact experiments are dissociated from startle reflexes [32], and are thought to reflect a direct subcortical release of motor programs from the pmRF [4, 21, 34]. The StartReact effect was absent in PD-patients with severe FOG performing a simple ballistic movement of the upper extremity, but was intact in non-freezers [32]. Remarkably, PPN-stimulation restored the SAS-induced movement onset acceleration [32]. Although restoration of this StartReact effect seemed to be associated with perceived improvements in gait [31], the question remains whether and how deficient StartReact effects of the upper extremity may relate to FOG. We reasoned that demonstration of an impaired StartReact effect in a gait-related task would provide stronger support for the relevance of upper brainstem dysfunction in FOG. We therefore examined gait initiation in freezers and non-freezers using the StartReact paradigm. We added an ankle dorsiflexion task as a control condition, aiming to reproduce the StartReact effect for a simple ballistic movement [32]. We predicted that the StartReact effect would be absent or reduced in freezers during gait initiation as well as ankle dorsiflexion.
Materials and methods
Participants
Twenty-six patients with PD participated: 12 with FOG and 14 without FOG (see below for definitions). Exclusion criteria were any other disorder or medication affecting gait and severe cognitive impairment. Patients were considered to have reached an OFF-state when they experienced an end-of-dose effect prior to the intake of their next medication dose. In addition, 15 healthy controls of similar age were included. The study was approved by the local medical ethics committee. All subjects gave their written informed consent prior to the experiment.
Clinical assessment
PD patients were clinically assessed with the motor subsection (Part III) of the MDS-Unified Parkinson’s Disease Rating Scale (UPDRS, score/132) [11]. Patients also completed the New Freezing of Gait Questionnaire (N-FOGQ, score/33) [20]. Additionally, they performed a series of walking tests to objectively verify subjects as freezers or non-freezers [28, 29], including eight rapid axial 360-degree turns in both directions and walking with 25 % of the preferred step length (at a normal pace, and as rapidly as possible). Based on the detailed physical examination, 12 persons were classified as ‘freezers’, and the 14 others were classified as ‘non-freezers’, as they did not show FOG episodes during examination and never experienced subjective gluing in daily life. The N-FOGQ revealed that all freezers had more frequent and more severe FOG during the OFF-medication state. Additionally, global executive function was assessed with the frontal assessment battery (FAB, score/18).
Experimental setup and protocol
First, participants performed a warned reaction task. For this test, participants sat in a chair placed in front of two blocks with light-emitting diodes (LEDs). Illumination of the first LED array served as a warning signal and participants were instructed to perform ankle dorsiflexion as soon as the second LED array was lit. The latter was the imperative stimulus (IS). Patients performed ankle dorsiflexion with their most affected side and all controls performed dorsiflexion with their right foot. Second, we examined gait initiation, while subjects were standing 4 m in front of the LED arrays. Again, illumination of the first LED array served as a warning signal, and illumination of the second array as the IS. Participants were instructed to perform rapid gait initiation at the IS, without further instruction about which foot to step with first.
In both tasks, the forewarning periods (1–3.5 s) and the inter-trial intervals (6–10 s) were variable. All subjects performed 16 dorsiflexion trials and 16 gait initiation trials. In 25 % of trials (four during each task) an SAS was given simultaneously with the IS. The SAS (50 ms white noise, 116 dB sound pressure level) was generated by a custom-made noise generator and delivered through binaural earphones. Prior to each task, subjects were allowed five practice trials.
Data collection
EMG
EMG data were collected from bilateral tibialis anterior (TA) and rectus femoris (RF) muscles and the left sternocleidomastoid (SCM) muscle (ZeroWire by Aurion, Italy). EMG signals were sampled at 2,000 Hz, full-wave rectified and low-pass filtered at 30 Hz (zero-lag, second-order Butterworth filter).
Motion analysis
Reflective markers were placed using a full-body model [8]. Marker positions were recorded by an 8-camera 3D motion analysis system (Vicon Motion Systems, United Kingdom) at a sample rate of 100 Hz. Furthermore, to determine movement onsets in the ankle dorsiflexion task, we placed a triaxial accelerometer on top of the foot. Accelerometer signals were sampled at 2,000 Hz.
Force plates
Ground reaction forces under both feet were recorded by two force plates (60 × 180 cm each; AMTI Custom 6-axis composite force platform, USA), embedded in the surface. The signals of the force plates were sampled at 2,000 Hz and low-pass filtered at 10 Hz (second order-Butterworth filter).
Data analysis
Simple reaction-time task
Two reaction-time parameters were assessed, accelerometer reaction time and EMG reaction time in the TA. Onset latencies were determined using a semi-automatic computer algorithm that selected the first instant at which the EMG-activity or foot accelerations exceeded a threshold of 2 SD above the mean baseline activity, as calculated over a 500-ms period just prior to the IS.
Gait initiation
The outcomes of the gait initiation task included the onset and amplitude of stepping-leg EMG activity in the TA and RF. Onset latencies were determined using the aforementioned algorithm. The average EMG response amplitude was calculated over a period of 100 ms following onset latency, after subtraction of average baseline activity [5, 22, 24]. For each trial, we also determined whether an anticipatory postural adjustment (APA) occurred prior to step onset. A weight shift was considered to be an APA if it met two criteria: first, the difference between the vertical loading underneath the stance and stepping leg had to rise above a threshold of 2 SD above the mean difference, as calculated over a 500-ms period prior to the IS. This moment was defined as the onset of the APA. Second, the increase in force under the stepping leg had to exceed 5 % of the total body weight. For each APA, we determined the maximum increase in vertical force under the stepping leg, normalized for body weight. We also determined whether multiple APAs occurred.
Furthermore, we determined step onset and length for each trial separately, using the horizontal displacement of the heel and toe markers.
Startle reflex
For each trial in which an SAS was applied, we determined whether a startle reflex occurred. A startle reflex was defined as a short latency response in the SCM-muscle, starting within 130 ms following the SAS.
Statistical analysis
Differences in the outcomes of the clinical assessment between freezers and non-freezers were tested using unpaired t tests. Outcome measures of the ankle dorsiflexion and gait initiation tasks were analyzed using a repeated-measures ANOVA, with SAS (SAS–non-SAS) as the within-subject factor and group (freezing–non-freezing controls) as the between-subjects factor. In the case of a significant SAS × group interaction, we used Tukey post-hoc tests to identify differences in SAS-induced effects between the groups. The latter post-hoc test was also performed in the case of significant group interactions.
To identify whether the SAS-effects on muscle onset latencies were independent of bradykinesia, we also conducted these analyses with UPDRS bradykinesia subscores as a covariate. As bradykinesia did not change any of the statistical outcomes, these results are not further reported. The alpha level was set at 0.05.
Results
Clinical assessment
Clinical characteristics of the participants are shown in Table 1. Freezers and non-freezers did not differ with respect to age [t(24) = −0.272, p = 0.788], nor did the non-freezers and controls [t(27) = 0.103, p = 0.919]. The total UPDRS-III score, UPDRS-bradykinesia items subscore, and FAB-score did not differ significantly between freezers and non-freezers [t(24) = −0.958, p = 0.348; t(24) = −0.424; p = 0.675 and t(24) = −0.542, p = 0.593, respectively]. Freezers had a significantly higher score on the N-FOGQ [t(24) = 10.846, p < 0.001], UPDRS-PIGD-subscore [t(24) = −2.900, p = 0.008] and a longer disease duration [t(24) = 2.501, p = 0.020].
Ankle dorsiflexion task
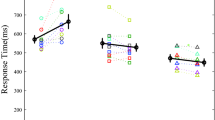
An SAS accelerated the onset of TA responses (SAS; F 1,38 = 226.256, p < 0.001), but the acceleration differed significantly between the groups (SAS × group; F 2,38 = 13.581, p < 0.001; Fig. 1). The acceleration was attenuated in the freezers (17 ms acceleration to 114 ± 15 ms) compared to the non-freezers (44 ms acceleration to 96 ± 16 ms, p < 0.001) and controls (42 ms acceleration to 96 ± 18 ms, p < 0.001), whereas non-freezers and controls did not differ from each other (p = 0.885). Without an SAS, the onset latencies did not differ between the groups (p > 0.175). This pattern was confirmed by the accelerometer data, yielding a significant SAS × group interaction (F 2,38 = 11.205, p < 0.001; Fig. 1), with less acceleration in the freezers.
Muscles responses in gait initiation
No FOG episodes were observed during the gait initiation task. Prior to step onset, we observed the consistent activation of TA in the stepping leg to initiate the APA, as well as the activation of RF in the vast majority of the participants (37/41). An SAS accelerated the onset of TA-response (SAS; F 1,38 = 284.554, p < 0.001; Fig. 2), but this effect differed significantly between groups (SAS × group; F 2,38 = 7.030, p = 0.003). The acceleration was less pronounced in the freezers (31 ms acceleration to 88 ± 119 ms) compared to the non-freezers (51 ms acceleration to 69 ± 13 ms, p = 0.012) and controls (54 ms acceleration to 75 ± 15 ms, p = 0.003), whereas non-freezers and controls did not differ from each other (p = 0.894). Without am SAS, the onset latencies of TA responses did not differ between the groups (p > 0.332).
Onset latencies of muscle responses involved in the anticipatory postural adjustments (APAs) prior to gait initiation. Mean latencies (SE) are shown for the tibialis anterior (left panel) and rectus femoris (right panel) of the stepping leg. Plus indicates significant SAS interaction, Delta indicates significant SAS × group interaction
The same pattern of results was found for RF onset latencies (SAS × group; F 2,34 = 4.771, p = 0.015; Fig. 2). A smaller SAS-induced acceleration was seen in the freezers (25 ms acceleration to 98 ± 33 ms) compared to the non-freezers (52 ms acceleration to 83 ± 19 ms, p = 0.012) and controls (45 ms acceleration to 82 ± 12 ms, p = 0.068). Without an SAS, there were no between-group differences in RF-onset latencies (p > 0.136).
The SAS increased the amplitude of TA responses by 41 % (SAS; F 1,38 = 18.503, p < 0.001). This effect did not differ between the groups (SAS × group; F 2,38 = 0.689, p = 0.508; Fig. 3). There was, however, a significant group effect (group; F 2,38 = 7.168, p = 0.002), with smaller overall TA responses in freezers compared to controls (p = 0.004).
The SAS increased the amplitude of RF responses by 40 % (SAS; F 1,34 = 9.184, p = 0.005), without differential group effects (SAS × group; F 2,34 = 0.274, p = 0.762; group; F 2,34 = 0.464, p = 0.632).
Anticipatory adjustments in gait initiation
APAs were detected in more than more than 90 % of trials, irrespective of group or SAS. We did not record any multiple APAs, which is in line with the absence of FOG episodes during the experiment.
The SAS significantly accelerated APA onsets (SAS; F 1,38 = 167.692, p < 0.001), but this effect differed between groups (SAS × group; F 2,38 = 7.245, p = 0.002). The acceleration was less pronounced in freezers (34 ms acceleration to 160 ± 60 ms) compared to non-freezers (73 ms acceleration to 119 ± 24 ms, p = 0.003) and controls (68 ms acceleration to 125 ± 26 ms, p = 0.010), whereas non-freezers and controls did not differ from each other (p = 0.885). In trials without an SAS, APA onset did not differ between the groups (p > 0.997).
The SAS increased APA amplitude by 10 % on average (SAS; F 1,38 = 4.722, p = 0.036; Fig. 3), and this effect did not differ between the groups (SAS × group; F 2,38 = 0.061, p = 0.941). Although the APA amplitude tended be smaller in freezers compared to non-freezers and controls, and smaller in non-freezers compared to controls, the group effect did not reach significance (group; F 2,38 = 3.012, p = 0.061).
Step onset and length in gait initiation
The SAS accelerated step onset (SAS; F 1,38 = 64.430, p < 0.001; Fig. 4). The effect of the SAS did not differ between the groups (SAS × group; F 2,38 = 1.697, p = 0.197), although the acceleration tended to be smaller in freezers (54 ms acceleration) compared to non-freezers (94 ms) and controls (93 ms). There was a significant group effect (group; F 2,38 = 4.012, p = 0.026). Without an SAS, step initiation was delayed in freezers (588 ± 119 ms) and non-freezers (585 ± 64 ms) compared to controls (503 ± 65 ms; p = 0.032 and p = 0.034, respectively), whereas step onset did not differ between freezers and non-freezers (p = 0.997).
The SAS shortened the length of the first step by on average 4 cm (SAS; F 1,38 = 11.747, p = 0.001; Fig. 4), which effect did not differ between the groups (SAS × group; F 2,38 = 0.797, p = 0.458). Step lengths differed between groups (group; F 2,38 = 8.089, p = 0.001), with shorter steps in freezers (30 ± 14 cm) compared to non-freezers (46 ± 15 cm; p = 0.013) and controls (52 ± 11 cm; p = 0.001). Step lengths did not differ between non-freezers and controls (p = 0.531).
Startle reflexes
In the gait initiation task, we found no differences in startle reflex occurrence between freezers (31 % of trials with SAS), non-freezers (25 % of trials with SAS), and controls (33 % of trials with SAS, F 2,40 = 0.178, p = 0.838). This pattern was confirmed by the ankle dorsiflexion task, where no difference in startle reflex occurrence was seen between freezers (25 % of trials with SAS), non-freezers (27 % of trials with SAS), and controls (38 % of trials with SAS, F 2,40 = 0.464, p = 0.632). Furthermore, a higher occurrence of startle reflexes was not associated with a larger StartReact effect, neither in the gait initiation task (r p = 0.146, p = 0.362), nor in the ankle dorsiflexion task (r p = 0.167, p = 0.297).
Discussion
We found that the accelerating effect of a startling auditory stimulus (SAS) was attenuated in PD patients with FOG, and this was seen for both gait initiation and for a simple, reactive ankle dorsiflexion movement. The SAS-induced accelerations were independent of the occurrence of startle reflexes in the sternocleidomastoid muscle. The reduced StartReact effect differentiated freezers from non-freezers with similar disease severity, whereas non-freezers did not differ from control subjects with regard to the effects of the SAS; this result was independent of the severity of bradykinesia. Furthermore, freezers had reduced step lengths of their first step to initiate gait.
Deficient StartReact effect in freezers
The present study is the first to apply the StartReact paradigm to gait initiation in PD patients with FOG, providing strong evidence for the coexistence of freezing and reduced StartReact effects. We were able to confirm the disturbed StartReact effect in freezers during simple reactive movements, shown previously for an upper-limb task [32], now replicated for a simple ankle dorsiflexion movement. Importantly, the present results extend these previous findings in three ways. First, we show that attenuation of the StartReact effect is not restricted to simple movements, but also occurs in gait initiation, a complex whole-body movement that can provoke freezing episodes. Second, the present results were obtained in a less-severely affected group of patients with predominantly OFF-period FOG, who are more representative of ‘typical’ PD patients compared to the group with severe ON-period freezing that was included by Thevathasan et al. [32]. Third, we included patients without prior PPN surgery, which allowed us to study the presumed StartReact effects without the possible influence of surgical microlesions or chronic after-effects of DBS.
We observed that in non-freezers, the SAS accelerated the EMG and movement onsets to the same extent as in controls. This confirms previous observations on simple reactive movements, as well as gait initiation in PD [3, 9, 26]. Apparently, the pre-programming of motor responses and their reflexive release by the SAS is still intact in these patients. In contrast, PD patients with FOG showed a consistently attenuated StartReact effect. The pmRF presumably plays a pivotal role in the StartReact effect [21, 34]. Hence, we suggest that in freezers, motor responses (including the APA to initiate a step) may be poorly represented in this brainstem reticular structure, or that the reflexive release of these motor responses may be deficient due to pmRF networks that encode the motor response being less responsive to excitatory stimuli. The latter could be the result of enhanced inhibitory drive from other structures, likely involving the PPN, as it has strong inhibitory projections on the pmRF [14, 15, 25]. This notion is coherent with the reported effects of PPN stimulation on StartReact effects [32].
Underscaling of gait parameters
In the current study, we confirmed the underscaling of step length in freezers that was observed previously [6, 19]. The underscaling of step length was independent of the presence of an SAS. Interestingly, an SAS did result in a small but significant reduction of step length, both in PD patients and in controls. The mechanisms underlying the reduction of step length by an SAS are not clear, and should be explored by future studies. In addition to the underscaling of step length, both freezers and non-freezers had a tendency for smaller amplitudes of anticipatory postural adjustments (APAs) compared to controls. This tendency is in line with the previously reported underscaling of APAs in PD [2, 7, 10, 17, 26, 35]. Both the reduced step length and the smaller APAs have been attributed to reduced brain activity in the supplementary motor area (SMA) [12, 30] and are thought to contribute to FOG [6]. It is conceivable that the mechanisms underlying the underscaling of movements are different from those underlying the deficient StartReact effect, as non-freezers showed underscaling APAs as well, but still exhibited an intact StartReact effect. Furthermore, freezers demonstrated intact augmentation of EMG-response amplitudes due to the SAS, but at the same time exhibited consistent delays in the onset of these responses.
Relation between disturbed StartReact and freezing of gait
The finding of attenuated SAS-induced accelerations of motor responses in freezers raises the question of whether it may be relevant to the causation of FOG. The neural structures most likely involved in the StartReact phenomenon (pmRF and PPN) are also thought to be involved in the integration of APAs with subsequent stepping movements [16, 18, 23, 27]. The results of our gait initiation task point at deficiencies in APA representation or release at brainstem level, which may compromise the integration with subsequent steps. This possibly leads to further underscaling and increased variability in step lengths, as previously reported in freezers [6, 13, 19]. With time-varying demands such as turning, or when exaggerating the underscaling of gait characteristics (e.g., when making small steps), these spatiotemporal gait abnormalities increase the computational load on the PPN and the pmRF. At such instances, these structures may no longer be able to coordinate the integration of APAs with steps, leading to FOG episodes. As this hypothesis remains speculative and largely based on indirect evidence, further studies are needed to corroborate whether brainstem structures are indeed unable to integrate the different motor programs during an FOG episode, for example by directly measuring the oscillatory activity of the PPN during an FOG episode. A first report has already associated the attenuation of PPN alpha activity with FOG [33], but this promising finding warrants further investigation.
References
Bloem BR, Hausdorff JM, Visser JE, Giladi N (2004) Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord 19:871–884
Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA (1997) Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord 12:206–215
Carlsen AN, Almeida QJ, Franks IM (2013) Using a startling acoustic stimulus to investigate underlying mechanisms of bradykinesia in Parkinson’s disease. Neuropsychologia 51:392–399
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004) Prepared movements are elicited early by startle. J Mot Behav 36:253–264
Carpenter MG, Allum JH, Honegger F, Adkin AL, Bloem BR (2004) Postural abnormalities to multidirectional stance perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:1245–1254
Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R (2009) Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain 132:2151–2160
Crenna P, Frigo C (1991) A motor programme for the initiation of forward-oriented movements in humans. J Physiol 437:635–653
Davis RB, Ounpuu S, Tyburski D, Gage JR (1991) A gait analysis data-collection and reduction technique. Hum Mov Sci 10:575–587
Fernandez-Del-Olmo M, Bello O, Lopez-Alonso V, Andres Sanchez J, Santos-Garcia D, Valls-Sole J (2012) The effects of auditory startle and nonstartle stimuli on step initiation in Parkinson’s disease. Mov Disord 27:1570–1573
Gantchev N, Viallet F, Aurenty R, Massion J (1996) Impairment of posturo-kinetic co-ordination during initiation of forward oriented stepping movements in parkinsonian patients. Electroencephalogr Clin Neurophysiol 101:110–120
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society URTF (2008) Movement disorder society-sponsored revision of the unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Hanakawa T, Katsumi Y, Fukuyama H, Honda M, Hayashi T, Kimura J, Shibasaki H (1999) Mechanisms underlying gait disturbance in Parkinson’s disease: a single photon emission computed tomography study. Brain 122(Pt 7):1271–1282
Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N (2003) Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Exp Brain Res 149:187–194
Inglis WL, Winn P (1995) The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol 47:1–29
Koch M, Kungel M, Herbert H (1993) Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res 97:71–82
la Fougere C, Zwergal A, Rominger A, Forster S, Fesl G, Dieterich M, Brandt T, Strupp M, Bartenstein P, Jahn K (2010) Real versus imagined locomotion: a [18F]-FDG PET-fMRI comparison. Neuroimage 50:1589–1598
Mille ML, Johnson Hilliard M, Martinez KM, Simuni T, Rogers MW (2007) Acute effects of a lateral postural assist on voluntary step initiation in patients with Parkinson’s disease. Mov Disord 22:20–27
Musienko PE, Zelenin PV, Lyalka VF, Orlovsky GN, Deliagina TG (2008) Postural performance in decerebrated rabbit. Behav Brain Res 190:124–134
Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E (2001) Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord 16:1066–1075
Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N (2009) Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their careers. Gait Posture 30:459–463
Nonnekes J, Oude Nijhuis LB, de Niet M, de Bot ST, Pasman JW, van de Warrenburg BP, Bloem BR, Weerdesteyn V, Geurts AC (2014) StartReact restores reaction time in HSP: evidence for subcortical release of a motor program. J Neurosci 34:275–281
Nonnekes J, Scotti A, Oude Nijhuis LB, Smulders K, Queralt A, Geurts AC et al (2013) Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience 245:109–120
Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A (2011) Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 10:734–744
Queralt A, Weerdesteyn V, van Duijnhoven HJ, Castellote JM, Valls-Sole J, Duysens J (2008) The effects of an auditory startle on obstacle avoidance during walking. J Physiol 586:4453–4463
Reese NB, Garcia-Rill E, Skinner RD (1995) The pedunculopontine nucleus-auditory input, arousal and pathophysiology. Prog Neurobiol 47:105–133
Rogers MW, Kennedy R, Palmer S, Pawar M, Reising M, Martinez KM, Simuni T, Zhang Y, MacKinnon CD (2011) Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol 106:915–924
Schepens B, Stapley P, Drew T (2008) Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol 100:2235–2253
Snijders AH (2012) Summary and discussion. In: Tackling freezing of gait in Parkinson’s disease. Ipskamp Drukkers, Enschede, pp 196–197
Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR (2012) Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord 18:149–154
Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I (2011) Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain 134:59–72
Thevathasan W, Cole MH, Graepel CL, Hyam JA, Jenkinson N, Brittain JS, Coyne TJ, Silburn PA, Aziz TZ, Kerr G, Brown P (2012) A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain 135:1446–1454
Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Bogdanovic M, Coyne TJ, Silburn PA, Aziz TZ, Brown P (2011) A block to pre-prepared movement in gait freezing, relieved by pedunculopontine nucleus stimulation. Brain 134:2085–2095
Thevathasan W, Pogosyan A, Hyam JA, Jenkinson N, Foltynie T, Limousin P, Bogdanovic M, Zrinzo L, Green AL, Aziz TZ, Brown P (2012) Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 135:148–160
Valls-Sole J, Kumru H, Kofler M (2008) Interaction between startle and voluntary reactions in humans. Exp Brain Res 187:497–507
Vaugoyeau M, Viallet F, Mesure S, Massion J (2003) Coordination of axial rotation and step execution: deficits in Parkinson’s disease. Gait Posture 18:150–157
Acknowledgments
This research was funded by a Radboud University Medical Centre Research Grant to JH Nonnekes, a grant from the European Commission to LB Oude Nijhuis and BR Bloem (FP7-PEOPLE-2012-ITN 316639), and a Netherlands Organization for Scientific Research Veni Research Grant 916.10.106 to V. Weerdesteyn. We thank Roland Loeffen for technical assistance.
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nonnekes, J., Geurts, A.C.H., Oude Nijhuis, L.B. et al. Reduced StartReact effect and freezing of gait in Parkinson’s disease: two of a kind?. J Neurol 261, 943–950 (2014). https://doi.org/10.1007/s00415-014-7304-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7304-0