Abstract
Freezing of gait is an episodic gait disorder, characterized by the inability to generate effective forward stepping movements. The pathophysiology underlying freezing of gait remains insufficiently understood, and this hampers the development of better treatment strategies. Preliminary evidence suggests that impaired force control during walking may contribute to freezing episodes, with difficulty to unload the swing leg and initiate the swing phase. Here, we used external loading to manipulate force control and to investigate its influence on freezing of gait. Twelve Parkinson’s disease patients with freezing of gait performed three contrasting tasks: (1) loaded gait while wearing a belt fortified with lead weights; (2) weight-supported gait using a parachute harness connected to a rigid metal cable running above the gait trajectory; and (3) normal gait. Gait tasks were used to provoke freezing episodes, including rapid 360° turns. Freezing episodes were quantified using blinded, videotaped clinical assessment. Furthermore, ground reaction forces and body kinematics were recorded. Loading significantly increased the mean number of freezing episodes per trial compared to the normal gait condition (P < 0.05), but the effect of weight support was not consistent. Loading particularly increased the number of freezing episodes during rapid short steps. Step length was significantly smaller during loaded gait compared to normal gait (P < 0.05), but changes in anticipatory postural adjustments were not different. Our results may point to impaired force control playing a key role in freezing of gait. Future studies should further investigate the mechanism, i.e., the contribution of deficient load feedback, and evaluate which forms of weight support might offer treatment opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Freezing of gait (FOG) is an episodic gait disorder characterized by a sudden inability to generate effective forward stepping movements, leading to falls and a reduced quality of life [1, 2]. It is a common and debilitating phenomenon in Parkinson’s disease [3]. Known triggers for FOG include challenging events that require precise regulation of step length and gait timing, such as turning or step initiation [4, 5]. However, the pathophysiology underlying FOG remains insufficiently understood, and this hampers development of better treatment strategies [6, 7].
Force control is impaired in Parkinsonian gait [8, 9], and is thought to contribute to the gait disturbances [10], such as FOG. During normal gait, it is suggested that feedback of the forces generated during the stance phase of the gait cycle may regulate the timing and amount of loading of the stance leg and unloading of the swing leg needed for the initiation of the swing phase [11, 12]. However, previous studies that used loading to investigate force control in Parkinson’s disease reported reduced load sensitivity and decreased lower-leg extensor activity [13]. Therefore, our primary hypothesis is that additional load contributes to FOG by augmenting the need for generating forces to initiate the swing phase. In contrast, weight support may diminish FOG. Secondly, we hypothesize that additional load increases FOG by reducing step length. Loading reduces step length in healthy subjects and patients with osteoarthritis [14]. Furthermore, patients with Parkinson’s disease with FOG already display difficulties in generating and maintaining an appropriate step length as compared to both controls and patients without freezing [3, 15]. Reduced load sensitivity may interact with the defective step amplitude generation, and load may therefore affect freezing even beyond the effect on step size reduction. However, FOG is an intermittent phenomenon, which is elicited more during complex gait tasks than during normal walking. Therefore, we hypothesize that additional load may lead to a reduced step length without showing increased FOG during normal walking, while simultaneously demonstrating more FOG during gait tasks that are known to elicit more FOG, i.e., turning and gait initiation. If additional load leads to more FOG during gait initiation, we also expect to record abnormalities in anticipatory postural adjustments (APAs), i.e., the sequential postural shifts that unload the swing leg just prior to gait initiation by propelling the body mass over the stance limb and into forward motion [16, 17]. Parkinson’s disease patients show abnormal APAs following postural perturbations [18] and specifically show a delay between their APAs and voluntary movements, such as rising onto the toes [19] or a voluntary step [20, 21]. In mild Parkinson’s disease, the APA’s can be normal, but interestingly, there is a lack of APA refinement in some patients when trials are repeated [22]. In the context of the present study, it can be argued that this may be attributed to a deficient use of force feedback signals [22]. In patients with FOG, inappropriate APAs are present in the majority of trials and are observed more often than in patients without FOG and elderly controls [23]. In particular, inappropriate APAs in freezers are longer and more ample than in parkinsonian non-freezers and controls [23, 24].
Here, we examine the influence of force control on FOG to increase our understanding of the pathophysiology underlying FOG, which could ultimately lead to new therapeutic options, such as alternative walking procedures. We manipulated loading responses, using three contrasting tasks: (1) loaded gait while wearing a belt fortified with lead weights, to create greater demand for unloading forces, thereby worsening FOG; (2) weight-supported gait (walking while suspended by a supporting harness), to reduce the demand for unloading forces and thus, alleviate FOG; and (3) normal gait. We used gait tasks known to provoke FOG, such as rapid 360° turns and walking with steps smaller than the self-preferred step length [15, 25]. FOG episodes were quantified using videotaped clinical assessment. Furthermore, ground reaction forces and lower body kinematics were measured.
Materials and methods
Study population
Twelve patients with Parkinson’s disease participated (Table 1). Patients were diagnosed according to the UK Brain Bank criteria [26]. All patients had a previous history of freezing of gait (FOG) that was assessed subjectively using the new freezing of gait questionnaire (NFOG-Q). The presence of FOG was also determined objectively during performance of rapid, full turns [5, 27]. All patients reported FOG during subjective OFF-medication periods, but 11 patients also reported FOG during ON-phases. Nine patients reported more FOG during OFF-phases, whereas three patients reported no difference in freezing either during ON or OFF. None of the patients had predominant ON-state freezing. Exclusion criteria were unstable medical conditions that negatively affected balance and gait. The study was approved by the local medical ethics committee and was conducted in accordance with the Declaration of Helsinki. All subjects gave their written informed consent prior to the experiment.
Protocol
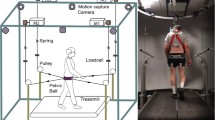
Patients were examined in the afternoon, during a subjective OFF-phase, at least 4 h after intake of the last dose of dopaminergic medication, to increase the likelihood of FOG episodes to occur [28]. Following the baseline clinical assessments, subjects performed various gait tasks during three conditions: (1) while being partially loaded with lead weights attached to a belt, corresponding to 15 % of their body weight (loaded gait); (2) while being partially unloaded by a supporting harness that reduced 15 % of their weight (weight-supported gait); and (3) while walking normally (normal gait) (Fig. 1). Because of the episodic occurrence of FOG and the possible occurrence of flooring and ceiling effects, we used different gait tasks known to provoke FOG, such as rapid 360° turning and walking with short steps [3, 15], as well as tasks that are less provoking, such as walking normally [4, 29]. Patients started each gait task following a verbal instruction (ready-start).
Loading was achieved by wearing a scuba-diving belt with the correct amount of lead weights distributed evenly around the waist. Weight support was achieved by suspending the subjects from a parachute harness connected to a rigid metal cable running above the gait trajectory. Force plates underneath the feet were used to calculate 15 % load or weight support. All three conditions (loaded, weight supported, and normal gait) were counterbalanced across patients to avoid the possible effects of fatigue, learning, and residual effects of medication. A pilot study showed that loading participants with 15 % extra weight was feasible in our patients, similar to a previous study in healthy subjects [30].
Data collection
We collected analogue video data, and both kinematic and kinetic data.
Video data
Two video cameras captured the gait tasks at a sample rate of 50 Hz. One was positioned sideways and captured both the starting position of the subject and the complete area needed for rapid 360° turning. This camera captured only part of the gait trajectories for normal gait and short steps. The second camera was positioned behind the subject and captured the complete gait trajectory.
Motion analysis
Reflective markers were placed at anatomical landmarks using a model including the lower body and trunk [31]. Marker positions were recorded by a 6-camera 3D motion analysis system (Vicon Motion Systems, United Kingdom) at a sample rate of 100 Hz.
Force plates
Ground reaction forces beneath both feet were recorded by two force plates (AMTI Custom 6-axis composite force platform, USA), which were embedded within the walkway. The signals of the force plates were sampled at 1,000 Hz and low-pass filtered at 10 Hz (second-order Butterworth filter).
Data analysis
Freezing of gait episodes
The frequency and duration of the FOG episodes during the different conditions and tasks were scored by two blinded and experienced raters in FOG (AS and JN) using offline video analysis, which is the current gold standard for FOG assessment [6]. To assure blinding, videos were cropped, such that the hips and upper body could not be seen, and raters could not distinguish between the different conditions. Raters were asked to score each trial as showing either ‘definite FOG’ or ‘no FOG’ by defining FOG as an obvious episode with ineffective stepping and the characteristic FOG phenotype. Whenever several consecutive episodes occurred, these were counted as separate FOG episodes if intervals between effective stepping were more than one second. Freezing was differentiated from a voluntary stop or hesitation, overall akinesia, or festination based on the phenotypical presentation. If the raters disagreed, trials were sent back to them for consensus. We calculated the mean number of FOG episodes per trial and the duration of FOG, both as the percentage of time a patient froze (cumulative duration of FOG episodes within a task/total duration of the task) and as the mean duration per episode in seconds [6].
Kinematic analyses
We used the displacement of the heel and toe markers to determine the mean step length and the step-to-step length variability (within-trial standard deviation (SD)). Calculation of these measures was only performed for normal walking and normal walking rapid trials where the step length was self-preferred. To measure the influence of both loaded and weight-supported conditions on body posture, we calculated the trunk flexion angle relative to the pelvic tilt angle and the hip flexion angle. When these angles are smaller, and therefore posture is more stooped, a biomechanical consequence may be a smaller step length. The angles were calculated prior to the start of the gait tasks, while subjects were standing.
Kinetics
Prior to each first step in both the normal walking task and the normal walking rapid task, we determined whether an anticipatory postural adjustment (APA) occurred [32]. APA amplitudes were calculated according to previously used techniques and both normalized for body weight and corrected for the amount of loading (Fig. 2) [33]. In brief, a weight shift was considered an APA if it met two criteria. First, the difference between the vertical force underneath the stance and stepping leg had to exceed a threshold of 2 SD above the mean difference, as calculated over 100 ms prior to the actual step. Second, an increase in force directed towards the stepping leg had to exceed 5 % of the total body weight, thereby taking into account the applied load, or weight support. As a result, normal changes due to minor weight shifts were not classified as an APA. After determining an APA, we calculated the maximum increase in vertical force directed towards the stepping leg, normalized for body weight and corrected for the amount of loading. We also determined whether multiple APAs occurred. Furthermore, we calculated the forward impulse of the APA by integrating the cumulative anterior–posterior ground reaction force on both force plates during the APA period [30]. For this calculation, the end of the APA period was defined by the final heel-off of the swing leg [34].
Vertical weight shifts. Representative traces of vertical weight shifts (forces beneath the right foot minus the left foot) during a single trial within the normal walking task for the (a) normal gait, (b) loaded, and (c) weight supported condition. The vertical lines represent step onset. The horizontal grey lines depict the mean response and outer limits for calculation of the APAs. In a–c, only one APA was calculated
Statistical analyses
Inter-rater reliability was assessed using Cohen’s kappa for the rating of presence of FOG in each trial. We used the data-values of each rater, before they had reached consensus on the presence of FOG. Furthermore, the correlation between the two raters for both number and duration of FOG episodes was analyzed using Pearson’s correlation coefficients. Our primary analysis concentrated on between-condition comparisons of the number and duration of FOG episodes across all gait tasks. Therefore, we used one-way repeated measures analysis of variance (ANOVA) models for condition (loaded, weight support, and normal gait). Next, we investigated the effect of gait task on FOG episodes during the normal gait condition, because certain tasks are known to provoke FOG, such as 360° rapid turning and short steps [5, 15], while others are less informative, possibly leading to a flooring effect. First, a one-way, repeated measures ANOVA model for gait task was performed during the normal gait condition. Tasks that produced significantly less FOG compared to the other tasks were removed from a secondary, repeated measures ANOVA model for condition and task, to determine the effect of load within the most informative tasks. Before the analyses, we ascertained that the data were normally distributed. Furthermore, significant main and interaction effects were further explored using post-hoc Student’s paired t-tests. Data were analyzed using IBM SPSS Statistics 20, using a significance level of P < 0.05. All data in the text represent means ± standard deviations.
Results
Freezing of gait (FOG)
Inter-rater reliability
Prior to consensus, the raters reached a high degree of agreement for the presence of FOG within each trial (agreement 94 %, Cohen’s kappa = 0.88 (P < 0.01)). The correlation between the two raters was high for both the number (r = 0.87, P < 0.01) and duration of FOG episodes (r = 0.98, P < 0.01).
Number of FOG episodes
Across all gait tasks, loading significantly influenced the mean number of FOG episodes per trial (F (2,22) = 4.03, P < 0.05). The mean number of FOG episodes per trial was significantly larger during the loaded condition (0.86 ± 0.45) compared with the normal gravity condition (0.68 ± 0.36; P < 0.01) (Fig. 3). No significant differences were recorded for the weight-supported condition (0.71 ± 0.53) compared to normal gait or the loaded condition.
During the normal gravity condition, the type of gait task showed a significant effect on the mean number of FOG episodes per trial (F (5,55) = 3.53, P < 0.05). As expected [15, 25], rapid 360° turning and short, rapid steps elicited significantly more FOG episodes compared to the other tasks (Table 2). Furthermore, the short, rapid steps task demonstrated a main effect of load on the mean number of FOG episodes (F (2,22) = 6.06, P < 0.01) (Table 3).
Duration of FOG episodes
Load did not significantly affect the relative percentage of time that a patient froze (cumulative duration of FOG episodes within each task/total duration of that task) (F (1.07,11.74) = 0.20, P = 0.68). Furthermore, load did not significantly affect the mean duration of the determined FOG episodes (F (1.02,11.27) = 0.12, P = 0.74).
No residual effect of medication and fatigue on the number of FOG episodes
The ordering of the conditions across patients and the number of FOG episodes were not correlated (r = −0.02, n = 36, P = 0.91). We did find a moderate, but significant negative correlation between the amount of time since last medication intake and the number of FOG episodes (r = −0.42, n = 36, P < 0.05), i.e., patients who experienced less FOG during the experiment had a longer interval between stopping their medication and onset of the experiment. Presumably patients with more marked FOG require less time to reach an OFF-phase with FOG after postponing their medication.
Effect of weight load on anticipatory postural adjustments (APAs) and forward impulse
Although amplitudes of forces were different in an absolute sense (Fig. 2), we found no difference between conditions for APA amplitudes when corrected for load. The amplitude of the last APA (in case of multiple APAs) prior to a step did not differ between loaded gait (171.74 ± 133.36 N s), normal gait (175.29 ± 92.96 N s), and weight-supported gait (187.70 ± 116.94 N s) (F (2,18) = 0.19, P = 0.83). Furthermore, the mean number of APAs per trial did not differ between loaded gait (0.79 ± 0.54), normal gait (0.72 ± 0.39), and weight-supported gait (0.82 ± 0.43) (F (2,20) = 0.16, P = 0.85). Table 4 shows the number of trials in which we recorded either no APA, one APA, or multiple APAs during each condition. The forward impulse of the APA was also not different between normal gait (8.11 ± 3.46 N s), loaded gait (9.15 ± 5.04 N s), and weight-supported gait (8.68 ± 3.89 N s) (F (2,16) = 1.43, P = 0.27).
Effect of weight load on FOG during gait initiation
We specifically investigated the effect of load on the number of FOG episodes during gait initiation, because we observed a significant effect of load on the number of FOG episodes during the entire gait task, but no effect on the APAs that were calculated prior to gait initiation. During gait initiation, there was no significant effect of load on the mean number of freezing episodes per trial across all tasks (F (2,22) = 1.64, P = 0.22), although the number of episodes appeared largest during loaded gait (0.24 ± 0.20), followed by weight-supported gait (0.15 ± 0.20) and normal gait (0.13 ± 0.17).
Effect of weight load on kinematic outcome measures
Step length
Load had a significant effect on the mean step length during normal gait for step cycles in which no FOG occurred (F (2,22) = 3.72, P < 0.05). The mean step length was significantly smaller during loaded gait (0.38 ± 0.11 m) compared with normal gait (0.44 ± 0.09 m; P < 0.05). Step-to-step length variability was not different between loaded gait (57.17 ± 26.80), normal gait (51.01 ± 19.46), and weight-supported gait (46.82 ± 12.97) (F (1.36, 14.91) = 1.03, p = 0.35).
Trunk flexion relative to pelvis
No significant differences were found in stooped posture (measured as trunk flexion relative to pelvis angle) during stance prior to each trial between loaded gait (6.72° ± 9.83°), normal gait (5.20° ± 8.45°), and weight-supported gait (6.56° ± 11.77°) (F (1.08,10.75) = 0.89, P = 0.37).
Hip angle
Load significantly influenced hip-flexion angle, measured during stance prior to each trial (F (2,18) = 4.41, P < 0.05). The hip-flexion angle during the weight-supported condition (3.92° ± 7.64°) was significantly larger compared with normal gait (1.21° ± 7.59°; P < 0.01) and loaded gait (1.46° ± 9.82°; P < 0.05).
Discussion
We studied the influence of weight load on FOG in Parkinson’s disease. Additional load increased the number of FOG episodes. Furthermore, during step cycles without FOG, the step length was significantly shorter during the loaded condition compared to normal gait. When investigating specifically the effect of additional load during tasks that are known to provoke FOG consistently, such as rapid turning and rapid short steps (to circumvent a flooring effect that may have affected simpler gait tasks where few FOG episodes occurred), the results were less clear. In addition, the opposite effect—less FOG during weight-supported gait—was not observed. Despite these exceptions, the increase in FOG induced by loading remains a robust finding and raises some intriguing questions about the mechanisms underlying FOG.
Influence of weight load on the occurrence of FOG
We hypothesized that additional load, applied evenly around the waist, would augment the need for generating forces to initiate the swing phase, and would therefore elicit more FOG. Our main outcome supports this hypothesis. However, the results of our secondary outcome measures and analyses are less clear, complicating the interpretation of the results, concerning both the underlying mechanism and the suggestions for future clinical applications to reduce FOG.
Deficient use of automated load feedback
Deficient use of automated load feedback is thought to contribute to gait impairments in PD [10]. We hypothesized that FOG is also related to this deficient use of automated load feedback. Initiation of the swing phase critically depends on the unloading of extensors at the end of the stance phase [12]. Adding extra weight should therefore increase the difficulty in initiating the swing. This might be especially problematic for patients with Parkinson’s disease since their load feedback pathways are deficient, and their Ib inhibition is replaced by Ib facilitation [8, 35]. This would imply that during the stance phase, the unloading is not achieved easily because load receptors (mechanoreceptors and Ib afferents from Golgi Tendon Organs) keep the extensor activity increased, thereby blocking the automated onset of swing [8, 13]. Continuous overactivation apparently exists in pathways that inhibit the centers for generating flexion movements (flexor “half-center”) during gait [10]. This is compensated for by “voluntary” activations of the tibialis anterior muscles, which are abnormally large in the swing phase of Parkinson’s disease patients. If this unloading hypothesis is correct, one would expect that interventions that facilitate gait in Parkinson’s disease would also restore the balance between inhibitory and excitatory Ib feedback. Indeed, autogenic inhibition is reduced in Parkinson’s disease patients, but can be restored by high-frequency stimulation of the subthalamic nucleus [36]. Furthermore, these changes in autogenic inhibition correlated with a clinical improvement of gait. In our study, additional load increased the number of freezing episodes, which may suggest that deficient use of automated load feedback contributes to FOG. However, a limitation of the current study was that autogenic inhibition was not investigated. Therefore, future studies may investigate the relation between autogenic inhibition and FOG by recording lower-leg extensor activity and voluntary activations of tibialis anterior muscles during the pre-swing phases of the gait cycle.
Influence of weight load on anticipatory postural adjustments (APAs)
With the observed effect of loading on FOG, we also expected a reduction of generated forces recorded as APAs [32]. However, our results do not demonstrate an effect of load on the amplitude and occurrence of APAs, which may be explained by the limitation that we only assessed APAs prior to gait initiation. In that case, we found no difference in the number of FOG episodes between conditions. This was disappointing since gait initiation is a common provoking condition for FOG [37]. We suspect that the absent effect on APAs may be a consequence of the smaller number of FOG episodes during gait initiation compared to, e.g., the short steps rapid task. In addition, during our experiments, FOG events were perhaps less common upon gait initiation because the verbal starting command acted as an auditory cue that facilitated gait initiation [21, 28]. Future studies should therefore study the effect of loading and unloading on self-selected gait initiation. Furthermore, future studies should record forces throughout the stance phase of a gait cycle.
Influence of weight load on step length; interaction with the sequence effect?
We hypothesized that additional load would decrease step length, because maintaining the normal step length would require increased generation of forward forces. Our results indeed show that additional load both leads to more FOG and to a smaller step length. However, whether additional load leads to a failure in augmenting the generation of forces needed for stepping remains unclear, due to previously discussed limitations in study design concerning, i.e., the results of the APA amplitudes and forward impulse of the APAs. Furthermore, it is currently unclear whether a smaller step length was merely a side effect of the induced increase in FOG due to additional load, or whether the shortening of step length was the main cause of FOG. Loading is known to reduce step length in healthy subjects and patients with osteoarthritis [14]. Furthermore, several studies indicated that shortening the step length promotes the induction of FOG [15, 38]. Hence, it is tempting to conclude that the sequence effect—a failure to steadily generate adequate amplitudes for a series of intended steps, leading to a progressive reduction of step size that may ultimately culminate into an FOG event [15, 38]—may have played a role in the increased incidence of FOG. However, a closer look at the data shows that this is unlikely. In the present study, loaded gait reduced step length by 14 % following loading, which is too small to have much effect on FOG. A similar reduction in step length (14 %) led even to a small reduction in FOG incidence in a previous study (see Fig, 1A in Chee et al., 2009) [15]. Only when there were reductions to about half the preferred step length was a clear increase in FOG noted in the same study. Hence, the present data are unlikely to be explained solely by a sequence effect. Whether and to what extent impaired force generation contributes to FOG and whether it interacts with the sequence effect remains a subject for future studies.
Influence of weight support on the occurrence of FOG
We expected weight support to decrease the need for the generation of weight shifting and unloading forces that help to initiate the swing phase. Consequently, this should be associated with less FOG events. For weight-supported gait, we indeed found a significantly smaller number of FOG episodes compared to the loaded condition during the gait tasks that are best known to provoke FOG. However, the weight-supported condition was not significantly different from normal gait, and this may have been caused by methodological limitations that led to changes of the normal gait pattern during weight-supported gait. We achieved weight support by suspending patients from a parachute harness connected to a rigid metal cable running above the gait trajectory. Although this method may have reduced the need for generating forces that unload the swing leg, it also changed the biomechanical properties of gait by increasing hip flexion. Such biomechanical changes may have complicated gait performance and worsened FOG, due to a change in the smoothness of the vertical and lateral body movements that are normally achieved during gait [39]. Furthermore, the biomechanical changes may have increased attention or arousal during weight support, and this in turn may have influenced FOG occurrence [40, 41]. However, during the gait tasks that did not involve turning, several patients indicated that it was easier to perform the tasks, as they felt secured by the harness and even actively leaned forward, thereby presumably overcoming FOG in a number of trials. Future studies should further investigate the role of weight support on FOG without changing the normal gait pattern. Furthermore, possible treatment opportunities such as alternative walking procedures may be investigated.
Abbreviations
- FOG:
-
Freezing of gait
- APA:
-
Anticipatory postural adjustment
- NFOG-Q:
-
New freezing of gait questionnaire
- H&Y:
-
Hoehn & Yahr rating scale
- MDS-UPDRS III:
-
Unified Parkinson’s disease rating scale part 3 (motor scale)
- ABC-6:
-
Activities-specific balance confidence scale-6
- FAB:
-
Frontal assessment battery
References
Bloem BR, Hausdorff JM, Visser JE, Giladi N (2004) Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord 19(8):871–884
Giladi N, Nieuwboer A (2008) Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord 23(Suppl 2):S423–S425
Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik E (2001) Abnormalities of the spatiotemporal characteristics of gait at the onset of freezing in Parkinson’s disease. Mov Disord 16(6):1066–1075
Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A (2010) Freezing of gait in Parkinson’s disease: the impact of dual-tasking and turning. Mov Disord 25(15):2563–2570
Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR (2012) Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord 18(2):149–154
Morris TR, Cho C, Dilda V, Shine JM, Naismith SL, Lewis SJ, Moore ST (2012) A comparison of clinical and objective measures of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord 18(5):572–577
Shine JM, Naismith SL, Lewis SJ (2011) The pathophysiological mechanisms underlying freezing of gait in Parkinson’s Disease. J Clin Neurosci 18(9):1154–1157
Delwaide PJ, Pepin JL, Maertens de Noordhout A (1991) Short-latency autogenic inhibition in patients with Parkinsonian rigidity. Ann Neurol 30(1):83–89
Dietz V, Zijlstra W, Prokop T, Berger W (1995) Leg muscle activation during gait in Parkinson’s disease: adaptation and interlimb coordination. Electroencephalogr Clin Neurophysiol 97(6):408–415
Dietz V, Duysens J (2000) Significance of load receptor input during locomotion: a review. Gait Posture 11(2):102–110
Duysens J, Clarac F, Cruse H (2000) Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80(1):83–133
Duysens J, Pearson KG (1980) Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187(2):321–332
Dietz V, Colombo G (1998) Influence of body load on the gait pattern in Parkinson’s disease. Mov Disord 13(2):255–261
Kubinski AJ, Higginson JS (2012) Strategies used during a challenging weighted walking task in healthy adults and individuals with knee osteoarthritis. Gait Posture 35(1):6–10
Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R (2009) Gait freezing in Parkinson’s disease and the stride length sequence effect interaction. Brain 132(Pt 8):2151–2160
Burleigh AL, Horak FB, Malouin F (1994) Modification of postural responses and step initiation: evidence for goal-directed postural interactions. J Neurophysiol 72(6):2892–2902
Elble RJ, Moody C, Leffler K, Sinha R (1994) The initiation of normal walking. Mov Disord 9(2):139–146
King LA, St George RJ, Carlson-Kuhta P, Nutt JG, Horak FB (2010) Preparation for compensatory forward stepping in Parkinson’s disease. Arch Phys Med Rehabil 91(9):1332–1338
Frank JS, Horak FB, Nutt J (2000) Centrally initiated postural adjustments in parkinsonian patients on and off levodopa. J Neurophysiol 84(5):2440–2448
King LA, Horak FB (2009) Delaying mobility disability in people with Parkinson disease using a sensorimotor agility exercise program. Phys Ther 89(4):384–393
Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA (1997) Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord 12(2):206–215
Hall LM, Brauer SG, Horak F, Hodges PW (2013) The effect of Parkinson’s disease and levodopa on adaptation of anticipatory postural adjustments. Neurosci 250:483–492
Tard C, Dujardin K, Bourriez JL, Destee A, Derambure P, Defebvre L, Delval A (2013) Attention modulates step initiation postural adjustments in Parkinson freezers. Parkinsonism Relat Disord. pii: S1353-8020(13)00416-1
Jacobs JV, Lou JS, Kraakevik JA, Horak FB (2009) The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson’s disease. Neuroscience 164(2):877–885
Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I (2011) Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain 134(Pt 1):59–72
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N (2009) Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 30(4):459–463
Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, Bloem BR (2008) Clinimetrics of freezing of gait. Mov Disord 23(Suppl 2):S468–S474
Rogers MW, Kennedy R, Palmer S, Pawar M, Reising M, Martinez KM, Simuni T, Zhang Y, MacKinnon CD (2011) Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol 106(2):915–924
Caderby T, Dalleau G, Leroyer P, Bonazzi B, Chane-Teng D, Do MC (2013) Does an additional load modify the Anticipatory Postural Adjustments in gait initiation? Gait Posture 37(1):144–146
Davis RB, Ounpuu S, Tyburski D, Gage JR (1991) A gait analysis data-collection and reduction technique. Hum Movement Sci 10(5):575–587
Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB (2009) Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol 215(2):334–341
Nonnekes J, Scotti S, Oude Nijhuis LB, Smulders K, Queralt A, Geurts ACH, Bloem BR, Weerdesteyn V (2013) Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience 245:109–120
Mickelborough J, van der Linden ML, Richards J, Ennos AR (2000) Validity and reliability of a kinematic protocol for determining foot contact events. Gait Posture 11(1):32–37
Tatton WG, Bedingham W, Verrier MC, Blair RD (1984) Characteristic alterations in responses to imposed wrist displacements in parkinsonian rigidity and dystonia musculorum deformans. Can J Neurol Sci 11(2):281–287
Potter M, Illert M, Wenzelburger R, Deuschl G, Volkmann J (2004) The effect of subthalamic nucleus stimulation on autogenic inhibition in Parkinson disease. Neurology 63(7):1234–1239
Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N (2003) Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease. Eur J Neurol 10(4):391–398
Iansek R, Huxham F, McGinley J (2006) The sequence effect and gait festination in Parkinson disease: contributors to freezing of gait? Mov Disord 21(9):1419–1424
Stokes VP, Andersson C, Forssberg H (1989) Rotational and translational movement features of the pelvis and thorax during adult human locomotion. J Biomech 22(1):43–50
Vandenbossche J, Deroost N, Soetens E, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E (2011) Freezing of gait in Parkinson disease is associated with impaired conflict resolution. Neurorehabil Neural Repair 25(8):765–773
Nanhoe-Mahabier W, Delval A, Snijders AH, Weerdesteyn V, Overeem S, Bloem BR (2012) The possible price of auditory cueing: influence on obstacle avoidance in Parkinson’s disease. Mov Disord 27(4):574–578
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L, Movement Disorder Society Task Force on Rating Scales for Parkinson’s D (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19(9):1020–1028
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N, Movement Disorder Society URTF (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23(15):2129–2170
Oude Nijhuis LB, Arends S, Borm GF, Visser JE, Bloem BR (2007) Balance confidence in Parkinson’s disease. Mov Disord 22(16):2450–2451
Lima CF, Meireles LP, Fonseca R, Castro SL, Garrett C (2008) The Frontal Assessment Battery (FAB) in Parkinson’s disease and correlations with formal measures of executive functioning. J Neurol 255(11):1756–1761
Acknowledgements
We thank Roland Loeffen for expert technical assistance. This research was performed as part of the Moving Beyond Industrial Academic Training Network toward focused treatment of age-related motor symptoms, and was funded from the European Community‘s Seventh Framework Programme FP7/2012 (under grant agreement No. 316639 to LBON, VW and BRB). Support was also received from the Netherlands Organization for Scientific Research (Veni Research Grant 916.10.106 to VW, Vidi research grant 016.076.352 to BRB) and the Radboud University Medical Centre Research (grant to JN).
Conflicts of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Ethical standard
The study was approved by the medical ethics committee of the region Arnhem-Nijmegen, the Netherlands and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects gave their written informed consent prior to the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mensink, S.H.G., Nonnekes, J., van Bon, G. et al. Additional weight load increases freezing of gait episodes in Parkinson’s disease; an experimental study. J Neurol 261, 999–1008 (2014). https://doi.org/10.1007/s00415-014-7274-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7274-2