Abstract
Cerebral arterioveneous malformations (AVM) can cause neurological symptoms and carry a risk of hemorrhage. Therapeutic options to cure or reduce AVM include surgery, embolization, irradiation, and combinations thereof. Prompted by three index cases treated in our center, we studied whether AVM embolization is associated with an increased risk of subsequent amyotrophic lateral sclerosis (ALS). In a monocenter series, we retrospectively analyzed the new development of ALS in patients who had been treated with embolization of cerebral AVM from 1986 to 2010 (n = 1,114). After a median follow-up of 11 years (range, 0–25 years) after first embolization, seven patients developed ALS with a median latency of 14 years (range, 12–17 years) and a median age of ALS onset of 38 years (range, 28–52 years). In all cases, the initial limb of ALS symptom onset was ipsilateral to the AVM. Five patients died within the follow-up period, with a range of 1–4 years after the onset of ALS symptoms. The seven patients belonged to a subgroup of 34 patients who had in common a rare AVM architecture characterized by significant perinidal angiogenesis. All cases were partially treated by at least three embolization sessions. As there is no known association between AVM and ALS, AVM embolization must be taken into account to have contributed to the development of ALS in the seven patients with this rare AVM architecture. Searching for underlying mechanisms, we compared frozen serum samples that were available from four of the patients who developed ALS, from eight patients with AVM of other architecture, and less than three embolizations who did not develop ALS, and of 20 controls. The concentration of vascular endothelial growth factor (VEGF) in the serum was lowest in AVM patients who developed ALS (245 ± 154 pmol/l) and highest in controls (409 ± 178 pmol/l). Although this difference was not statistically significant in the small sample, it suggests that low VEGF production by AVM with significant angiogenesis, possibly due to multiple embolization procedures, might have contributed to ALS development. ALS should be considered as a late complication of multiple embolizations of cerebral AVM characterized by significant perinidal angiogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral arterioveneous malformations (AVM) are characterized by multiple shunts between feeding arteries and draining veins via a nidus, without interposition of a capillary bed. Brain AVM are detected in approximately 1.1/100,000 person-years and carry a life-long risk of hemorrhage of approximately 2–4 % per year [3, 4]. Further symptoms include headache, seizures, and focal neurological deficits. While the majority of AVM do not induce perilesional angiogenesis (=AVM proper), a small proportion of AVM induce hypoxia in the surrounding brain parenchyma, leading to perinidal angiogenesis (Fig. 1) [1, 2].
Treatment of AVM includes microsurgical resection, embolization, stereotactic radiosurgery, or combinations thereof. Embolization is used to reduce nidal size prior to surgical resection or radiosurgery, to selectively eliminate areas at high risk of hemorrhage, or to completely obliterate the nidus. Cyanoacrylate, polyvinyl alcohol microparticles and, more recently, ethylene vinyl alcohol copolymers are frequently used agents for embolization [5]. The known long-term complications of embolization of cerebral AVM include hemorrhage, edema, and ischemia.
Methods
Within 1 year, we prospectively diagnosed new-onset ALS in three patients who had been treated with embolization of brain AVM several years before. To retrospectively search for further cases of ALS after AVM embolization, we systematically reviewed our electronic patient records of all patients treated with embolization for AVM at the Institute of Neuroradiology between 1986 and 2010.
Arterioveneous malformations size and shape, the diameter of the nidus, dural supply, and proportion of AVM occlusion were defined as earlier described in detail [2].
Diagnosis of definite or probable ALS followed the modified Airlie House El Escorial criteria [6]. Vascular endothelial growth factor (VEGF) concentrations were measured in serum and cerebrospinal fluid (CSF) by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, UK). Twenty CSF samples of patients with acute headache punctured for the exclusion of meningitis or subarachnoid bleeding, but without CSF abnormalities or AVM served as controls.
ANOVA, Pearson’s χ2 tests, and Pearson’s correlation were calculated with SPSS version 16 (SPSS, Somers, NY, USA). This retrospective analysis of data derived from patients treated by the authors did not require written consent of the patients or an ethics committee vote: http://www.kek.zh.ch/internet/gesundheitsdirektion/kek/de/vorgehen_gesuchseinreichung.html#subtitle-content-internet-gesundheitsdirektion-kek-de-vorgehen_gesuchseinreichung-jcr-content-contentPar-textimage_3.
Results
Population
At the Institute of Neuroradiology, University Hospital Zurich, 1,114 patients with brain AVM were treated with embolization from 1986 until the end of 2010. The total sample had a median follow-up of 11 years (range, 0–25 years).
ALS cases
Including the three index cases, seven out of the 1,114 patients had been diagnosed with definite ALS according to the electronic patient records (1:159; exact 95 % confidence interval 1:395–1:78; Table 1; Fig. 2). All diagnoses had been made at our institution. No further patients were diagnosed with probable ALS. In two additional patients who developed tetraparesis, ALS could not be ruled out, but was considered not likely: one was diagnosed with tetraparesis due to encephalitis, and the other had venous congestion caused by the AVM. Both were lost to follow-up and were not included in further analyses. Further, nine deaths due to spontaneous intracerebral hemorrhage supposedly related to the AVM were documented.
AVM and signs of ALS in MRI. a Axial T2-weighted MRI showing the AVM in the left frontal gyrus. At this stage, the patient (ID = 4; Table 1) had no symptoms of motor neuron disease. b Four years later, when the patient developed symptoms suggestive of ALS, MRI shows decreased size of the AVM after partial embolization. There are abnormal low signal intensities in the right motor cortex compatible with ALS (arrow)
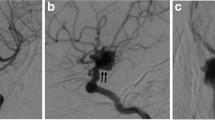
Of the seven patients with definite ALS appearing after AVM embolization, three were female and four were male (Table 1). For the ALS cases, a detailed family history had been documented. None of the patients’ parents or siblings had ALS or other neurodegenerative diseases. Median age of onset of ALS was 38 years (range, 28–52 years). In three of the seven patients, AVM embolization was performed because they had refused any other type of AVM treatment. In the other four patients, AVM was classified as inoperable, and AVM size was beyond the range of irradiation therapy. Location, size, and first symptoms of the AVM as well as the embolization agent were heterogeneous (Table 1). Three of the seven patients presented with AVM-related hemorrhage as the first symptom. However, the seven patients also shared several attributes: All AVMs were characterized by neopallial location, marked additional dural supply, and significant perinidal angiogenesis (Fig. 1). In addition, all patients underwent at least three embolization sessions (median 4; range, 3–6). No AVMs were completely obliterated (approximately 40–90 % as estimated by angiography; Fig. 3). No patient developed sustained ischemic or hemorrhagic damage due to the embolization procedure itself as analyzed clinically and by follow-up MRI. The seven patients developed the first ALS symptoms after a median latency of 14 years (range, 12–17 years) from the first embolization. In all cases, the initial limb of ALS symptom onset was ipsilateral to the AVM, before deficits spread to the other body site within weeks or months and progressed in a rather symmetrical spinal distribution type. In addition, one patient presented with additional bulbar symptoms (Table 1). MRI did not show an affection of the motor segment of the corpus callosum. Three of the seven had MRI abnormalities in motor cortex consistent with ALS findings. The later clinical course did not differ from patients with sporadic ALS. All patients had signs of both upper and lower motor neuron degeneration confirmed in standardized electrophysiological analyses. Five patients had died by the end of 2010, with a range of 1–4 years after the onset of ALS symptoms. Necropsies were not obtained.
Characteristics of all AVM patients treated with embolizations in Zurich (1986–2010). In the group of 34 patients with AVM with perinidal angiogenesis, partial embolizations, and ≥3 embolizations, seven patients developed ALS. In the other groups, no diagnosis or symptoms equivocal for ALS were documented in the electronic patient records
Analysis of the entire 1,114 AVM patients treated with embolization revealed that those seven who developed ALS belonged to a small subgroup of 34 patients (1:4.9, exact 95 % confidence intervals 1:11.5–1:2.64) characterized by AVM with significant perinidal angiogenesis, incomplete embolizations, and multiple (at least three) embolization sessions (Fig. 3). Whereas 7,606 embolization procedures were performed in our hospital in 5,754 patients with diagnoses including hyper-vascularized tumors, arteriovenous malformations, and intradural aneurysms in the time period of 1986–2010, no further diagnoses of ALS or symptoms suspicious for ALS were documented in our database.
VEGF
In the CSF laboratory, residual CSF and serum samples are routinely stored as a backup at −80 °C after analyses. Such samples were available from four patients who had developed ALS after AVM embolization. These samples had been ascertained during the diagnostic work-up of the first ALS symptoms, i.e., after the last of 3–5 embolizations (Table 1). Eight residual samples were available from other AVM patients, i.e., who had AVM without perinidal angiogenesis and did not develop ALS. Those patients had had lumbar punctures for the exclusion of subarachnoid hemorrhage at first presentation or up to 15 years after 0–2 embolizations. Although samples from AVM patients were not collected in a standardized manner and the number was too small for valid statistical analysis, we analyzed VEGF in these samples, as they are the only source for any information on the possible association of VEGF with ALS development in AVM patients. The concentrations of VEGF in the CSF samples were below the lower limit of accurate detection (15 pg/ml). The concentrations of VEGF in the serum were numerically lowest in AVM patients who developed ALS (245 ± 154 pmol/l) and highest in controls (409 ± 178 pmol/l), but in this small group, those differences were not statistically significant (Table 2).
Discussion
Amyotrophic lateral sclerosis is a sporadic or occasionally genetic disease with an annual incidence rate of approximately 2.5 per 100,000 characterized by progressive degeneration of upper and lower motor neurons in the motor cortex, brainstem, and spinal cord leading to progressive limb weakness, bulbar palsy, and respiratory insufficiency [7]. No effective therapy exists. The median survival time from ALS onset to death, mostly from respiratory failure, ranges from 20 to 48 months [8].
The patients reported here developed ALS symptoms 12–17 years after the first session of embolization treatment of cerebral AVM. The appearance of ALS in seven cases out of a population of 1,114 patients or, more precisely, out of a subgroup of 34 patients harboring the same AVM subtype cannot be explained by chance alone. Further, the median age at onset of 38 years was much lower than in sporadic ALS (approximately 65 years) [8]. Various hypotheses to explain this observation may be considered:
AVM and ALS could be genetically or pathophysiologically linked
However, no linkage of ALS to genes that may be involved in generating predispositions to AVM has been identified. The family history of the seven patients was free from ALS and AVM, and a clinical association of ALS with AVM has not been reported.
The agents used for embolization may induce motor neuron degeneration
Used as embolic agents in mammals, cyanoacrylate and polyvinyl alcohol have been reported to induce chronic inflammation in vessel walls and adjacent brain parenchyma characterized by an infiltration with leukocytes and giant cell reactions [9–12]. In chronic inflammatory CNS diseases like multiple sclerosis, inflammation may precede clinical symptoms by many years, and neuroinflammation is observed in the CNS from both human ALS patients and mouse models of the disease [13]. Thus, the embolic agents might have initiated a cascade of CNS inflammation yielding degeneration of upper and lower motor neurons via an as-yet-unknown mechanism. However, in all our cases, the initial ALS manifestation was on the body’s ipsilateral side of the AVM, i.e., resulting from impairment of the hemisphere contralateral to the embolized AVM, which makes a direct toxic effect of the embolization agent on the surrounding brain parenchyma as cause of neuronal degeneration improbable. Moreover, the lower motor neurons were in large physical distance to the location were the embolic agents were applied, but were nevertheless affected together with the upper motor neurons.
Repetitive incomplete reduction of cerebral AVM with perinidal angiogenesis by embolization may induce ALS
VEGF promotes cerebral perfusion and contributes to motor neuron integrity [14, 15]. After latency, mice with low systemic VEGF levels develop an ALS-like phenotype [16–18]. Accordingly, the significant association of the low-VEGF-2578AA genotype with increased susceptibility to ALS in males reappraises the link between reduced VEGF concentrations and ALS [17]. Further, ALS patients have decreased plasma and CSF levels of VEGF and cannot up-regulate VEGF as normally observed in the CSF in response to hypoxemia [19–22].
In patients with cerebral AVM, VEGF synthesis is also abnormal. VEGF expression in the endothelium, subendothelium, and adventitia of AVM specimens is much higher than in normal brain [23]. This local VEGF production inhibits systemic VEGF production as indicated by lower VEGF plasma levels in AVM patients compared with controls [24, 25]. In AVM with perinidal angiogenesis, arterioveneous shunts lead to hypoxia in the brain tissue surrounding the AVM. This promotes VEGF production and VEGF-derived angiogenesis (Fig. 1) [2]. After embolization, local VEGF production decreases as a consequence of both AVM reduction and improvement of perfusion. Consequently, systemic VEGF production increases, but does not compensate for the already-inhibited AVM VEGF production [24]. Accordingly, VEGF levels in the four available serum samples of our AVM patients who developed ALS tended to be lower than in AVM patients who did not develop ALS or in non-AVM controls (Table 2). In all seven patients who developed ALS (Fig. 2), multiple incomplete embolizations were necessary. It remains speculative as to whether multiplicity of embolizations or of other procedure-related factors like multiple anesthetics or any characteristics of that specific AVM that made multiple embolizations necessary are relevant risk factors. We did not find any difference between the seven patients who developed ALS and the 21 patients with AVM with similar AVM who did not. It is uncertain whether longer follow-up will show more patients with ALS. Necropsies of our patients were not available. Thus, we can only speculate about reasons of the clinically obvious onset of pathology contralateral to the AVM. Residual VEGF production of the AVM might have delayed ALS pathology in the surrounding of the AVM. For possible future cases, respective analysis as well as ALS pathology staging, e.g., by the analysis of the spreading neuronal inclusions of phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) as suggested by Brettschneider et al. [26], might be valuable.
In summary, in a cohort of 1,114 embolized AVM patients, seven out of a subgroup of 34 patients who had an AVM with perinidal angiogenesis and who were treated by ≥3 incomplete embolizations developed ALS. We cannot exclude additional undiagnosed or new ALS cases that have not yet appeared in any of the AVM patients. ALS should be considered as a risk of embolization of cerebral AVM. This risk may be restricted to a well-defined small subgroup of AVM patients. It may be useful to get genetic testing in these patients concerning the VEGF genotype [17]. Whether a limitation of the number of embolization sessions reduces this risk in respective patients remains to be shown, but it may be necessary to be cautious with multiple embolizations in respective future patients.
References
Valavanis A (1996) The role of angiography in the evaluation of cerebral vascular malformations. Neuroimaging Clin N Am 6:679–704
Valavanis A, Yasargil MG (1998) The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg 24:131–214
Al-Shahi R, Fang JS, Lewis SC, Warlow CP (2002) Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture-recapture analysis. J Neurol Neurosurg Psychiatry 73:547–551
Kondziolka D, McLaughlin MR, Kestle JR (1995) Simple risk predictions for arteriovenous malformation hemorrhage. Neurosurgery 37:851–855
The n-BCA Trial Investigators (2002) N-butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi-center trial. AJNR Am J Neuroradiol 23:748–755
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Piemonte and Valle d’Aosta Register for Amyotrophic Lateral Sclerosis (PARALS) (2001) Incidence of ALS in Italy: evidence for a uniform frequency in Western countries. Neurology 56:239–244
Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D et al (2009) Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler 10:310–323
Berenstein A, Hieshima G (1987) Clinical versus experimental use of isobutyl-2-cyanoacrylate. J Neurosurg 67:318–320
Cromwell LD, Freeny PC, Kerber CW, Kunz LL, Harris AB et al (1986) Histologic analysis of tissue response to bucrylate-pantopaque mixture. AJR Am J Roentgenol 147:627–631
Kunstlinger F, Brunelle F, Chaumont P, Doyon D (1981) Vascular occlusive agents. AJR Am J Roentgenol 136:151–156
Vinters HV, Debrun G, Kaufmann JC, Drake CG (1981) Pathology of arteriovenous malformations embolized with isobutyl-2-cyanoacrylate (bucrylate). Report of two cases. J Neurosurg 55:819–825
McGeer PL, McGeer EG (2002) Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 26:459–470
Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P (2009) Role and therapeutic potential of VEGF in the nervous system. Physiol Rev 89:607–648
Segura I, De Smet F, Hohensinner PJ, Ruizde Almodovar C, Carmeliet P (2009) The neurovascular link in health and disease: an update. Trends Mol Med 15:439–451
Dodge JC, Treleaven CM, Fidler JA, Hester M, Haidet A et al (2010) AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Ther 18:2075–2084
Lambrechts D, Poesen K, Fernandez-Santiago R, Al-Chalabi A, Del Bo R et al (2009) Meta-analysis of vascular endothelial growth factor variations in amyotrophic lateral sclerosis: increased susceptibility in male carriers of the -2578AA genotype. J Med Genet 46:840–846
Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D et al (2001) Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet 28:131–138
Just N, Moreau C, Lassalle P, Gosset P, Perez T et al (2007) High erythropoietin and low vascular endothelial growth factor levels in cerebrospinal fluid from hypoxemic ALS patients suggest an abnormal response to hypoxia. Neuromuscul Disord 17:169–173
Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F et al (2003) VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet 34:383–394
Moreau C, Devos D, Brunaud-Danel V, Defebvre L, Perez T et al (2006) Paradoxical response of VEGF expression to hypoxia in CSF of patients with ALS. J Neurol Neurosurg Psychiatry 77:255–257
Devos D, Moreau C, Lassalle P, Perez T, De Seze J et al (2004) Low levels of the vascular endothelial growth factor in CSF from early ALS patients. Neurology 62:2127–2129
Hashimoto T, Wu Y, Lawton MT, Yang GY, Barbaro NM et al (2005) Coexpression of angiogenic factors in brain arteriovenous malformations. Neurosurgery 56:1058–1065 (discussion 1058–1065)
Kim GH, Hahn DK, Kellner CP, Hickman ZL, Komotar RJ et al (2008) Plasma levels of vascular endothelial growth factor after treatment for cerebral arteriovenous malformations. Stroke 39:2274–2279
Lee JK, Hong YJ, Han CJ, Hwang DY, Hong SI (2000) Clinical usefulness of serum and plasma vascular endothelial growth factor in cancer patients: which is the optimal specimen? Int J Oncol 17:149–152
Brettschneider J, Del Tredici K, Toledo JB, Robinsons JL, Irwin DJ et al (2013) Stages of pTDP-43 pathology in amyothrophic lateral sclerosis. Ann Neurol 74:20–38
Acknowledgments
We are grateful to the patients and their relatives who gave information on their complete medical histories, and we thank Claudio Bassetti, Ansgar Studer, Hans Jung, Susanne Wegener, and many other colleagues who were involved in the examination, treatment, and characterization of the AVM patients. Without their help, this study would not have been possible.
Conflicts of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest related to this work.
Ethical standard
This study was done in accordance with national and institutional ethical standards and with the Helsinki Declaration of 1975, as revised in 2000 and 2008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valavanis, A., Schwarz, U., Baumann, C.R. et al. Amyotrophic lateral sclerosis after embolization of cerebral arterioveneous malformations. J Neurol 261, 732–737 (2014). https://doi.org/10.1007/s00415-014-7260-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7260-8