Abstract
To compare the effects of intravenous amantadine and placebo therapy on freezing of gait in patients with Parkinson’s disease, this randomized, double-blind, placebo-controlled, multicenter trial compared the efficacy of 5 days intravenous amantadine and placebo treatments on freezing of gait in 42 subjects randomly allocated 2:1 to amantadine or placebo groups. Changes in freezing of gait questionnaire (FOG-Q) scores and in unified Parkinson’s disease rating scale (UPDRS) scores, from baseline to immediately (V1) and 1 month (V2) after treatments, were assessed. Among the 42 patients (amantadine n = 29, placebo n = 13, a mean age 65.5 ± 9.4 years and a mean FOG-Q score 17.4 ± 3.2), 40 subjects completed treatment. There was no significant group difference on the primary outcome measure as total FOG-Q score changes at V1. However a significant beneficial effect of amantadine on freezing was seen at V2 in the UPDRS Part II freezing and FOG-Q item 3 scores, and there was significant improvement in the UPDRS Part IV total score and in the UPDRS Part II getting out of bed score in the amantadine group at both V1 and V2. There was no serious adverse event reported during the study. The intravenous amantadine therapy did not show a significant improvement on overall FOG-Q scores in patients with moderate-to-severe freezing; however, it might be beneficial by attenuating freezing severity and improving patients’ mobility. To prove this finding further studies with larger sample sizes are warranted in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amantadine treatment has been reported to be effective in patients with Parkinson’s disease (PD) since 1969 [1, 2]. The anti-parkinsonian effect of amantadine was initially suggested to be related to its anti-cholinergic action and modulation of dopamine metabolism in the striatum, but later the effect was reported to be related to its blocking action on the glutamate N-methyl-d-aspartate (NMDA) receptor, a voltage-dependent ion channel [3, 4]. In PD, the glutamatergic pathway in the basal ganglia circuitry is hyperactive and the dopaminergic pathway is hypoactive [5]. Thus, amantadine is expected to reverse the pathologic overactivation of the glutamatergic pathway in PD.
Freezing of gait (FOG) is a troublesome symptom in patients with advanced stage PD which is often refractory to dopaminergic drug therapy [6, 7]. The glutamate pathway is a non-dopaminergic mechanism of locomotion and gait in PD [8], and systemic administration of a glutamate antagonist can decrease akinesia and facilitate locomotion in parkinsonian animal models [5, 9]. There are a few clinical studies into amantadine treatment for FOG in PD that have produced a variety of results [10–13].
This multicenter, double-blind, placebo-controlled, randomized trial was designed to compare the effects of high-dose, intravenous amantadine treatment with those of placebo on moderate-to-severe FOG in patients with advanced PD.
Methods
Subjects
Patients diagnosed as PD according to the UK PD brain bank criteria [14], being treated with anti-parkinsonian drugs for 5 years or more, exhibiting FOG at outpatient clinic visit, FOG-Questionnaire (FOG-Q) [15] total scores ≥7, aged 30–79 years, and able to understand and agree to participate were enrolled in this study. Subject exclusion criteria included: clinically significant cognitive dysfunction (Korean version of Mini-mental Status Examination (K-MMSE) score <20), behavioral disturbance or psychiatric disorder using psychoactive drug treatment; clinically significant medical illness; cardiac diseases such as decompensated heart failure, cardiomyopathy, second-degree or third-degree atrioventricular block, bradycardia <55/min, congenital QT syndrome, Torsades de Pointes, ventricular arrhythmia, use of budipine or other drugs potentially resulting in QT interval prolongation; disorders such as renal disease, seizures, peptic ulcer disease, liver disease, pheochromocytoma, and malignancy; pregnancy or breast feeding; participation in other clinical trials in the 4 weeks preceding this trial; history of a hypersensitivity reaction to amantadine-containing drugs; and a history of heavy metal intoxication.
Standard protocol approvals, registration and patient consents
This study was approved by the institutional review boards of the four participating sites. Written informed consent was obtained from all patients. This trial is registered at ClinicalTrials.gov under the identifier NCT01313845.
Study design
The study was a randomized, double-blind, placebo-controlled, multicenter trial comparing the efficacy of intravenous amantadine and placebo treatment for moderate-to-severe FOG in patients with PD. After baseline selection, subjects were classified into Hoehn and Yahr (HY) stages ≤2.5 and ≥3. Subsequently, randomization was carried out based on a random permuted block design, separately for the two strata, 2:1 to amantadine or placebo. A patient allocation table developed by the Medical Research Collaborating Center (MRCC) at Seoul National University Hospital was delivered to the pharmacist at the clinical trial center of each of the four participating hospitals. The investigators and participants were unable to identify the medication as amantadine or placebo, and that blindness was maintained until completion of this study. Those who were taking oral amantadine stopped it, but maintained all other anti-parkinsonian drugs during intravenous therapy. After completion of intravenous infusion, they restarted it at the day of V1 evaluation.
Treatment consisted of amantadine 200 mg/500 mL or normal saline 500 mL (placebo). The medications were delivered in identical dark brown bags. Treatments were infused for 3 h, twice a day for 5 days. Assessments were conducted at baseline (V0), after 5 days of treatment (V1), and at 1 month after treatment (V2). Assessments were based on FOG-Q, K-MMSE, Movement Disorders Society Task Force Revised Unified PD Rating Scale (UPDRS), HY stage, clinical global improvement (CGI), patient global improvement (PGI), and adverse event data. The FOG-Q [15] measures patient’s experience over the last week; thus in this study, V1 measure was based on the patient’s experience over a 6-day treatment period (from admission to completion), and V2 measure was based on 1 week experience prior to V2. For investigational purposes, subjects were categorized into “ON” freezers or not, using an a priori defined subgroup of those having FOG at their best on condition through a 1-day observation at the admission day and by history taking.
Sample size
A sample size of 42 randomized patients was determined to be necessary in order to demonstrate a significant difference between amantadine and placebo groups at α level of 0.05 and a power of 0.80 to produce a clinically meaningful outcome that results in a change in FOG-Q score of 2.0 or larger in treatment group and a FOG-Q score standard deviation of 2.0 based on a previous report [12]. Sample size determination also considered the potential of a 15 % drop-out rate.
Outcome measures
The primary outcome measure was a change in FOG-Q score from V0 to V1. Secondary outcome measures were changes in FOG-Q score from V0 to V2 and changes in UPDRS Part III total score from V0 to V1 and from V0 to V2. Other investigative outcome measures were changes from V0 to V1 and from V0 to V2 in FOG-Q sub-item scores; UPDRS Part II items 11 (getting out of bed, a car, or a deep chair), 12 (walking and balance), and 13 (freezing) scores; UPDRS Part III items 10 (gait), 11 (freezing), and 12 (postural instability) scores; UPDRS Part IV total score; changes in HY stage, K-MMSE score, CGI score, and PGI score.
Statistical analysis
Efficacy measures were analyzed within an intention-to-treat paradigm that included all available data on all randomized subjects. Missing values in the primary and secondary outcomes were imputed by using the last observation carried forward method. Continuous variables in two treatment groups collected at each time point were compared by using the t test or the Mann–Whitney U test based on a normality assumption. Categorical variables in the demographic data of the two treatment groups were examined by using the χ 2 test. A mixed-effect model for repeated measures was conducted with covariates of baseline scores, two treatment groups, time, and treatment-by-time interaction. The treatment-by-time interaction term was included to test the differential treatment effect on outcome measures by time. If there was no evidence of interaction effect, treatment effect at each time point was tested. For investigational purposes, we finally examined the FOG group-by-time interaction to test the differential effect of amantadine in a predefined subgroup of patients described above, and this examination was only conducted in the amantadine treatment group. The significance level for all analyses was set at a p-value of 0.05 (two-tailed). All statistical analyses were performed using IBM SPSS (version 20.0; IBM Inc., USA) and R (version 2.15.1; http://www.r-project.org) statistics software.
Results
Disposition of subjects
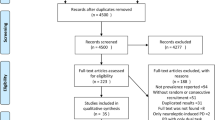
Initially, 47 subjects were enrolled in the study. Five of those subjects (10.6 %) were excluded from the study before the baseline phase (Fig. 1 includes exclusion rationale), leaving 42 subjects for randomization. One subject dropped out of the study after randomization and another subject was excluded because of protocol violation during intravenous treatment. As a result, 40 subjects (21 men, mean age 65.5 ± 9.4 years, FOG-Q mean score 17.4 ± 3.1, mean daily levodopa equivalent dose 820 ± 304.0 mg/d) completed the treatment.
After treatment, data from four subjects in the placebo group and one subject in the amantadine group were excluded during 1-month follow-up, resulting in a significantly higher drop-out rate in the placebo group (46.2 %, n = 6 of 13) than in the amantadine group (3.4 %, n = 1 of 29). Drop-outs (n = 3) commonly occurred after V1, and the reason for ceasing participation was patient dissatisfaction with symptom improvement (Fig. 1). The baseline characteristics of the subjects are comparatively summarized in Table 1. There were no significant differences between the amantadine and the placebo groups.
Mixed-effect model for repeated measure analysis
To determine whether the amantadine and placebo treatment effects were different at different times, the analysis included a treatment-by-time interaction term. The results showed a significant interaction for FOG-Q item 3 and UPDRS Part II item 13 (freezing) scores (Fig. 2). This indicated that the response to treatment was significantly different between the two treatment groups from V0 to V1 and from V0 to V2 (interaction p = 0.029 and 0.032, respectively). For this measurement, the amantadine effect was not significantly different from those of placebo at V1, however at V2, the effect of amantadine was significantly better than that of placebo for UPDRS Part II item 13 (p = 0.011) and showed a tendency to a better effect for FOG-Q item 3 (p = 0.087) (See Table 2).
Score changes from baseline to immediately after treatment and to 1 month after treatment for a item 3 of the freezing of gait questionnaire (FOG-Q) and b freezing score of the unified Parkinson’s disease rating scale (UPDRS) Part II. Based on inclusion of a treatment-by-time interaction term, there was significant treatment-time interaction in both scores (mixed-effect model repeated-measure analysis, interaction p = 0.029 and 0.032, respectively)
Since we found no significant treatment-by-time interaction for any of the other measures which meant equal effectiveness at both V1 and V2, we analyzed the amantadine treatment effect for these measures. The significant beneficial effect of amantadine was found for the UPDRS Part II item 11 (getting out of bed) and the total UPDRS Part IV scores (p = 0.004 and 0.015, respectively) at both V1 and V2. There was also marginal significance for the improvement of the UPDRS Part III total scores (p = 0.066) in the amantadine group. A mixed-effect model analysis data summary is presented in Table 2.
Investigational analysis for the treatment effect and the freezing of gait subtype, “ON” freezing
To assess differential effect on “ON” freezers, data from the amantadine-treated group was entered into additional mixed-model analyses. Among the baseline characteristics, there was no significant difference in age, duration of PD, daily medication dosages, the FOG-Q, and the K-MMSE scores between the ON freezers and non-ON freezers. There were significant differences in UPDRS Part III items 10, 11, and 12 (p = 0.006, <0.001, and 0.001, respectively), but there was no difference in the UPDRS parts I and II total and sub-item scores or in the total Part III and Part IV scores between the two groups. To determine whether the treatment effect was different at different times, we included a FOG group-by-time interaction term in the analysis. A significant FOG group-by-time interaction (interaction p = 0.041) was detected for FOG-Q item 4 (duration of FOG at worst condition), which indicated that the amantadine treatment effect was seen for both FOG groups, but at V2 the beneficial effect was more pronounced in the subjects with ON-freezing than in the non-ON freezers (p = 0.034, Fig. 3). On the other hand, significantly greater improvement was observed in the non-ON freezers at both V1 and V2 in the UPDRS Part II item 12 (walking and balance) and Part III item 11 (freezing) scores (p = 0.004 and 0.029, respectively, Fig. 3). With regard to other outcome measures, there were no significant differences in the response to amantadine between the two freezing groups. A summary of the mixed-effect model results is presented in Supplementary Table 1.
Different responses to amantadine treatment in ON- and non-ON freezers. Outcome measures exhibiting significant differences from the mixed effect repeated measure analysis are shown. a Freezing of gait questionnaire (FOG-Q) item 4 score changes. b Unified Parkinson’s disease rating scale (UPDRS) Part II walking and balance score changes; interaction p = 0.607. c UPDRS Part III freezing score changes; interaction p = 0.122. *p < 0.05 a comparison between the two groups
Adverse events
There were no significant adverse events reported during the study period. Two subjects reported side effects during intravenous infusion; one reporting mild nonspecific dizziness and the other reporting palpitations. Both of these symptoms improved after a brief cessation of intravenous infusion and did not reappear upon re-administration. Three subjects, who were randomized to placebo group, dropped out after 5 days of infusion since they did not want to continue the study due to no change in their freezing symptoms.
Discussion
This randomized, double-blind, placebo-controlled trial of intravenous amantadine on moderate-to-severe FOG in advanced PD patients failed to show a significant beneficial effect on primary outcome measures. However, through investigational analysis, intravenous amantadine treatment might be potentially beneficial for improving freezing severity and patients’ mobility as measured on FOG-Q item 3 scores (freezing frequency), the UPDRS Part II item 13 (severity and frequency of freezing), the UPDRS Part II item 11 (getting out of bed), and the total UPDRS Part IV scores. Notably the intravenous amantadine effect on FOG severity was significantly demonstrated at a one-month follow-up after treatment, a time when the placebo effect was expected to be absent.
During the study, three subjects in the placebo group discontinued participation due to a lack of subjective symptomatic change after intravenous treatment, whereas there was no such drop-out in the amantadine group. Upon completion of this study, the three placebo patients received free-of-charge open label intravenous amantadine, and subjective improvement of their freezing symptoms was reported. Another notable finding was that the amantadine treatment effect was more pronounced in the non-”ON” freezers than “ON” freezers, as shown in the UPDRS Part II walking and balancing scores and Part III freezing score changes (Fig. 3). However, changes in the total FOG-Q and UPDRS scores did not show any difference; thus, further studies with larger sample sizes are warranted to confirm and interpret these observations.
There are a few clinical studies into FOG in PD. A retrospective study of the relationships between FOG and therapeutic modalities in PD reported that longer duration amantadine treatments may decrease the incidence of FOG [10]. A small-scale, double-blind, controlled trial of oral amantadine treatment showed a beneficial effect on FOG in pure akinesia [11]. An open label trial of intravenous amantadine treatment improved FOG in patients with PD, whereas it did not improve FOG in subjects with Parkinson-plus syndrome [12]. Recently, a small-scale, double-blind crossover trial involving intravenous amantadine therapy of 200 mg for 2 days has reported no significant beneficial effect of amantadine treatment compared to that of placebo on PD patients with severe dopa-resistant ON-freezing [13]. That crossover trial differed from ours with respect to study population, therapy design, and daily amantadine dose, which was half the amount in our study. Their negative-benefit results might also be explained by the amantadine effect on severe ON-freezing subjects being less than that for those not having ON-freezing. Alternatively, ON-freezing subjects might tend to have a more pronounced placebo response than that of non-ON freezing subjects. Interestingly we found that the placebo effect was significantly greater in our subgroup, ‘ON’ freezers, who received placebo (Supplementary Table 2).
A possible mechanism for the beneficial effect on FOG in our subjects might be related to an improvement in motor fluctuation and off symptoms since the UPDRS Part III motor scores and Part IV motor complication scores tended to be improved following intravenous amantadine therapy. A beneficial effect of intravenous amantadine on levodopa-related motor complications has already been reported in other controlled trials [16, 17]. In addition, anti-glutamatergic action is reported to affect parkinsonian motor symptoms as well as cognitive functioning [18–20], with the latter being associated with FOG in PD [21]. Thus, intravenous amantadine therapy might affect the mechanism controlling glutamatergic signals from the subthalamic nucleus to the pedunculopontine nucleus and other brainstem locomotor centers, an action that could relieve gait problems. Alternatively, enhanced cognitive functioning as a result of amantadine treatment might be related to a cognitive-based mechanism for improving FOG. In our study, the MMSE scores at baseline, V1 and V2 were not significantly different between amantadine (25.3 ± 3.6, 26.5 ± 3.1 and 26.3 ± 2.7, respectively) and placebo (25.2 ± 3.4, 24.7 ± 5.7 and 26.3 ± 3.8, respectively) groups. However, since detailed evaluation of cognitive functions was not conducted in the current work, further studies are needed to confirm cognitive effect in relation to FOG.
It has been reported that intravenous amantadine can rapidly improve akinetic crisis within the first 3 days of infusion in PD [22]. In addition, it has been reported that the plasma concentration of amantadine during 10 days of daily intravenous administration of 200 mg amantadine sulfate was approximately half of that in subjects receiving 600 mg of oral amantadine daily [23]. The clinical response was not clearly correlated with the serum concentration since responses were highly variable among individuals [23]; however, within the same individuals, differences in pharmacokinetic properties and effects on serum concentrations at the same dosages between the intravenous and oral drugs may explain the different responses. In the present study, daily intravenous amantadine dose was 400 mg which is a maximum dose allowed by the Korea Food and Drug Administration. Interestingly no serious adverse events were reported during our infusion therapy, and subjects tolerated it well. The usual prescribed dosage of oral amantadine by physicians is 150–300 mg daily; thus, based on the plasma concentrations of amantadine mentioned above [23], the serum concentration achieved by the present intravenous study regimen would be expected to result in approximately 2–4 times higher concentrations than those achieved by usual oral dosages. Therefore, intravenous amantadine is thought to have two advantages compared to oral drugs: rapid action and potential to produce high plasma concentrations. Among the study participants, 69 % (29 of 42) had taken oral amantadine previously (mean dosages 208.7 ± 74.9 mg/days) and maintained oral therapy at the same dosage level after completing the treatment. A comparison of our subjects with and without a priori exposure to oral amantadine detected no significant differences in outcome measures. There was also no difference in the drop-out rates of placebo group between those who were taking oral amantadine and those who were not (50 vs. 50 %).
The present study has several limitations. The sample size was small and was established based on determining differences in the primary outcome measure (FOG-Q score change); thus, the improvements detected in the UPDRS sub-item scores need to be tested further in other trials with larger sample sizes. Throughout the study, a significant number of subjects randomized to the placebo group dropped out due to reported worsening or lack of improvement of symptoms (n = 5), whereas only one amantadine group subject dropped out. The unexpectedly high drop-out rate (38 %) in the placebo group might have reduced the statistical power of this study. In addition, the definition of ‘ON’ freezers in our study was limited. According to a recent paper, ON-freezing is different from ‘pseudo ON state’ freezing [24]. Most of our patients also had FOG at their off condition, and they were not tested using a supratherapeutic dose of levodopa. Thus, future studies with more robust criteria on classifying FOG in PD are needed to confirm the differential effects of amantadine on ON-freezing versus OFF-freezing. Lastly, the primary outcome measure (FOG-Q) may inadequately represent symptom change in our patients, and investigative outcome measures used in this study (the UPDRS Part II items 11, 12 and Part III items 10, 12) were not specific to freezing. Another evaluation tool that represents the severity of freezing during everyday activity such as an accelerometric assessment of FOG needs to be used in clinical trials regarding freezing in PD, especially trials that include subjects with severe motor fluctuations. Nevertheless, our results indicate that a 5-day infusion of amantadine is safe for PD subjects and might potentially be a considerable therapeutic option for the treatment of moderate-to-severe FOG in PD. Further trials with larger sample sizes are needed to confirm the efficacy of this treatment in PD.
References
Schwab RS, England AC Jr, Poskanzer DC, Young RR (1969) Amantadine in the treatment of Parkinson’s disease. JAMA 208:1168–1170
Parkes JD, Calver DM, Zilkha KJ, Knill-Jones RP (1970) Controlled trial of amantadine hydrochloride in Parkinson’s disease. Lancet 1:259–262
Lupp A, Lucking CH, Koch R, Jackisch R, Feuerstein TJ (1992) Inhibitory effects of the antiparkinsonian drugs memantine and amantadine on N-methyl-D-aspartate-evoked acetylcholine release in the rabbit caudate nucleus in vitro. J Pharmacol Exp Ther 263:717–724
Blanpied TA, Clarke RJ, Johnson JW (2005) Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J Neurosci 25:3312–3322
Lange KW, Kornhuber J, Riederer P (1997) Dopamine/glutamate interactions in Parkinson’s disease. Neurosci Biobehav Rev 21:393–400
Okuma Y (2006) Freezing of gait in Parkinson’s disease. J Neurol 253(supp7):VII27–VII32
Giladi N (2008) Medical treatment of freezing of gait. Mov Disord 23(supp 2):S482–S488
Devos D, Defebvre L, Bordet R (2010) Dopaminergic and non-dopaminergic pharmacological hypotheses for gait disorders in Parkinson’s disease. Fundam Clin Pharmacol 24:407–421
Skuza G, Rogoz Z, Quack G, Danysz W (1994) Memantine, amantadine, and l-deprenyl potentiate the action of l-dopa in monoamine-depleted rats. J Neural Transm Gen Sect 98:57–67
Giladi N, Treves TA, Simon ES et al (2001) Freezing of gait in patients with advanced Parkinson’s disease. J Neural Transm 108:53–61
Kondo T (2006) Drug intervention for freeizng of gait resistant to dopaminergic therapy: a pilot study. Parkinsonism Relat Disord 12:S63–S66
Kim YE, Yun JY, Jeon BS (2011) Effect of intravenous amantadine on dopaminergic-drug- resistant freezing of gait. Parkinsonism Relat Disord 17:491–492
Kim YE, Yun JY, Yang HJ et al (2012) Intravenous amantadine for freezing of gait resistant to dopaminergic therapy: a randomized, double-blind, placebo-controlled, crossover clinical trial. PLoS One 7(11):e48890
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Giladi N, Shabtai H, Simon ES, Biran S, Tal J, Korczyn AD (2000) Construction of freezing of gait questionnaire for patients with parkinsonism. Parionsonism Relat Disord 6:165–170
Růzicka E, Streitová H, Jech R et al (2000) Amantadine infusion in treatment of motor fluctuations and dyskinesias in Parkinson’s disease. J Neural Transm 107:1297–1306
Del Dotto P, Pavese N, Gambaccini G et al (2001) Intravenous amantadine improves levodopa-induced dyskinesias: an acute double-blind placebo-controlled study. Mov Disord 16:515–520
Pinter MM, Birk M, Helscher J, Binder H (1999) Short-term effect of amantadine sulphate on motor performance and reaction time in patients with idopathic Parkinson’s disease. J Neural Transm 106:711–724
Muller T, Kuhn W, Schulte T, Przuntek H (2003) Intravenous amantadine sulphate application improves the performance of complex but not simple motor tasks in patients with Parkinson’s disease. Neurosci Lett 339:25–28
Inzelberg R, Bonuccelli U, Schechtman E et al (2006) Association between amantadine and the onset of dementia in Parkinson’s disease. Mov Disord 21:1375–1379
Amboni M, Cozzolino A, Longo K, Picillo M, Barone P (2008) Freezing of gait and executive functions in patients with Parkinson’s disease. Mov Disord 23:395–400
Müller T, Kuhn W, Quack G, Przuntek H (1995) Intravenous application of amantadine and antiparkinsonian efficacy in Parkinsonian patients. J Neural Transm Suppl 46:407–413
Brenner M, Haass A, Jacobi P, Schimrigk K (1989) Amantadine sulphate in treating Parkinson’s disease: clinical effects, psychometric tests and serum concentrations. J Neurol 236:153–156
Espay AJ, Fasano A, van Nuenen BF, Payne MM, Snijders AH, Bloem BR (2012) “On” state freezing of gait in Parkinson disease: a paradoxical levodopa-induced complication. Neurology 78:454–457
Acknowledgments
This study is partially supported by the grant of National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0014451). The investigational drugs were unconditionally sponsored by Hanwha Pharmaceuticals.
Conflicts of interest
There is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration Clinical Trials NCT01313845.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, J.Y., Oh, S., Kim, J.M. et al. Intravenous amantadine on freezing of gait in Parkinson’s disease: a randomized controlled trial. J Neurol 260, 3030–3038 (2013). https://doi.org/10.1007/s00415-013-7108-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7108-7