Abstract
Additional autoimmune diseases in people with multiple sclerosis (MS) and their relatives have been studied many times. Studies have employed different designs, and yielded conflicting results. We performed a systematic review, and calculated overall risk of additional autoimmune diseases in people with MS and their first-degree relatives. PubMed and Web of Science were searched. Thyroid disease, diabetes, inflammatory bowel disease, psoriasis, rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) were studied. A generic inverse variance model was used, and subgroup analysis was used to explore heterogeneity. The OR of thyroid disease was increased in both people with MS (OR 1.66; p < 0.00001) and their relatives (OR 2.38; p < 0.00001). A similar association was seen between MS and inflammatory bowel disease (OR 1.56; p < 0.0001) and psoriasis (OR 1.31; p < 0.0001), although not in relatives. There was no increase in the rate of either SLE or RA. Studies examining diabetes showed significant heterogeneity and evidence of publication bias. There is an increase in the rate of certain autoimmune diseases in people with MS and their first-degree relatives. However, this does not extend to all conditions studied. Given the nonspecific clinical presentation of thyroid disease, it should be considered in all people with MS presenting with nonspecific symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) has been described as the archetypal autoimmune disease of the central nervous system. In common with many other autoimmune diseases, MS risk appears to be influenced by both genetics and environment [1, 2]. Whilst the gene most strongly associated with MS is the HLA-DRB1*1501 MHC class II haplotype, a number of other genes, many associated with immune function, have been associated with MS in large-scale genome-wide association studies [3]. In addition, MS has been associated with vitamin D deficiency [1], which is in turn associated with a number of other autoimmune diseases [4].
It is well known that the risk of MS is increased in relatives of probands with MS, emphasizing the genetic contribution to disease [5]. The study of the risk of additional autoimmune diseases in both people with MS, and their first-degree relatives has been pursued over many years, with studies employing a variety of designs and yielding conflicting results [6].
The most recent large-scale study to attempt to address this question [7] used the Swedish National MS register together with the Swedish National Patient Register. Roshanisefat et al. [7] found no consistent evidence for an increased risk of autoimmune disease in the parents of people with MS; additionally they found that the risk of a second autoimmune disease appeared to be increased only after the diagnosis of MS [7]. This finding, which suggests that the increased risk seen in MS may be a result of the increased contact that people with MS have with health-care professionals, implies that there may be either surveillance or reporting bias underlying previous reports of an increased risk of additional diagnoses. However, the study by Roshanisefat et al. [7] is not the only one using a national database to attempt to answer the question regarding MS and autoimmune disease. National databases from Denmark [8, 9], the UK [10], California [11] and Taiwan [12] have also been employed to address this question.
There is therefore a large amount of information available examining the frequency of autoimmune disease in both people with MS and their first-degree relatives. We performed a systematic review of the frequency of selected autoimmune diseases, including (autoimmune thyroid disease, type 1 diabetes mellitus, inflammatory bowel disease, psoriasis, systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA), in both people with MS and their first-degree relatives. Heterogeneity between studies was assessed, and, where possible, overall estimates of the frequency of these diseases in both people with MS and their first-degree relatives were calculated.
Methods
Inclusion criteria
Inclusion criteria were prespecified. Papers selected for inclusion were those published after 1980 which gave figures for the prevalence of specified autoimmune diseases in both MS and healthy control populations. The control population had to be matched to the MS population in terms of age and sex, or alternatively a precise local population prevalence of autoimmune disease had to be given (approximations of overall population rates were not felt to be sufficiently precise). The control population for the “relatives of MS” population could be either directly matched, or alternatively the probands matched and their families compared.
Search strategy
PubMed and Web of Science were searched using the terms “multiple sclerosis” AND “thyroid”, “multiple sclerosis” AND “diabetes”, “multiple sclerosis” AND “Crohn’s”, “multiple sclerosis” AND “ulcerative colitis”, “multiple sclerosis” AND “inflammatory bowel disease”, “multiple sclerosis” AND “psoriasis”, “multiple sclerosis” AND “lupus”, “multiple sclerosis” AND “SLE”, “multiple sclerosis” AND “rheumatoid” and “multiple sclerosis” AND “arthritis”. The resulting abstracts were hand-searched for publications meeting the inclusion criteria. The results from each search were cross-referenced, as many of the included papers examined more than one autoimmune disease.
Statistical analysis
A generic inverse variance fixed or random effects model was used for the statistical analysis as appropriate. A random effects model was applied unless I 2 was ≤25 %, in which case a fixed effects model was used [13]. Between-study heterogeneity was assessed for each calculation using Cochran’s Q Chi-squared test and I 2 [14]. Where present, heterogeneity was explored using subgroup analysis. Risks are reported as pooled odds ratios (OR) and 95 % confidence intervals (CI). Bias was assessed using visual inspection of funnel plots, and where more than ten studies were included, quantified using an Egger p value [15]. A p value of <0.05 was considered statistically significant. Analyses were conducted using RevMan 5.1 (Cochrane Information Management Systems).
Results
Included papers
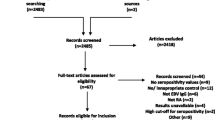
Following the initial searches 254 papers and four conference abstracts were assessed in order to ascertain whether the inclusion criteria were met. All four conference abstracts were rejected, as the same cohorts were used in later published articles. 41 unique papers were initially selected for inclusion (Fig. 1). Two studies were later excluded from the analysis as the number of relatives was not given, only the number of index MS cases [16, 17]. The remaining 39 papers, details of which are given in Table 1, were used in the analysis.
Autoimmune disease
Overall results are given in Table 2, and discussed in more detail below.
Thyroid autoimmunity
Clinical thyroid dysfunction Hypothyroidism in iodine-replete areas is generally autoimmune in nature [18], but the same cannot be said of hyperthyroidism. Some studies have therefore used hypothyroidism as a surrogate diagnosis for autoimmune thyroid dysfunction. There were 19 studies examining thyroid function in MS [9–12, 19–33]. In five of these studies [9, 19–21, 26] there were no cases of thyroid dysfunction in either MS patients or controls, and so these could not be included in the analysis. The remaining 14 studies gave an overall increased risk of thyroid dysfunction in people with MS (OR 1.66, 95 % CI 1.35–2.05, p < 0.00001), without between-study heterogeneity (Cochran’s Q p = 0.16, I 2 = 27 %; Fig. 2a). A funnel plot demonstrated no significant publication bias (Fig. 3a), with an Egger p value of 0.76. When those studies using hypothyroidism as a marker of autoimmune thyroid disease were selected [10, 12, 22, 28, 32], there was an increased risk in people with MS (OR 1.72, 95 % CI 1.00–2.97, p = 0.05) with no heterogeneity. A similar effect was seen when only those studies specifying “autoimmune thyroid disease” were selected [23–25, 27, 31, 33] (OR 1.72, 95 % CI 1.46–2.04, p < 0.00001). When cases of Hashimoto’s thyroiditis were analysed separately [11, 25, 31], there was no increased risk in people with MS (OR 1.42, 95 % CI 0.72–2.79, p = 0.31).
Concerning thyroid function in first-degree relatives of people with MS, seven studies were identified [9, 17, 28, 30, 31, 34, 35]. One study was excluded [17] as only the number of MS index cases was given, rather than the number of relatives, and one included no patients with thyroid dysfunction [9]. There was an overall increased risk of thyroid dysfunction in first-degree relatives of people with MS (OR 2.38, 95 % CI 1.95–2.91, p < 0.00001; Fig. 2b) with no significant heterogeneity or evidence of publication bias (Fig. 3b).
Thyroid autoantibodies
Ten studies examined thyroid autoantibodies in patients with MS [19, 21, 23, 26, 29, 32, 36–39]. One study [19] did not detect any antibodies in either MS patients or controls, and was excluded from the analysis. There was an overall increased rate of thyroid autoantibodies in patients with MS compared to healthy controls (OR 2.36, 95 % CI 1.32–4.20, p = 0.004) but with significant heterogeneity (Cochran’s Q p = 0.0001, I 2 = 74 %). There was no evidence of publication bias (Egger p value = 0.56). Heterogeneity was explored by examining each thyroid autoantibody individually, but each attempt at subgroup analysis resulted in a small number of studies being examined. No studies gave data regarding the rate of thyroid autoantibodies in relatives of MS patients compared to healthy controls.
Type 1 diabetes mellitus
There were 17 studies [7, 10–12, 24, 27, 28, 30–33, 40–45] examining coexisting diabetes in in MS. One study [30] included no cases of diabetes in either people with MS or controls. There was an increased risk of diabetes associated with MS overall (OR 2.02, 95 % CI 1.22–3.40, p = 0.006; Fig. 4a). However, this was associated with significant heterogeneity when all studies were considered together (Cochran’s Q p < 0.00001, I 2 = 91 %). There was no evidence of publication bias (Egger p value = 0.16; supplementary Fig. 1a). The potential reasons for the heterogeneity seen were explored by only including those studies that specified type 1 diabetes or insulin-dependent diabetes (four studies were excluded [33, 43–45]). A study using patients with stroke as the control group was also excluded [40], given the association between stroke and diabetes. This strengthened the relationship between MS and diabetes (OR 2.69, 95 % CI 1.43–5.04), but heterogeneity remained (Cochran’s Q p < 0.00001, I 2 = 94 %). Separating studies using large databases from those using questionnaires did not affect heterogeneity. Funnel plots of the subgroup analyses did not reveal any evidence of publication bias, but supported the high degree of heterogeneity found.
The risk of diabetes in first-degree relatives of people with MS was examined in 11 studies [7, 8, 27, 28, 30, 31, 34, 35, 41, 44, 45]. There was an overall increased risk of diabetes in relatives of people with MS (OR 1.49, 95 % CI 1.15–1.94, p = 0.002; Fig. 4b). This was associated with significant heterogeneity (Cochran’s Q p < 0.0001, I 2 = 74 %), and there was evidence of publication bias (Egger p value = 0.003; supplementary Fig. 1b), with the smaller studies showing a greater effect size. When the two studies not specifying type 1 diabetes were excluded [44, 45], a similar result was obtained (OR 1.48, 95 % CI 1.10–2.00, p = 0.01). Only two studies [7, 8] used databases to examine the OR of type 1 diabetes in first-degree relatives of people with MS. When these studies were examined separately no increase in diabetes risk was seen (OR 1.13, 95 % CI 0.82–1.57), with no significant heterogeneity and no evidence of publication bias (supplementary Fig. 1c). Interestingly, studies using a questionnaire design [27, 28, 30, 31, 34, 35, 41] appeared to show an increase in the risk of type 1 diabetes in first-degree relatives of people with MS (OR 1.65, 95 %CI 1.17-2.35, p = 0.005) with no significant heterogeneity between studies. However, a funnel plot revealed evidence of publication bias amongst these studies (supplementary Fig. 1d), severely limiting the applicability of the results.
Inflammatory bowel disease
Crohn’s disease
Four studies [7, 9, 24, 27] examined the number of people with MS and Crohn’s disease. There was a significantly increased risk of Crohn’s disease in people with MS (OR 1.37, 95 % CI 1.12–1.69, p = 0.003). Three studies [7, 9, 27] examined the risk of Crohn’s disease in first-degree relatives of people with MS, but showed no increased risk (OR = 1.13, 95 % CI 0.90–1.41, p = 0.31). There was no significant heterogeneity, and publication bias did not appear to be present in either analysis.
Ulcerative colitis
Six studies examined the number of people with MS and ulcerative colitis [7, 9, 24, 27, 32, 46]. Again, there was an increased risk of ulcerative colitis in people with MS (OR 2.26, 95 % CI 1.23–4.14, p = 0.009), but with significant heterogeneity (Cochran’s Q p = 0.003, I 2 = 72 %). Three studies [7, 9, 27] examined the risk of ulcerative colitis in relatives of people with MS, but showed no increased risk (OR 1.15, 95 % CI 0.95–1.40, p = 0.15). There was no publication bias.
All inflammatory bowel disease
Data from six studies [7, 9, 11, 24, 27, 28] were used to calculate the overall OR associated with MS for inflammatory bowel disease. There was an increased risk of inflammatory bowel disease with MS (OR 1.56, 95 % CI 1.28–1.90, p < 0.0001; Fig. 5a). No increase in risk was seen in relatives of people with MS (OR 1.29, 95 % CI 0.92–1.82, p = 0.14) [7, 9, 27, 28, 34, 35]; Fig. 5b). There was no significant publication bias (supplementary Fig. 2a, b), although heterogeneity was observed between those studies examining relatives (Cochran’s Q p = 0.008, I 2 = 68 %).
Psoriasis
Eight studies examined the risk of psoriasis in people with MS [7, 9, 11, 24, 27, 28, 30, 31]. There was a significant increase in the risk of psoriasis in people with MS (OR 1.31, 95 % CI 1.09–1.57, p < 0.0001). There was no significant between-study heterogeneity (Cochran’s Q p = 0.16, I 2 = 34 %; Fig. 6). Six studies examined the risk of psoriasis in first-degree relatives of people with MS [7, 9, 28, 30, 34, 35] but showed no increased risk (OR 1.17, 95 % CI 0.94–1.46, p = 0.16), and no heterogeneity or publication bias was detected (supplementary Fig. 3).
Systemic lupus erythematosus
Studies examining the risk of SLE in MS used either clinical diagnosis or serology (i.e. the presence of autoantibodies). Five studies using clinical diagnosis [9, 11, 12, 27, 28] did not appear to show an increased risk of SLE in MS (OR 2.80, 95 % CI 0.76–10.25, p = 0.12), although heterogeneity was high (Cochran’s Q p < 0.00001, I 2 = 88 %). There appeared to be an increased risk of detectable ANA [21, 32, 47–49] (OR 6.36, 95 % CI 1.36–29.69), but with a high 95 % CI and heterogeneity. There was no increased risk of detectable dsDNA antibodies [49, 50] (OR 1.26, 95 % CI 0.29–5.47) in MS. No significant publication bias was seen in either analysis. All studies examining the risk of SLE in first-degree relatives of people with MS [9, 28, 34, 35] used a clinical diagnosis, but showed no increase in risk (OR 1.53, 95 % CI 0.87–2.69).
Rheumatoid arthritis
Eleven studies examined the risk of RA in MS [7, 9–12, 24, 27, 28, 30–32]. There was no association between MS and RA seen in either MS patients (OR 1.15, 95 % CI 0.77–1.73, p = 0.49) or relatives (OR 0.98, 95 % CI 0.80–1.20, p = 0.87). There was significant heterogeneity between studies examining patients with MS, but no publication bias (Egger p value = 0.28; supplementary Fig. 4). When studies using a questionnaire design were selected [24, 27, 28, 30, 31], the lack of association between MS and RA persisted (OR 1.29, 95 % CI 0.98-1.71, p = 0.07), but without heterogeneity (Cochran’s Q p = 0.43, I 2 = 0 %).
Conclusions and discussion
This study demonstrates a consistent increase in the rate of clinical thyroid disorders amongst both people with MS and their first-degree relatives. This finding remained during most of the subgroup analyses, although not when those studies specifying Hashimoto’s thyroiditis were selected, possibly due to the small number of patients. A similar association was seen between MS and inflammatory bowel disease and MS and psoriasis, although this did not appear to extend to relatives of people with MS. Neither SLE nor RA demonstrated a significantly increased rate in either people with MS or their relatives, but significant heterogeneity was demonstrated, limiting the interpretation of these results.
Aside from clinical thyroid disease, the only autoimmune disease showing a significantly increased risk in relatives of people with MS was type 1 diabetes. However, there was a high degree of heterogeneity between studies examining this relationship. Whilst it was possible to reduce this heterogeneity by separately analysing studies that used a questionnaire design and those that used large databases, these two subgroup analyses yielded opposing results, complicating interpretation. Additionally, publication bias clearly affected the results, with the two studies using large databases showing neither publication bias nor heterogeneity; this is in contrast to the smaller-scale studies in which there was evidence of persisting bias. It is possible that poor differentiation between type 1 and type 2 diabetes may have led to an additional significant error in those studies examining diabetes prevalence, and efforts to overcome this were not made in all studies. Detection and reporting bias may also have contributed to this result.
Thyroid disease is relatively common in the general population. The symptoms of thyroid disease tend to be nonspecific and progress insidiously. The finding of a consistent increase in the rate of thyroid disease in both patients with MS and their relatives should prompt the consideration of baseline testing of thyroid function in people with MS, and alert clinicians to consider thyroid dysfunction in those patients reporting nonspecific symptoms who have not had thyroid function checked recently. The increase in the rate of thyroid autoantibodies, although of interest, should not prompt screening for these in MS patients. Thyroid autoantibodies may be present in healthy people with normal thyroid function, with the prevalence of thyroid peroxidase antibodies reported to be as high as 12 % in some series of healthy individuals [51].
Ultimately, the findings of a meta-analysis such as this are limited by the quality of the studies included. Despite the best efforts of the authors of the studies included here, it is highly likely that diseases were misclassified in the studies included here. Comparing self-reports to diagnoses verified by general practitioners, Broadley et al. [34] found that the positive predictive value of a patient-reported condition varied from 32 % for RA to 85 % for thyroid disease [34]. This is a major limitation of questionnaire-based studies. Similarly, reporting bias may have led to over-estimation of autoimmune disease prevalence amongst people with MS and their relatives. This is particularly apparent in those studies examining the frequency of diabetes in relatives of people with MS, where there was clear evidence of publication bias. However, the majority of the more recent studies used large-scale databases, potentially minimizing these sources of bias. Interestingly, in the case of diabetes in relatives, the effect of MS disappeared when studies using databases were analysed separately, highlighting the benefits of such studies.
This study does not address the potential cause(s) of the increased rate of autoimmune diseases demonstrated. This is likely to be multifactorial, as the diseases studied have differing underlying aetiologies and pathogenesis. Common factors in the development of MS and these diseases include both genetic and environmental factors, including smoking and vitamin D deficiency. However, the conditions studied do not have a single underlying pathogenesis, and as such it is difficult to use this study to shed light on the mechanisms underlying MS development. However, it demonstrates the importance of study design when addressing epidemiological questions such as these, and highlights the need to be vigilant for a second diagnoses in people who have an existing diagnosis of MS.
References
Ebers G (2008) Environmental factors and multiple sclerosis. Lancet Neurol 7(3):268–277
Ramagopalan SV, Dobson R, Meier UC, Giovannoni G (2010) Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol 9(7):727–739
Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L et al (2011) Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476(7359):214–219
Moroni L, Bianchi I, Lleo A (2012) Geoepidemiology, gender and autoimmune disease. Autoimmun Rev 11(6–7):A386–A392
Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC (2003) Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci USA 100(22):12877–12882
Disanto G, Ramagopalan SV (2012) Multiple sclerosis and co-morbid autoimmune disease: the final nail in the coffin? Mult Scler 18(10):1370–1371
Roshanisefat H, Bahmanyar S, Hillert J, Olsson T, Montgomery S (2012) Shared genetic factors may not explain the raised risk of comorbid inflammatory diseases in multiple sclerosis. Mult Scler 18(10):1430–1436
Nielsen NM, Westergaard T, Frisch M, Rostgaard K, Wohlfahrt J, Koch-Henriksen N et al (2006) Type 1 diabetes and multiple sclerosis: a Danish population-based cohort study. Arch Neurol 63(7):1001–1004
Nielsen NM, Frisch M, Rostgaard K, Wohlfahrt J, Hjalgrim H, Koch-Henriksen N et al (2008) Autoimmune diseases in patients with multiple sclerosis and their first-degree relatives: a nationwide cohort study in Denmark. Mult Scler 14(6):823–829
Somers EC, Thomas SL, Smeeth L, Hall AJ (2009) Are individuals with an autoimmune disease at higher risk of a second autoimmune disorder? Am J Epidemiol 169(6):749–755
Langer-Gould A, Albers KB, Van Den Eeden SK, Nelson LM (2010) Autoimmune diseases prior to the diagnosis of multiple sclerosis: a population-based case-control study. Mult Scler 16(7):855–861
Kang JH, Chen YH, Lin HC (2010) Comorbidities amongst patients with multiple sclerosis: a population-based controlled study. Eur J Neurol 17(9):1215–1219
Ried K (2006) Interpreting and understanding meta-analysis graphs – a practical guide. Aust Fam Physician 35(8):635–638
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Dogan S, Atakan N, Kurne A, Karabudak R (2011) High frequency of psoriasis in relatives in a Turkish multiple sclerosis cohort. Skinmed 9(1):11–13
Annunziata P, Morana P, Giorgio A, Galeazzi M, Campanella V, Lore F et al (2003) High frequency of psoriasis in relatives is associated with early onset in an Italian multiple sclerosis cohort. Acta Neurol Scand 108(5):327–331
Braverman LE, Utiger RD (1991) Introduction to hypothyroidism. In: Braverman LE, Utiger RD (eds) The thyroid, a fundamental and clinical text, 6th edn. JB Lippincott, Philadelphia, pp 919–920
Iwasaki Y, Kinoshita M (1988) Thyroid function in patients with multiple sclerosis. Acta Neurol Scand 77(3):269
Wei T, Lightman SL (1997) The neuroendocrine axis in patients with multiple sclerosis. Brain 120(Pt 6):1067–1076
Spadaro M, Amendolea MA, Mazzucconi MG, Fantozzi R, Di Lello R, Zangari P et al (1999) Autoimmunity in multiple sclerosis: study of a wide spectrum of autoantibodies. Mult Scler 5(2):121–125
Karni A, Abramsky O (1999) Association of MS with thyroid disorders. Neurology 53(4):883–885
Niederwieser G, Buchinger W, Bonelli RM, Berghold A, Reisecker F, Koltringer P et al (2003) Prevalence of autoimmune thyroiditis and non-immune thyroid disease in multiple sclerosis. J Neurol 250(6):672–675
Edwards LJ, Constantinescu CS (2004) A prospective study of conditions associated with multiple sclerosis in a cohort of 658 consecutive outpatients attending a multiple sclerosis clinic. Mult Scler 10(5):575–581
Sloka JS, Phillips PW, Stefanelli M, Joyce C (2005) Co-occurrence of autoimmune thyroid disease in a multiple sclerosis cohort. J Autoimmun Dis 2:9
Munteis E, Cano JF, Flores JA, Martinez-Rodriguez JE, Miret M, Roquer J (2007) Prevalence of autoimmune thyroid disorders in a Spanish multiple sclerosis cohort. Eur J Neurol 14(9):1048–1052
Ramagopalan SV, Dyment DA, Valdar W, Herrera BM, Criscuoli M, Yee IM et al (2007) Autoimmune disease in families with multiple sclerosis: a population-based study. Lancet Neurol 6(7):604–610
Henderson RD, Bain CJ, Pender MP (2000) The occurrence of autoimmune diseases in patients with multiple sclerosis and their families. J Clin Neurosci 7(5):434–437
Seyfert S, Klapps P, Meisel C, Fischer T, Junghan U (1990) Multiple sclerosis and other immunologic diseases. Acta Neurol Scand 81(1):37–42
Midgard R, Gronning M, Riise T, Kvale G, Nyland H (1996) Multiple sclerosis and chronic inflammatory diseases. A case-control study. Acta Neurol Scand 93(5):322–328
Laroni A, Calabrese M, Perini P, Albergoni MP, Ranzato F, Tiberio M et al (2006) Multiple sclerosis and autoimmune diseases: epidemiology and HLA-DR association in North-east Italy. J Neurol 253(5):636–639
De Keyser J (1988) Autoimmunity in multiple sclerosis. Neurology 38(3):371–374
Wynn DR, Rodriguez M, O’Fallon WM, Kurland LT (1990) A reappraisal of the epidemiology of multiple sclerosis in Olmsted county, Minnesota. Neurology 40(5):780–786
Broadley SA, Deans J, Sawcer SJ, Clayton D, Compston DA (2000) Autoimmune disease in first-degree relatives of patients with multiple sclerosis. A UK survey. Brain 123(Pt 6):1102–1111
Deretzi G, Kountouras J, Koutlas E, Zavos C, Polyzos S, Rudolf J et al (2010) Familial prevalence of autoimmune disorders in multiple sclerosis in Northern Greece. Mult Scler 16(9):1091–1101
Ioppoli C, Meucci G, Mariotti S, Martino E, Lippi A, Gironelli L et al (1990) Circulating thyroid and gastric parietal cell autoantibodies in patients with multiple sclerosis. Ital J Neurol Sci 11(1):31–36
Annunziata P, Lore F, Venturini E, Morana P, Guarino E, Borghi S et al (1999) Early synthesis and correlation of serum anti-thyroid antibodies with clinical parameters in multiple sclerosis. J Neurol Sci 168(1):32–36
Durelli L, Ferrero B, Oggero A, Verdun E, Ghezzi A, Montanari E et al (2001) Thyroid function and autoimmunity during interferon beta-1b treatment: a multicenter prospective study. J Clin Endocrinol Metab 86(8):3525–3532
Wei Q, Bundell C, Wu JS, Castley A, James I, Hollingsworth P et al (2010) Low level of systemic autoimmunity in Western Australian multiple sclerosis patients. Mult Scler 16(3):351–354
Dallmeijer AJ, Beckerman H, de Groot V, van de Port IG, Lankhorst GJ, Dekker J (2009) Long-term effect of comorbidity on the course of physical functioning in patients after stroke and with multiple sclerosis. J Rehabil Med 41(5):322–326
Marrosu MG, Cocco E, Lai M, Spinicci G, Pischedda MP, Contu P (2002) Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: a cohort study. Lancet 359(9316):1461–1465
Wertman E, Zilber N, Abramsky O (1992) An association between multiple sclerosis and type I diabetes mellitus. J Neurol 239(1):43–45
Lindegard B (1985) Diseases associated with multiple sclerosis and epilepsy. A population cohort study of 159,200 middle-aged, urban, native Swedes observed over 10 years (1970–79). Acta Neurol Scand 71(4):267–277
Warren S, Warren KG (1981) Multiple sclerosis and associated diseases: a relationship to diabetes mellitus. Can J Neurol Sci 8(1):35–39
Warren SA, Warren KG (1982) Multiple sclerosis and diabetes mellitus: further evidence of a relationship. Can J Neurol Sci 9(4):415–419
Pokorny CS, Beran RG, Pokorny MJ (2007) Association between ulcerative colitis and multiple sclerosis. Intern Med J 37(10):721–724
Michielsens B, Walravens M, Vermylen J, Carton H (1991) Diagnostic significance of antinuclear antibodies in neurologic patients. Acta Neurol Scand 84(2):102–106
Dore-Duffy P, Donaldson JO, Rothman BL, Zurier RB (1982) Antinuclear antibodies in multiple sclerosis. Arch Neurol 39(8):504–506
Szmyrka-Kaczmarek M, Pokryszko-Dragan A, Pawlik B, Gruszka E, Korman L, Podemski R et al (2012) Antinuclear and antiphospholipid antibodies in patients with multiple sclerosis. Lupus 21(4):412–420
Hyypia T, Viander M, Reunanen M, Salmi A (1982) Antibodies to nuclear and smooth muscle antigens in multiple sclerosis and control patients. Acta Neurol Scand 65(6):629–635
Hawkins BR, Cheah PS, Dawkins RL, Whittingham S, Burger HG, Patel Y et al (1980) Diagnostic significance of thyroid microsomal antibodies in randomly selected population. Lancet 2(8203):1057–1059
Acknowledgments
The authors would like to thank Dr. Sreeram Ramagopalan for invaluable assistance with locating archived manuscripts, and comments on the final text. R.D. is funded by an Association of British Neurologists/MS Society of Great Britain Clinical Research Fellowship. G.G. receives grant support from the Medical Research Council, National MS Society, MS Society of Great Britain and Northern Ireland, AIMS2CURE and the Roan Charitable Trust.
Conflicts of interest
R.D. has no conflicts of interest to declare. G.G. has received research grant support from Bayer-Schering Healthcare, Biogen-Idec, GW Pharma, Merck Serono, Merz, Novartis, Teva and Sanofi-Aventis. G.G. has received personal compensation for participating on advisory boards in relation to clinical trial design, trial steering committees and data and safety monitoring committees from: Bayer-Schering Healthcare, Biogen-Idec, Eisai, Elan, Fiveprime, Genzyme, Genentech, GSK, Ironwood, Merck-Serono, Novartis, Pfizer, Roche, Sanofi-Aventis, Synthon BV, Teva, UCB Pharma and Vertex Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

415_2012_6790_MOESM1_ESM.jpg
Supplementary material 1 (a) Funnel plot demonstrating the lack of publication bias when examining the frequency of diabetes in people with MS. (b) Funnel plot demonstrating the lack of publication bias when examining the frequency of diabetes in relatives of people with MS (JPEG 20 kb)




415_2012_6790_MOESM5_ESM.jpg
Supplementary material 5 (a) Funnel plot demonstrating the lack of publication bias when examining the frequency of inflammatory bowel disease in people with MS. (b) Funnel plot demonstrating the lack of publication bias when examining the frequency of inflammatory bowel disease in relatives of people with MS (JPEG 17 kb)


415_2012_6790_MOESM7_ESM.jpg
Supplementary material 7 (a) Funnel plot demonstrating the lack of publication bias when examining the frequency of psoriasis in people with MS. (b) Funnel plot demonstrating the lack of publication bias when examining the frequency of psoriasis in relatives of people with MS (JPEG 18 kb)


415_2012_6790_MOESM9_ESM.jpg
Supplementary material 9 (a) Funnel plot demonstrating the lack of publication bias when examining the frequency of RA in people with MS. (b) Funnel plot demonstrating the lack of publication bias when examining the frequency of RA in relatives of people with MS (JPEG 19 kb)

Rights and permissions
About this article
Cite this article
Dobson, R., Giovannoni, G. Autoimmune disease in people with multiple sclerosis and their relatives: a systematic review and meta-analysis. J Neurol 260, 1272–1285 (2013). https://doi.org/10.1007/s00415-012-6790-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6790-1