Abstract
The study aimed to investigate the volume of the olfactory bulb (OB) in patients with temporal lobe epilepsy (TLE). Specifically, we wanted to see whether the olfactory deficit typically found in TLE patients also exerts a top-down influence on the OB. Twenty patients, and 20 age- and sex-matched healthy controls underwent olfactory testing by means of the Sniffin′ Sticks testing device (measurement of odor threshold, and identification abilities). In addition, they underwent an MR scan with 2-mm-thick T2-weighted fast spin-echo images without interslice gap in the coronal plane covering the anterior and middle segments of the base of the skull. Olfactory function was significantly impaired in TLE patients compared to healthy controls both at threshold level and for odor identification (p < 0.001); in addition, OB volumes were smaller than in controls (p = 0.013). The deficit seen at the level of the OB did not correlate with the side of the epileptic focus. Assuming that the olfactory deficit in TLE patients is due to the central nervous epileptic focus it appears that the OB volume is not only subject to changes in the periphery of the olfactory system, but also changes as a consequence to changes at a cortical level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The olfactory bulb (OB) changes with olfactory function [20, 23, 28, 32]. Most of the observations relate to bottom-up influences. This means that the OB volume decreases in relation to missing input, either following experimental severing of the olfactory fibers or lateralized blockage of the naris [28], destruction of olfactory receptor neurons following infections of the upper respiratory tract [31], or the assumed rupture of olfactory fibers following head trauma [6]. There are only a few examples which allow us to hypothesize that this change in volume is also the case in central nervous diseases affecting the sense of smell, e.g. Parkinson’s disease [29], major depression [21], Alzheimer’s disease [25] or schizophrenia [27]. However, at least for Alzheimer’s disease [3, 7] or schizophrenia the function of olfactory receptor neurons has been shown to be compromised [2, 26]. Accordingly, the change of the OB volume may be due to either peripheral or central nervous damage.
The aim of the present study was to investigate olfactory function and OB volume in patients with temporal lobe epilepsy (TLE). This group of patients has been shown repeatedly to have a reduced sense of smell [5, 9, 14, 18, 30]. Assuming that the olfactory deficit was also present in our group of patients, we hypothesized that they should also exhibit a decreased OB volume. This was based on the assumption that OB volume is not only due to bottom-up but also to top-down influences with central nervous processing being affected in TLE.
Materials and methods
Participants
The participants included 20 patients (10 men, 10 women; mean age, 46.6 years; age range, 22–69 years) with unilateral temporal lobe epilepsy and 20 healthy normal control subjects (10 men, 10 women; mean age, 46.8 years; age range, 22–67 years) matched for gender, and age. Unilaterality of the symptomatology was established by unilateral seizure origin and unilateral focal preponderance of interictal epileptiform discharges in the EEG, unilateral MRI findings, and the results of neuropsychological testing during routine clinical workup; all of the patients participated in a presurgical epilepsy program. All patients were on therapeutic levels of antiepileptic medication. None had ever experienced olfactory auras.
Twenty healthy controls without history of chemosensory disorders were selected to match the patient group in gender and age; these subjects were randomly selected from a previous investigation [4] that utilized exactly the same techniques for investigating the OB and olfactory function.
Detailed information about the experiment was given to all participants and written consent was obtained. All aspects of the study were performed in accordance with the Declaration of Helsinki. The study protocol was approved by the local Ethics Board of the Faculty of Medicine of the Technical University of Dresden (numbers EK190207).
Olfactory testing
A detailed history was taken from all subjects. They were instructed not to eat or to drink anything but water 1 h prior to the measurements, in order to avoid chemosensory desensitization. Odor thresholds for phenlethylalcohol (a rose-like odor) were assessed monorhinally in all individuals by means of the “Sniffin’ Sticks” test kit [15, 17]. In addition, odor identification was measured in a birhinal fashion using the 16-item kit from “the Sticks”.
Magnetic resonance imaging (MRI)
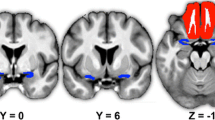
All examinations were performed using a 1.5-Tesla magnetic resonance imaging system (Sonata Sonata; Siemens, Erlangen, Germany) with a cp-head coil. Volumes of the right and left OB were determined using 2-mm-thick T2-weighted fast spin-echo images without interslice gap in the coronal plane covering the anterior and middle segments of the base of the skull. Measurements of OB volume were performed by manual segmentation of the coronal slices through the OBs using the AMIRA 3D visualization and modeling system (Visage Imaging, Carlsbad, USA) (Fig. 1). As suggested by Yousem and colleagues (1996, 1997, 1998), the sudden change of diameter at the beginning of the olfactory tract was used as the proximal demarcation of the OB. Measurements were performed “blinded” to the individual status. OB volumes were calculated by planimetric manual contouring (surface in mm2) and all surfaces were added and multiplied by two because of the 2-mm slice thickness to obtain a volume in mm3 (see Fig. 2 for a series of coronal slices through a pair of OBs).
Series of coronal slices through a pair of olfactory bulbs, from the frontal beginning of the OB until its transition into the olfactory tract which is delineated by a sudden change in diameter of the structure. MRI measurements were performed with a 1.5-Tesla scanner (Sonata Vision; Siemens, Erlangen, Germany) using an 8 channel-head coil. The investigation protocol included one whole brain anatomical sequence without interslice gap (5-mm-thick standard T1-weighted 3D sequance) for every participant to rule out any organic brain disorders. The OB sequence included acquisition of 2-mm-thick T2-weighted fast spin-echo images without interslice gap in the coronal plane covering the anterior and middle segments of the base of the skull. Images were offline processed and left and right OB limits were drawn manually on each coronal slice using the AMIRA 3D visualization and modeling system (Visage Imaging, Carlsbad, USA). OB volumes were calculated by planimetric manual contouring (surface in mm2) and all surfaces were added and multiplied by two (2-mm slice thickness) to obtain a volume in mm3. The change of diameter at the beginning of the olfactory tract was used as the distal demarcation of the OB
Statistical analyses
Data were analyzed by means of SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Analyses of variance for repeated measures and t tests were used wherever appropriate. The level of significance was set at 0.05.
Results
Psychophysics: TLE patients versus controls
When comparing birhinal measurements for odor identification, controls (M = 14.4, SD = 1.3) scored significantly higher than TLE patients (M = 11.0, SD = 2.2) (t test: t[38] = 5.81, p < 0.001). Also, controls scored higher for odor thresholds (controls: left M = 7.2, SD = 2.1; right M = 8.0, SD = 2.7; TLE patients: left M = 4.6, SD = 2.4; right M = 4.1, SD = 2.4) (factor “group”: F[1,38] = 21.7, p < 0.001).
Psychophysics: side of focus versus “healthy” side in TLE patients
When running comparisons in TLE patients only with regard to the side of focus, patients with left-sided focus (n = 11) were less sensitive on the left side compared to people with a right-sided focus (n = 9) (thresholds focus left side: M = 3.43, SD = 2.04; thresholds focus right side: M = 5.97, SD = 2.03; t test: t[18] = 2.78, p = 0.012). For the right side no significant group difference was found (thresholds focus left side: M = 3.55, SD = 2.57; thresholds focus right side: M = 4.86, SD = 2.22; t test: t[18] = 1.21, p = 0.24).
Correlations across all participants
Across all participants we found a significant correlation for the left OB volume and odor identification (r 28 = 0.43, p = 0.024) but not odor threshold (r 28 = 0.31, p = 0.11); for the right OB we found a similar constellation (odor identification: r 28 = 0.42, p = 0.027; odor threshold: r 28 = 0.26, p = 0.18).
The OB was only measurable in 14 patients; in six patients, either imaging of the OB had not been performed or the quality of the images, probably due to movement artifacts, was considered too poor to allow reliable analysis of the OB. Data from these 14 patients were compared to the results from 14 controls matched for age and gender (TLE patients: 8 men, 6 women; mean age, 44.5 years; age range, 25–69 years; controls: 8 men, 6 women; mean age, 46.9 years; age range, 26–67 years).
OB volume: TLE patients versus controls
Controls exhibited larger OB volumes than TLE patients (controls: left M = 68.3, SD = 13.9; right M = 69.4, SD = 14.4; TLE patients: left M = 54.6, SD = 13.7; right M = 54.9, SD = 15.4) (factor “group”: F[1, 26] = 7.04, p = 0.013).
OB volume: side of focus versus “healthy” side in TLE patients
When running comparisons in TLE patients only with regard to the side of focus, no significant difference was found (left-sided OB volume: patients with a left-sided focus (n = 7): M = 50.2, SD = 7.0; patients with a right-sided focus (n = 7): M = 59.0, SD = 17.8; right-sided OB volume: patients with a left-sided focus: M = 50.9, SD = 12.0; patients with a right-sided focus: M = 58.9, SD = 18.3) (t[12] < 1.23, p > 0.23) (Fig. 3).
MRI from a female patient with left-sided hippocampal sclerosis (age 21 years) and left-sided epileptic focus. Odor thresholds for phenyl ethyl alcohol were 2.75 for left-sided testing and 1.5 for right-sided testing, which is not indicative of a significant difference [36]. Olfactory bulbs were slightly smaller on the left (46.9 mm3) compared to the right side (58.5 mm3)
Discussion
Our results indicated that (1) olfactory function was significantly impaired in TLE patients compared to healthy controls both at threshold level and for odor identification, and (2) in addition, OB volumes were smaller in TLE patients than in controls. The deficit seen at the level of the OB did not correlate with the side of the focus.
These results confirm major portions of the literature on olfactory function in epilepsy. While differences are found in some investigations for odor identification only and not for odor thresholds, other studies—at the present—report a deficit at both threshold and suprathreshold level [5, 9, 14, 18, 30]. Interestingly, in contrast to work provided by others [8, 16, 35] the present findings indicate a significant decrease of olfactory sensitivity at the side of the focus which appears to be intuitive, and also corresponds to previous electrophysiological data [14]. These differences between studies may be due to, for example, differences in sample size or olfactory tests used.
The major finding of this study was that OB volume is decreased in TLE patients compared to controls. This is similar to changes in the OB volume observed in patients with olfactory loss due to head trauma [10, 12, 20, 34], infections of the upper respiratory tract [20, 24], or sinunasal disease [11]. In addition, changes in OB volume have been observed in depression [21], Alzheimer′s disease and schizophrenia [27]. In healthy subjects the OB also decreases with age [4, 33].
Considering the changes we see in TLE patients, there is increasing evidence for changes of olfactory function secondary to changes at a cortical level. A number of investigations in patients with peripheral olfactory loss revealed correlations between OB volume and odor thresholds, but not as frequently between OB volume and suprathreshold measures of olfactory function, i.e. odor discrimination and odor identification abilities. Provided that odor thresholds are more closely related to peripheral function [13, 19], these results support the idea that OB volume is more directly related to peripheral input than to higher central nervous processing of olfactory information. This hypothesis has to be reassessed, as top-down projections from the olfactory cortex to the OB is reported to be much heavier than bulbo-cortical afferent projection [22]. The specific functional role of these projections however, are not yet known in detail.
A drawback of the present study is that the volume of the olfactory cortex as without such assessment one has to be more careful with speculations on the present findings especially with regard to the question whether the observed OB atrophy would be related to a possible atrophy of the olfactory cortex. In addition, it is interesting to note that function was impaired dependent on the side of the epileptic focus while OB volume was not. This maybe due to the variability of the OB measures; however, it may also be hypothesized that anti-epileptic medication exerted an effect on OB volume.
In conclusion, when assuming that the olfactory deficit in TLE patients is due to the central nervous epileptic focus, it appears that the OB volume is not only subject to changes in the periphery of the olfactory system [1], but also changes in response to changes at a cortical level. However, it has to be acknowledged that the number of patients and controls was relatively small (14 patients and 14 controls) and thus, these findings need further confirmation.
References
Arisi GM, Foresti ML, Mukherjee S, Shapiro LA (2012) The role of olfactory stimulus in adult mammalian neurogenesis. Behav Brain Res 227:356–362 (Epub 2011 Mar 2029)
Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ, Hahn CG (2001) Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry 58:829–835
Arnold SE, Smutzer GS, Trojanowski JQ, Moberg PJ (1998) Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer’s disease and schizophrenia. Ann NY Acad Sci 855:762–775
Buschhüter D, Smitka M, Puschmann S, Gerber JC, Witt M, Abolmaali ND, Hummel T (2008) Correlation between olfactory bulb volume and olfactory function. Neuroimage 42:498–502
Campanella G, Filla A, De Michele G (1978) Smell and taste acuity in epileptic syndromes. Eur Neurol 17:136–141
Delank KW, Fechner G (1996) Zur Pathophysiologie der posttraumatischen Riechstörungen. Laryngol Rhinol Otol 75:154–159
Duda JE, Shah U, Arnold SE, Lee VMY, Trojanowski JQ (1999) The Expression of [alpha]-, [beta]-, and [gamma]-Synucleins in Olfactory Mucosa from Patients with and without neurodegenerative diseases. Exp Neurol 160:515–522
Eskenazi B, Cain WS, Novelly RA, Friend KB (1983) Olfactory functioning in temporal lobectomy patients. Neuropsychologia 21:365–374
Eskenazi B, Cain WS, Novelly RA, Mattson R (1986) Odor perception in temporal lobe epilepsy patients with and without temporal lobectomy. Neuropsychologia 24(4):553–562
Farshchi S, Seraj JM, Kashani SS, Amir Farshchi A (2012) Direction of head trauma and its effect on olfactory bulb volume in post-traumatic anosmia. Tehran Univ Med J 70:365–370
Gudziol V, Buschhuter D, Abolmaali N, Gerber J, Rombaux P, Hummel T (2009) Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis–a longitudinal study. Brain 132:3096–3101
Haehner A, Rodewald A, Gerber JC, Hummel T (2008) Changes of the volume of the human olfactory bulb with olfactory function. Arch ORL 134:621–624
Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T (2010) Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol 30:1–6
Hummel T, Pauli E, Schuler P, Kettenmann B, Stefan H, Kobal G (1995) Chemosensory event-related potentials in patients with temporal lobe epilepsy. Epilepsia 36:79–85
Hummel T, Sekinger B, Wolf S, Pauli E, Kobal G (1997) “Sniffin’Sticks”: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52
Jones-Gotman M, Zatorre RJ (1988) Olfactory identification deficits in patients with focal cerebral excision. Neuropsychologia 26:387–400
Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T (2000) Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol 257:205–211
Kohler CG, Moberg PJ, Gur RE, O’Connor MJ, Sperling MR, Doty RL (2001) Olfactory dysfunction in schizophrenia and temporal lobe epilepsy. Neuropsychiatry Neuropsychol Behav Neurol 4:83–88
Lötsch J, Reichmann H, Hummel T (2008) Different odor tests contribute differently to the diagnostics of olfactory loss. Chem Senses 33:17–21
Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T (2005) Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. NeuroReport 16:475–478
Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T (2010) Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience 169:415–421
Neville KR, Haberly LB (2004) Olfactory cortex. In: Shepherd GM (ed) The synaptic organization of the brain. Oxford University Press, New York, pp 415–454
Rombaux P, Huart C, De Volder AG, Cuevas I, Renier L, Duprez T, Grandin C (2010) Increased olfactory bulb volume and olfactory function in early blind subjects. NeuroReport 21:1069–1073
Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T (2006) Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope 116:436–439
Thomann PA, Dos Santos V, Toro P, Schönknecht P, Essig M, Schröder J (2009) Reduced olfactory bulb and tract volume in early Alzheimer's disease—a MRI study. Neurobiol Aging 30:838–841
Turetsky BI, Hahn CG, Arnold SE, Moberg PJ (2009) Olfactory receptor neuron dysfunction in schizophrenia. Neuropsychopharmacology 34:767–774 (Epub 2008 Aug 2027)
Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE (2000) Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry 157:828–830
von Gudden B (1870) Experimentaluntersuchungen ueber das periphere und zentrale Nervensystem. Archiv f Psychiatrie u Nervenkrankheiten 2:693–723
Wang J, You H, Liu JF, Ni DF, Zhang ZX, Guan J (2011) Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. Am J Neuroradiol 32:677–681
West SE, Doty RL (1995) Influence of epilepsy and temporal lobe resection on olfactory function. Epilepsia 36:531–542
Yamagishi M, Fujiwara M, Nakamura H (1994) Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology 32:113–118
Yousem DM, Geckle RJ, Bilker W, McKeown DA, Doty RL (1996) MR evaluation of patients with congenital hyposmia or anosmia. Am J Radiol 166:439–443
Yousem DM, Geckle RJ, Bilker WB, Doty RL (1998) Olfactory bulb and tract and temporal lobe volumes. Normative data across decades. Ann NY Acad Sci 855:546–555
Yousem DM, Geckle RJ, Doty RL, Bilker WB (1997) Reproducibility and reliability of volumetric measurements of olfactory eloquent structures. Acad Radiol 4:264–269
Zatorre RJ, Jones-Gotman M (1991) Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain 114:71–84
Gudziol V, Lötsch J, Hähner A, Zahnert T, Hummel T (2006) Clinical significance of results from olfactory testing. Laryngoscope 116:1858–1863
Acknowledgments
We are thankful to Dorothee Buschhüter as she provided data from healthy subjects. This research was supported from a grant from the Roland-Ernst-Stiftung to TH.
Conflicts of interest
None of the authors has any conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hummel, T., Henkel, S., Negoias, S. et al. Olfactory bulb volume in patients with temporal lobe epilepsy. J Neurol 260, 1004–1008 (2013). https://doi.org/10.1007/s00415-012-6741-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6741-x