Abstract
Inconsistent results regarding the association between statin use and risk of Parkinson’s disease (PD) have been reported. We therefore examined the association between statin use and risk of PD by conducting a detailed meta-analysis of all observational studies published regarding this subject. A literature search in the PubMed database was undertaken through April 2012, looking for observational studies evaluating the association between statin use and risk of PD. Combined relative risk (RR) estimates and 95 % confidence intervals (CIs) were calculated using a random-effects model. Subgroup and sensitivity analyses were also performed. A total of eight (five case–control and three cohort) studies contributed to the analysis. There was heterogeneity and publication bias among the studies. Statin use significantly reduced the risk of PD by 23 % (RR 0.77, 95 % CI 0.64–0.92, p = 0.005). However, long-term statin use did not significantly affect the risk of PD (RR 0.72, 95 % CI 0.45–1.13, p = 0.15). Stratification of studies by age and smoking status significantly affected the final estimate (age-adjusted RR 0.61, 95 % CI 0.42–0.86, p = 0.005; age-not-adjusted RR 0.93, 95 % CI 0.83–1.05, p = 0.23 and smoking-adjusted RR 0.60, 95 % CI 0.42–0.87, p = 0.007; smoking-not-adjusted RR 0.92, 95 % CI 0.82–1.02, p = 0.10). Furthermore, sensitivity analysis confirmed the stability of results. Our meta-analysis supports the hypothesis that statin use reduced the risk of PD. Nevertheless, more randomized clinical trials and observational studies are required to confirm this association with underlying biological mechanisms in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurological disorder characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta [1]. The etiology of PD is suggested with the involvement of oxidative stress, neuroinflammation, and mitochondrial dysfunction [2]. Primary or idiopathic PD is the second most common late-onset neurodegenerative disease, characterized by several clinical features such as resting tremor, rigidity, akinesia, depression, and sleep disturbances. The prevalence of idiopathic PD in industrialized countries has been reported to be 0.3 % in the entire population and 1 % in those older than 60 years; in individuals aged 80 years or above, the prevalence can be higher than 4 % [3].
Statins [3-hydroxy-3-methyl glutaryl-coenzyme A (HMG-CoA) reductase inhibitors] are a therapeutic class of drugs that reduce plasma cholesterol levels and used to manage and prevent coronary heart disease. As such, statins are among the most commonly prescribed drugs worldwide [4]. Recently, statins have been found to have potent anti-inflammatory and immunomodulating effects, which led to the hypothesis that statins could be neuroprotective agents [5–7]. Additionally, statins have been shown to increase striatal dopamine concentration in animal models of PD [8]. Several observational studies have been conducted to examine the association between statin use and PD risk and have generated mixed results [9–16]. Some randomized clinical trials (RCTs) on statin use in coronary heart disease [17–19] reported incidence of PD, but most of the results were ambiguous because of inadequate power.
Until now, no definite conclusion on this topic has been made. In the present meta-analysis, we examined the statin use in relation to risk of PD using epidemiological studies published up to April 2012. RCTs have been excluded, since to the best of our knowledge, there were no RCTs published specifically related to this topic.
Materials and methods
Search strategy
Two authors independently performed the literature search by using the PubMed database up to April 2012. Search terms included: “statin(s)” or “HMG-CoA reductase inhibitor(s)” or “lipid-lowering agent(s)” or “atorvastatin” or “cerivastatin” or “fluvastatin” or “lovastatin” or “mevastatin” or “pravastatin” or “rivastatin” or “rosuvastatin” or “simvastatin” and “PD” with limits, humans and English. The titles and abstracts of the resulting articles were examined to exclude irrelevant studies. The full texts of the remaining articles were read to extract information on the topic of interest. Bibliographies and citation sections of retrieved articles were also reviewed for additional pertinent studies.
Inclusion and exclusion criteria
The studies considered in this meta-analysis were all observational (case–control or cohort) studies that evaluated exposure to statins and risk of PD. Any discrepancies between authors on inclusion of a study were resolved by joint evaluation of the manuscript. Articles were excluded if they were reviews, letters to the editor without original data, editorials, and or case reports. When there were multiple publications from the same population, only data from the most recent report were included in the meta-analysis and the remaining were excluded [20].
Data extraction
Two authors independently reviewed the primary studies to assess the appropriateness for inclusion in the present meta-analysis and data were extracted. The following information was obtained from each study: (1) first author’s last name, year of publication, and country of the population studied, (2) study design, (3) number of subjects and number of PD cases, (4) effect estimates and 95 % confidence intervals (CIs), (5) assessment of statin usage, (6) PD assessment, (7) control for confounding factors by matching or adjustments, if applicable. We extracted the effect estimates that reflected the greatest degree of control for potential confounders.
Quality assessment
The quality of each study was assessed independently by two authors by using the Newcastle-Ottawa Scale (NOS) [21]. The NOS consists of three parameters of quality: selection, comparability, and outcome (cohort studies) or exposure (case–control studies). The NOS assigns a maximum of four points for selection, two points for comparability, and three points for exposure/outcome. Therefore, nine points reflects the highest quality, seven to eight points reflects medium quality, and greater than or equal to six points reflects low quality. Any discrepancies were addressed by a joint revaluation of the original article with the third author.
Data synthesis and analysis
Because the risk of PD is low, the relative risk (RR) in prospective cohort studies mathematically approximates the odds ratio [22], therefore permitting the combination of cohort and case–control studies. Publication bias was assessed using Begg and Mazumdar adjusted-rank correlation test and Egger regression asymmetry test [23, 24]. To assess the heterogeneity among studies, we used the Cochran Q and I 2 statistics: for the Q statistic, a p value <0.10 was considered statistically significant for heterogeneity; while for I 2, a value >50 % is considered a measure of heterogeneity [25]. The primary measure was combined RR of PD from individual studies calculated using the random-effects model (DerSimonian and Laird method), which accounts for heterogeneity among studies. Tests for interaction using summary estimates were performed using the method described by Altman and Bland [26]. All analyses were performed using STATA version 11.0 (StataCorp, College Station, TX). All statistical tests were two-sided and p < 0.05 was considered statistically significant, except as otherwise specified.
The primary outcome in this meta-analysis was reported as RR with 95 % CI of developing PD in statin users. To assess any link between (1) long-term statin use and risk of PD and (2) individual statin use and risk of PD, we used the available data from studies that reported RR estimates for these particular associations.
Subgroup analyses were performed according to (1) study design (case–control and cohort), (2) adjustment for age, (3) adjustment for smoking, and (4) quality of studies to examine the impact of these factors on the association. To evaluate the stability of our results, we also performed a one-way sensitivity analysis. The scope of this analysis was to evaluate the influence of individual studies by estimating the average RR in the absence of each study. The present work was performed as per the guidelines proposed by the meta-analysis of Observational Studies in Epidemiology group [27].
Results
Search results
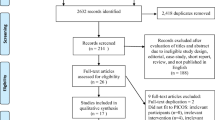
A total of 43 articles were identified during the initial search (Fig. 1). After reviewing the titles and abstracts of these articles, 31 were found to be ineligible as they were reviews, clinical trials, case reports, letters, and others that did not met the inclusion criteria. After reviewing the reference list of the remaining 12 articles, one more article was considered. After detailed evaluation of the remaining 13 full-text articles, five were excluded for reasons as described in Fig. 1.
Study characteristics
Eight relevant studies were identified, consisting of five case–control and three cohort studies involving a total of 1,472,938 subjects including 15,102 PD cases. Participants were followed-up for 2–14 years and the studies have been published between 2007 and 2012.
Five case–control studies [9, 12–15] were published between 2007 and 2010. These studies included 43,526 participants, followed-up for 2–11 years, reporting a total of 1,008 statin users among 10,760 PD cases and 3,610 statin users among 32,766 controls. Of the five, three studies reported a negative association between statin use and risk of total PD [9, 14, 15]. Statin use was ascertained by review of medical records in four studies [9, 12, 13, 15] and by self-report in one study [14]. Of them, two studies [9, 14] were conducted in North America, two [12, 15] in Europe, and one [13] in South America.
Three cohort studies [10, 11, 16] of statin use and risk of PD were published between 2007 and 2012. These included 1,429,412 participants who were followed-up for 2–14 years, reporting a total of 2,828 incident PD cases among 8,540,006 statin users. Two studies reported a negative association between statin use and risk of total PD [10, 16]. Statin use was ascertained by review of medical records in one study [10], using database in one study [11], and self-reported in one study [14]. All of these cohort studies were conducted in North America.
In a total of eight studies, six [10–14, 16] were population-based and two [9, 15] were hospital-based studies. All studies evaluated exposure to statins and the risk of PD. Four studies [9, 10, 14, 16] were controlled for potential confounding factors like age and smoking and only one study [9] was controlled for low-density lipoprotein cholesterol (LDL-C) by matching or adjustment. The characteristics of the selected studies are presented in Table 1.
Further, three studies [14–16] reported RR estimates on the association between long-term statin use and risk of PD (Table 2), and three studies [11, 12, 14] presented an examination of individual statin use in relation to risk of PD (Table 3).
Quality assessment results
According to the NOS, we found that one [12] study to be of high quality, five [9, 10, 14–16] of medium quality, and two [11, 13] of low quality.
Main analysis
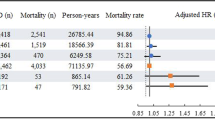
Publication bias was observed among studies using p value of the Begg’s (p = 0.03) and Egger’s (p = 0.01) tests and also the funnel plot did not have an expected funnel shape (Fig. 2). Because of significant heterogeneity (p heterogeneity = 0.009, I 2 = 63 %) was observed, a random-effects model was chosen over a fixed-effects model. A combined analysis of eight studies found statin use to be associated with significant reduction in the risk of PD (RR 0.77, 95 % CI 0.64–0.92, p = 0.005). Both the multivariable adjusted RR estimates with 95 % CIs of each study and combined RR are shown in Fig. 3.
Combined estimate of relative risk and 95 % confidence intervals of Parkinson’s disease associated with statin use based on eight studies (five case–control and three cohort) involving 1,472,938 participants including 15,102 PD cases. Squares indicate RR in each study. The square size is proportional to the weight of the corresponding study in the meta-analysis; the length of horizontal lines represents the 95 % CI. The unshaded diamond indicates the combined RR and 95 % CI (random-effects model)
Subgroup and sensitivity analyses
We found a significant inverse association between statin use and risk of PD among case–control studies (RR 0.77, 95 % CI 0.62–0.96, p = 0.02) as well as cohort studies (RR 0.74, 95 % CI 0.57–0.97, p = 0.02) as presented in Table 4. The combined RR of the studies that were able to control for age and smoking depicted a significant inverse association (RR 0.61, 95 % CI 0.42–0.86, p = 0.005 and RR 0.60, 95 % CI 0.42–0.87, p = 0.007, respectively) than studies that were not adjusted (RR 0.93, 95 % CI 0.83–1.05, p = 0.23 and RR 0.92, 95 % CI 0.82–1.02, p = 0.10, respectively). Subgroup of studies having medium quality presented significant inverse association (RR 0.60, 95 % CI 0.42–0.86, p = 0.005) than studies having low quality (RR 0.93, 95 % CI 0.81–1.07, p = 0.35) (Table 4). Tests for interaction were found to be non-significant for subgroups of study design (p interaction = 0.82) and significant for subgroups of age and smoking adjustments (p interaction = 0.03, for both).
To test the robustness of our findings, we also carried out a sensitivity analysis. To do this, the overall effect size was calculated by removing one study at a time. This analysis showed no significant variation in combined RR on excluding either outlier (very low sample size study) de Lau et al. [10] (RR 0.78, 95 % CI 0.65–0.96) or any of the other studies (RR was between 0.71 and 0.84), confirming the stability of the present results.
Results for long-term statin use
Long-term statin use (mostly ≥5 years of use) did not significantly affect the risk of PD (RR 0.72, 95 % CI 0.45–1.13, p = 0.15). However, there was high evidence of heterogeneity among these studies (p heterogeneity = 0.02, I 2 = 75 %) (Table 4). The multivariable-adjusted RR estimates with 95 % CIs of each study and combined RR are shown in Fig. 4.
Combined estimate of relative risk and 95 % confidence intervals of Parkinson’s disease associated with long-term statin use based on three studies (two case–control and one cohort) involving 141,302 participants including 56 PD cases. Squares indicate RR in each study. The square size is proportional to the weight of the corresponding study in the meta-analysis; the length of horizontal lines represents the 95 % CI. The unshaded diamond indicates the combined RR and 95 % CI (random-effects model)
Results for individual statin use
We found that among individual statins, only atorvastatin, lovastatin, and simvastatin decreased the risk of PD non-significantly (RR 0.72, 95 % CI 0.45–1.13, p = 0.15; RR 0.61, 95 % CI 0.16–2.35, p = 0.47 and RR 0.60, 95 % CI 0.35–1.03, p = 0.06, respectively) while pravastatin showed significant increased risk of PD (RR 2.22, 95 % CI 1.06–4.66, p = 0.03) (Table 4).
Discussion
In the past decade, the role of statins in the development of PD has been increasingly understood. With the present combined analysis of currently available eight observational studies, a 23 % reduction in PD risk among statin users as compared to non-users was observed and this association remained stable even after the sensitivity analysis.
The cause of neuronal death in PD remains largely unknown, but several mechanisms such as inflammation [28] or oxidative stress [29] are thought to play a major role in the pathogenesis of this disorder. In this context, statins may play a beneficial role because they attenuate neuro-inflammatory processes by inhibiting the expression of inflammatory mediators such as interleukin-6, tumor necrosis factor-α, and C-reactive protein [30], and protect cells against reactive oxygen species [31].
The present finding regarding decreased relative risk of PD among statin users is also supported by in vitro studies. In mice, simvastatin was reported to prevent striatal cells from dopamine depletion in a dose-dependent manner [8]. In another study, fluvastatin demonstrated antioxidant properties in the extra-cellular space of the rat striatum [32].
The decreased risk of PD in long-term statin users was found here to be non-significant. This is likely to be associated with varying patterns of statin use in the different study populations. In all the cases, drug use can be irregular, with months of non-use between periods of use [11, 12, 14]. Hence, cumulative amount of statin defined daily doses (DDDs) could be small despite its long duration. Conversely, other studies took into account the use of statins at high doses, which resulted in high cumulative amount of DDDs. From this point of view, it should be noted that the decreasing trend in PD relative risk has been found to be stronger for cumulative amount of statin use than for duration of its use [33]. Also, the varying definition of “long-term use” could have led to non-significant results. Moreover, the data on long-term statin use is available only in three studies among total eight studies.
On the other hand, analysis of those reports which specifically examined individual statin use in relation to PD (n = 3) suggested a non-significant protective association with atorvastatin, lovastatin, and simvastatin. Combined analysis of data from two studies involving pravastatin users found them to be 1.2 times more prone to incidence of PD than non-users. This may be due to the lipophilic character of atorvastatin, lovastatin, and simvastatin, whereas pravastatin and rosuvastatin are more hydrophilic [34]. Owing to their lipophilic structure, atorvastatin, lovastatin, simvastatin, and fluvastatin act as most powerful anti-inflammatory drugs on neurons due to their ability to penetrate more deeply into the phospholipid bilayer of cell membranes, and also have a higher affinity to the HMG-CoA reductase [35].
In the subgroup analyses, stratification by study design did not substantially affect the results. The potential confounding variables in detecting PD are age, smoking, and serum cholesterol levels [16]. Subgroup analysis of studies that were able to control for age [9, 10, 14–16] and smoking [9, 10, 12, 14, 16] were performed, and these revealed more robust inverse association as compared to the studies which are not adjusted. This represents the possible influence of age and smoking on the final combined effect estimate. Only one study [9], adjusted LDL-C, forbade us to perform a subgroup analysis on this confounding variable. An additional subgroup analysis of medium-quality studies showed a greater inverse association as compared to low-quality studies and this represents the influence of quality of studies on the final effect estimate.
The strength of the present analysis lies in inclusion of eight observational studies reporting data of more than 1.4 million participants, including 15,102 PD cases. However, our meta-analysis has several limitations. First, we did not search for unpublished studies for original data. Secondly, the included studies were different in terms of study design, confounder adjustments, and definitions of drug exposure and long-term statin use. Finally, our analysis was restricted to articles in the English language, which may have somewhat biased the results.
In conclusion, our results suggest a decreased relative risk of PD in statin users as identified by a combined meta-analysis of eight observational studies. The results support the hypothesis that cholesterol lowering with statins is beneficial for PD prevention. More RCTs and observational studies are needed to confirm this association with underlying biological mechanisms in the future. Larger studies involving more patients exposed to statins on a long-term basis may yield more information to answer the question of whether statins actually alter the PD risk to a material degree.
References
Lang AE, Lozano AM (1998) Parkinson’s disease. First of two parts. N Engl J Med 339:1044–1053
Schapira AH (2006) Etiology of Parkinson’s disease. Neurology 66:S10–S23
Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J (2011) Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol 26:S1–S58
Lamb E (2009) Top 200 drugs of 2008. http://www.pharmacytimes.com/issue/pharmacy/2009/2009-05/RxFocusTop200Drugs-0509. Accessed 2 May 2012
Becker C, Meier CR (2009) Statins and the risk of Parkinson disease: an update on the controversy. Expert Opin Drug Saf 8:261–271
Wood WG, Eckert GP, Igbavboa U, Muller WE (2010) Statins and neuroprotection: a prescription to move the field forward. Ann NY Acad Sci 1199:69–76
Wang Q, Yan J, Chen X, Li J, Yang Y, Weng J, Deng C, Yenari MA (2011) Statins: multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol 230:27–34
Selley ML (2005) Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res 1037:1–6
Huang X, Chen H, Miller WC, Mailman RB, Woodard JL, Chen PC, Xiang D, Murrow RW, Wang YZ, Poole C (2007) Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov Disord 22:377–381
de Lau LM, Stricker BH, Breteler MM (2007) Serum cholesterol, use of lipid-lowering drugs, and risk of Parkinson disease. Mov Disord 22:1985–1987
Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med 5:20
Becker C, Jick SS, Meier CR (2008) Use of statins and the risk of Parkinson’s disease: a retrospective case–control study in the UK. Drug Saf 31:399–407
Samii A, Carleton BC, Etminan M (2008) Statin use and the risk of Parkinson disease: a nested case control study. J Clin Neurosci 15:1272–1273
Wahner AD, Bronstein JM, Bordelon YM, Ritz B (2008) Statin use and the risk of Parkinson disease. Neurology 70:1418–1422
Ritz B, Manthripragada AD, Qian L, Schernhammer E, Wermuth L, Olsen J, Friis S (2010) Statin use and Parkinson’s disease in Denmark. Mov Disord 25:1210–1216
Gao X, Simon KC, Schwarzschild MA, Ascherio A (2012) Prospective study of statin use and risk of Parkinson disease. Arch Neurol 69:380–384
The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group (1998) Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 339:1349–1357
Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr (1998) Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 279:1615–1622
Heart Protection Study Collaborative Group (2002) MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360:7–22
Simon KC, Chen H, Schwarzschild M, Ascherio A (2007) Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 69:1688–1695
Ottawa Hospital Research Institute (2011) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2 May 2012
Zhang J, Yu KF (1998) What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 280:1690–1691
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Altman DG, Bland JM (2003) Interaction revisited: the difference between two estimates. BMJ 326:219
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Whitton PS (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150:963–976
Greenamyre JT, Hastings TG (2004) Biomedicine. Parkinson’s—divergent causes, convergent mechanisms. Science 304:1120–1122
Devaraj S, Chan E, Jialal I (2006) Direct demonstration of an antiinflammatory effect of simvastatin in subjects with the metabolic syndrome. J Clin Endocrinol Metab 91:4489–4496
Resch U, Tatzber F, Budinsky A, Sinzinger H (2006) Reduction of oxidative stress and modulation of autoantibodies against modified low-density lipoprotein after rosuvastatin therapy. Br J Clin Pharmacol 61:262–274
Obata T, Yamanaka Y (2000) Protective effect of fluvastatin, a new inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, on MPP(+)-induced hydroxyl radical in the rat striatum. Brain Res 860:166–169
Murtola TJ, Tammela TL, Mllttlnen L, Huhtala H, Platz EA, Ala-Opas M, Stenman UH, Auvinen A (2010) Prostate cancer and PSA among statin users in the Finnish Prostate Cancer Screening Trial. Int J Cancer 127:1650–1659
Schachter M (2004) Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19:117–125
Mason RP, Walter MF, Day CA, Jacob RF (2005) Intermolecular differences of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors contribute to distinct pharmacologic and pleiotropic actions. Am J Cardiol 96:11–23
Acknowledgments
The authors thank Dr. Dimple Kondal, Senior scientist (Biostatistician), Centre for excellence, Public Health Foundation, India for helping with the data analysis.
Conflicts of interest
No potential conflict of interest relevant to this article was reported. No funding was provided for this analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Undela, K., Gudala, K., Malla, S. et al. Statin use and risk of Parkinson’s disease: a meta-analysis of observational studies. J Neurol 260, 158–165 (2013). https://doi.org/10.1007/s00415-012-6606-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-012-6606-3